
A Review on: Current Diagnostic Techniques of Bovine Tuberculosis
*Corresponding Author(s):
Gashaw Enbiyale KasseCollege Of Veterinary Medicine And Animal Science, Gondar University, Gondar, Ethiopia
Tel:+31633844459,
Email:enbiyalegashaw@gmail.com
Abstract
Bovine tuberculosis is an infectious disease caused by M. bovis which belongs to MTBC that affects cattle, other domesticated animals and certain free or captive wild species. Bovine tuberculosis occurs worldwide and because of the zoonotic implications of the disease and production losses due to its chronic and progressive nature, eradication programs have been introduced in many countries. Infection of bovine tuberculosis is often sub clinical; when present, clinical signs are not specifically distinctive. In live animals diagnosed based on the basis of delayed hypersensitivity reactions using various tuberculin tests such as single intra-dermal, comparative intra-dermal, short thermal and stormont tests. After death, infection is diagnosed by bacteriological and histopathological examination and biochemical tests like nitrate reduction, niacin production, deamination of pyrazinamide and susceptibility to thiophen-2-carboxylic acid hydrazide. Tests based on cell mediated immunity like gamma interferon assay, the lymphocyte proliferation assay, the indirect enzyme-linked immune sorbent assay, LAM test, Myco dot antibody test and Molecular tests; polymerase chain reactions, spoligotyping and restriction fragment length polymorphism can be used. The central purpose of this review is to address diagnostic tests for tuberculosis. Considering the course of the infection in cattle, at least two tests, ideally complementary to one another, may be necessary for an adequate diagnosis: the first based on the cellular response, and the second capable of identifying anergic animals by detection of specific anti-M. bovis antibodies.
Keywords
INTRODUCTION
Existing strategies for long term B. tuberculosis control or eradication campaigns are being reconsidered in many countries because of the development of new testing technologies, increased global trade, continued struggle with wildlife reservoirs of B. tuberculosis, redistribution of international trading partner or agreements and emerging financial and animal welfare constraints on herd depopulation [8]. M. bovis shows a dysgenic colony shape on Lowenstein-Jensen medium and micro aerophilic growth, whileM. tuberculosis shows eugenic colony shape and aerophilic growth [9]. New molecular methods have been developed that provide clear criteria for the identification of M. bovis. These comprise a variety of Polymerase Chain Reaction (PCR) methods, which is based on DNA sequence variations in the direct repeat region of M. tuberculosis complex strains [10]. Not only molecular methods but also there are serological tests such as tuberculin test, gamma interferon assay, enzyme linked immune sorbent assay, mycodot antibody test and LAM test for detecting circulating antibodies and lymphocyte transformation [11].
Because of the dynamics of M. bovis transmission, the microscopic size of early lesions and the time it takes for an animal to mount a detectable immune response, no single ante (or post) mortem test for TB can be expected, on its own, to detect every infected herd and every infected animal in such herds [12]. As a result multidisciplinary approach must be employed based on current status of the disease [13]. Therefore; the objective of this review paper is to give concise review on various diagnostic techniques of bovine tuberculosis.
Specific objectives
• To address sensitivity and specificity of current diagnostic tests of bovine tuberculosis
• To identify the advantage and disadvantage of each test
CURRENT DIAGNOSTIC TECHNIQUES OF BOVINE TUBERCULOSIS
Conventional techniques
The luxuriant growth of M. tuberculosis on glycerol containing media, giving the characteristic ‘rough, tough, buff and hard to break up easily’ colonies is known as eugenic. Whereas M. bovis has sparse, small moist-sheen colonies that break up easily and thin growth on glycerol containing media that is called dysgenic, however, grows well on pyruvate containing media without glycerol [15]. Culture system is very sensitive, specific and can be used for drug sensitivity testing and for characterization of species but it requires characterization for species identification and take weeks for growing [16].
Histopathology/Microscopic examination: M. bovis can be demonstrated microscopically on direct smears from clinical samples or prepared tissue materials. The acid fastness of M. bovis is normally demonstrated with the classic Ziehl/Neelsen stain [17]. The stained slides are observed with an ordinary light microscope for the presence of acid-fast bacilli, which appear as red, colloidal or bacillary cells 1-3 microns in length occurring singly or in clumps and it considered as positive when 1 or more acid-fast bacteria were detected in at least 1 section of the sample.
The histopathologic findings were evaluated microscopically and classified as;
Positive: If tubercular granuloma displaying central necrosis with or without mineralization surrounded by macrophages, lymphocytes [18]
Inconclusive: Lesion characterized by irregular unencapsulated clusters of epithelioid macrophages but no multinucleated giant cells and necrosis, consistent with an initial stage
Negative: features not consistent with tubercular granuloma [19]
Histological examinations are practical, inexpensive and useful to make decisions on grossly suspect carcasses [20]. Despite those advantages, the requirement for obtaining postmortem samples limits the diagnostic process and most lesions can be paucibacillary leading to false-negative results [21].
Biochemical tests: Mycobacteria species identification is largely based on biochemical criteria [22].The most common biochemical tests are:
Nitrate reduction: Nitrate broth is used to determine the ability of an organism to reduce nitrate (NO3) to nitrite (NO2) using the enzyme nitrate reductase. The loop full cultured mycobacteria are added into the nitrate broth then examine for the development of a pink to red color. If the color is developed the organism is M. tuberculosis because M.bovis have not nitrate reductase enzyme [23].
Niacin test: The niacin test, which detects niacin (nicotinic acid) in aqueous extracts of the culture, is one of the elements in the identification of MTBC. M. tuberculosis excretes a large amount of niacin (nicotinic acid) into culture media or it is positive but M. bovis and M. avium are negative. The use of cyanogen bromide is an extremely hazardous and carcinogenic chemical that is banned in most countries, and should be discontinued. However, the niacin test can still be performed, using commercially available test-strips [24,25].
Deamination of pyrazinamide: When 0.1g pyrazinamide, 0.2g of pyruvic acid and 15.0g agar per liter and culture is incubate at 37°C for 4 days in a broth medium. A positive reaction is given by a pink band in the agar. M. tuberculosis is positive but M. bovis is negative because of resistance [26].
Susceptibility to Thiophen-2-Carboxylic acid Hydrazide (TCH): Thiophen-2-Carboxylic acid Hydrazide (TCH) is used to distinguish TCH-sensitivity and resistance. Mycobacterium strains by incorporated into a media such as Lowenstein-Jensen and compares the growth of bacteria. M. bovis is susceptible but M. tuberculosis is resistance [27].
Serological tests
Delayed hypersensitivity may not develop for a period of 3-6 weeks following infection. Thus, if herd/animal is suspected to have been in contact very recently with infected animals, delaying testing should be considered in order to reduce the probability of false-negatives. As the sensitivity of the test is less than 100%, it is unlikely that eradication of tuberculosis from a herd will be achieved with only a single tuberculin test. It should be recognized that when used in chronically infected animals with severe pathology, the tuberculin test may be unresponsive. The tuberculin test has not been well validated in most non-bovid and non-cervid species [29].
The two most common formats of the test used in cattle are single cervical tuberculin test and the single intra-dermal comparative cervical tuberculin test [30]. Both test formats use a purified protein derivative tuberculin prepared from a culture of M. bovis as the primary diagnostic antigen. Additionally, the SICCT test includes the use of a Mycobacterium avium-derived PPD (PPD-A) to provide a measure of environmental sensitization. It is the more specific of the two tests [31].
Single Cervical Tuberculin (SCT) test: The single cervical tuberculin test is a primary test used by intra-dermal injections of 0.1ml of PPD-B tuberculin in the mid cervical area with a reading by visual observation and palpation at 72 hours following injection. The positive reaction constitutes a diffuse swelling at the site of injection [32]. A major disadvantage of this test is that it requires animals to be handled twice, once for the tuberculin injection and a second time to read the test. Further, the person injecting and reading the test must also be adequately trained and sufficiently experienced to read the test accurately. Experience is critical; determining a response may be subjective, especially if the response to the injection is small [32].
The other disadvantage of the SID test is its lack of specificity and the number of No-Visible-Lesion reactors (NVLs) which occur [33] and failure to detect cases of minimal sensitivity, in old cows and in cows which have recently calved. As well as in early infection, in some cattle in an unresponsive state, referred to as anergy which is developed due to antigen excess or immune suppression which in-turn caused by non-specific factors such as malnutrition and stress [34].
Single Intradermal Comparative Cervical Test (SICCT): It measures hypersensitivity to tuberculin (both for M. bovis and M. avian) injected into the neck of cattle. The results are read after 72 hours. It compares the responses of M. bovis and M. avium. It only assumes that infection with M. bovis promotes a larger response to M. bovis tuberculin than to M. avium tuberculin and that infection with other types of mycobacterium promote the reverse relationship [35]. When the change in skin thickness is greater at the avian PPD injection site, the result is considered negative for BTB. Thus, if increased in the skin thickness at the injection site for the bovine (B) is greater than the increase in the 1+-skin thickness at the injection site at the avian (A) the animal is positive for bovine TB [33]. A maximum volume of 0.2 ml containing a minimum of 2000 IU of bovine and avian PPDs should be injected at cervical site [36].
Short thermal test: This type of tuberculin test is not commonly used but it used as additional test for the above tests. The principle is injection of tuberculin subcutaneously in the neck of the animal at normal temperature of not more than 39oC and for two hours later. If the temperature rise above 40oC at the time of 4, 6 and 8 hours after injection, the animal is classified as a positive reactor [32].
Stormont test: It is performed in the same way as single cervical tuberculin test in the neck with a second injection at the same site but after 7 days of first injection. After 24 hours of second injection, an increase in skin thickness of 5 mm or more should be considered as positive. It is more accurate than the Single cervical tuberculin test but a practical difficulty is the necessity for three visits to the farm [37].
Gamma interferon assay test: The interferon gamma (IFN-γ) blood test is a laboratory-based supplementary test for the diagnosis of TB in cattle. It is approved in the European Union for use in conjunction with the skin test in specific circumstances where it is needed to increase the overall diagnostic sensitivity (usually in known infected herds under TB restrictions). The assay is based on the release of IFN-? from sensitized lymphocytes during a 16-24 hours incubation period with specific antigen [36].
White blood cells from cattle that have come into contact with M. bovis release a protein (an immunological hormone) called interferon-gamma when stimulated with tuberculin in vitro. If the amount of interferon is over a certain level, the animal is classed as a reactor. The interferon-gamma test can identify a population of M. bovis infected animals that are missed by the skin test and it can do so a little earlier in the infection process than the skin test. Being a laboratory test, it is easier to standardize and quality control than the skin test and the readouts can be objectively measured. However, it also identifies a higher proportion of false positives and more expensive to perform than the skin test. Research has shown that the conventional interferon-gamma test has a sensitivity of 90% and specificity of 96.5% [38].
Disadvantages of the c-IFN test in relation to the skin tests apparent lower specificity. This may be partially overcome by replacing bovine tuberculin with defined mycobacterial antigens like the Early Secretory Antigenic Target (ESAT) 6 protein and Culture Filtrate Protein (CFP) 10 [39]. Both proteins are encoded by genes present in the M. bovis and M. tuberculosis genomes and absent from M. bovis, BCG and almost all environmental mycobacteria. But it allows for more rapid repeat testing. As a result, the test can be repeated in an animal with virtually no delay because no tuberculin is injected and there is no interference with the host’s immune system and the time taken to develop a c-IFN test response after infection (between 1 and 5 weeks) is marginally shorter than for the skin tests (3-6weeks) [40,41].
Lymphocyte proliferation assay: This test is used to determine proliferation in lymphocytes by measuring the incorporation of 3H-thymidine. The test compares the proliferation of peripheral blood lymphocytes to tuberculin PPD from M. bovis (PPD-B) and PPD from M. avium (PPD-A). The assay can be performed on whole blood or purified lymphocytes from peripheral blood samples [42]. These tests increase the specificity of the assay by removing the response of lymphocytes to non-specific or cross-reactive agents associated with non-pathogenic species of mycobacteria to which the animal may have been exposed [39].
This sero-diagnostic test have scientific value, but is not used for routine diagnosis because the test is time-consuming and the logistics and laboratory execution are complicated, meaning it requires long incubation times and the use of radio-active nucleotides. As compared with the IFN- ? test, this should be performed shortly after blood is collected. The test is relatively expensive and has not been subjected to inter-laboratory comparisons [43].
Enzyme-Linked Immuno Sorbent Assay (ELISA): In the contest of tests based on cellular immunity, ELISA has proven to be the best choice and can be a complement, rather than an alternative. It may be helpful in anergic animals for detection of antibody [44]. An advantage of the ELISA is its simplicity, but both specificity and sensitivity are limited in cattle, mostly due to the late and irregular development of the humoral immune response in cattle during the course of the disease and also poor in cattle when complex antigens such as tuberculin or culture filtrates are used respectively [45].
The two most antigens are MPB 70 and MPB 83, proteins derived from pathogenic strains of M. bovisculture filtrates, are both representative of a strong antigen-induced cell mediated immune response in the early stages of the tuberculosis infection. MPB 70 is the major secreted antigen of M. bovis, while MPB 83 is a cell wall lipoprotein. It is performed where no cellular immunity tests like the gamma-interferon test are available and where skin testing has been proven unreliable. However, its sensitivity in cattle is relatively low [22].
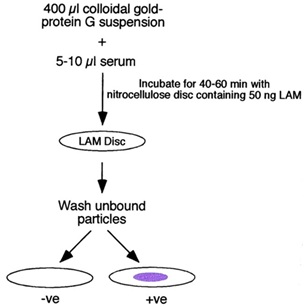
Myco dot antibody test: Myco dot antibody test is rapid sero-diagnostic test of Mycobacterium tuberculosis complex based on the detection of antimycobacterial antibodies in the serum samples of animals by employing plastic combs coated with LAM antigen which is a highly immunogenic lipopolysacharide presenting in the cell wall of all species of mycobacteria [46]. The air-dried discs were then examined for the presence of a light to deep purple spot indicative of the binding of the anti-LAM IgG–colloidal gold complex to the LAM blot [47] (Figure 1). This test is very quick, as fast as 60 minutes and easy to perform but its sensitivity is low, can only detect high levels of antibody as found in active diseases. It does not rule out TB in animals with poor antibody response as in immunocompromized and malnutrition. Not specific due to cross reactivity with other species of mycobacteria in the environment such as the Non-Tuberculosis Mycobacterium (NTM) which the animal is exposed to [46].
Molecular diagnostic techniques
It provides the possibility of detecting the presence of M. bovis in samples even when organisms have become nonviable [21] and can detect less than 10 bacteria in a clinical specimen. Their sensitivity of PCR ranges from 70-90% compared to the results of culture and its specificity varies between 90 and 95% [50]. PCR is only used on tissues that have histological (microscopic) evidence compatible with bovine tuberculosis. The result can typically be obtained within 7 days and are classified as either positive or negative. Optimal results are obtained when both PCR and isolation methods are used. DNA analysis techniques may prove to be faster and more reliable than biochemical methods for the differentiation of M. bovis from other members of the M. tuberculosis complex. PCR has been widely used in human patients evaluated for the detection of MTBC in clinical samples, mainly by sputum [8].
Spoligotyping: Spoligotyping is a useful PCR-based method to detect and type MTBC bacteria simultaneously. This method uses RLB (Reversed Line Blotting) and offers an alternative for typical Southern blotting when rapid results are required [51]. The method described based on DNA polymorphism present at one particular chromosomal locus, the "Direct Repeat" (DR) region, which is uniquely present in Mycobacterium tuberculosis complex bacteria [52]. The DR region in M. bovis BCG contains direct repeat sequences of 36 BP, which is interspersed by the non-repetitive DNA spacers of 35-41bp in length. Other MTC strains contain one or more IS6110 elements in DR-region [53]. DNA extracts from clinical samples or lysed bacteria (from freezer or Löwenstein) can also serve as template. The clinical importance of spoligotyping is determined by its rapidity, both in detecting causative bacteria and in providing epidemiologic information on strain identities. It can also be useful for identification of outbreak and can facilitate contact tracing of tuberculosis [54]. The specificity and sensitivity of this technique has been found to be 98 and 96%, respectively [55].
Restriction Fragment Length Polymorphism (RFLP): This is a gold standard for the molecular typing of M. tuberculosis due to its high discriminative power and reproducibility [56]. RFLP, as a molecular marker, is specific to a single clone/restriction enzyme combination and most RFLP markers are co-dominant (both alleles in heterozygous sample will be detected) and highly locus specific [57]. It requires large amount of Deoxyribonucleic Acid (DNA) and restricted to the mycobacterial cultures which take around 20 to 40 days to obtain sufficient DNA and applied to strains of all mycobacterium species for which suitable probes have been identified. International consensus has been achieved regarding the methodology of IS6110 RFLP typing of Mycobacterium tuberculosis complex isolates [58] and IS1245 RFLP typing of M. avium strains [50]. This is technically demanding, slow, and expensive and requires sophisticated analysis software for result analysis [53].
CONCLUSION AND RECOMMENDATIONS
Based on the above conclusions, the following recommendations are forwarded:
• Veterinarians and government should have to cooperate to widen the availability and accessibility of these diagnostic techniques as much as possible
• A great interaction among the livestock owners, medical and veterinary personnel is the prerequisites for the investigation of zoonotic importance of M. bovis and further investigations for minimizing its devastating effect in animals and humans
• Public health information campaigns are needed to raise community awareness about the risk of TB transmission through consumption of raw/undercooked meat and milk
• Detail research should be done on these diagnostic techniques
ACKNOWLEDGMENT
REFERENCES
- Noussair L, Bert F, Leflon-Guibout V, Gayet N, Nicolas-Chanoine MH (2009) Early Diagnosis of Extrapulmonary Tuberculosis by a New Procedure Combining Broth Culture and PCR. J Clin Microbiol 47: 1452-1457.
- Quinn PJ, Markey BK (2003) Concise Review of Veterinary Microbiology. Wiley, USA. Pg no: 34-37.
- Palomino JC, Leao SC (2007) Tuberculosis 2007: From Basic Science to Patient Care. Amedeo Challenge, Belgium. Pg no: 687.
- Le Roex N, Van Helden PD, Koets AP, Hoal EG (2013) Bovine TB in livestock and wildlife: What's in the genes? Physiol Genomics 45: 631-637.
- Hardstaff JL, Marion G, Hutchings MR, White PC (2013) Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Res Vet Sci 10: 86-93.
- Dhama K, Rajagunalan S, Chakraborty S, Verma AK, Kumar A, et al. (2013) Food-borne pathogens of animal origin-diagnosis, prevention, control and their zoonotic significance: a review. Pak J Biol Sci 16: 1076-1085.
- Rieder HL, VanDeun A, Kam KM, Kim SJ, Chonde TM, et al. (2007) Priorities for tuberculosis bacteriology services in low-income countries. (2ndedn). International Union against Tuberculosis and Lung Disease, Paris, France. Pg no: 28-36.
- Liébana E, Aranaz A, Mateos A, Vilafranca M, Gomez-Mampaso E, et al. (2010) Simple and rapid detection of Mycobacterium tuberculosis complex organisms in bovine tissue samples by PCR. J Clin Microbiol 33: 33-36.
- Kubica T, Agzamova R, Wright A, Rakishev G, Rushgerdes S, et al. (2006) Mycobacterium bovisisolates with Mycobacterium tuberculosis specific characteristic. Emerg Infect Dis 12: 763-765.
- Angela DP, Giuseppina C, Tony FV, Bijo B, Fatmira S et al. (2006) Detection of Mycobacterium tuberculosis complex in milk using polymerase chain reaction (PCR). Food Control 17: 776-780.
- Jeon BY, Kim SC, Je S, Kwak J, Cho JE, et al. (2010) Evaluation of enzyme-linked immunosorbent assay using milk samples as a potential screening test of bovine tuberculosis of dairy cows in Korea. Res Vet Sci 88: 390-393.
- Waters WR, Nonnecke BJ, Palmer MV, Robbe-Austermann S, Bannantine JP, et al. (2004) Use of recombinant ESAT6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by avium subsp. Avium and M. avium subsp paratuberculosis. Clin Diagn Lab Immunol 11: 729-735.
- Medeiros dos Santos L, Marrasi CD, Figueiredo SE, Lilenbuam W (2010) Potential application of new diagnostic methods for controlling bovine tuberculosis In Brazil. Braz J Microbiol 41: 531-541.
- Stenius R (1938) Differentiation by tuberculin testing of infection in cattle due to the human, bovine and avian types of tubercle bacilli. Vet Rec 50: 633-637.
- Patterson J, Grooms D (2000) Diagnosis of bovine tuberculosis: Gross necropsy, histopathology and acid fast staining. M State University Extension 35: 1-2.
- Cheesbrough M (1998) District Laboratory Practice in Tropical Countries: Part 1 (2ndedn). Cambridge University Press, New York, USA.
- International Office of Epizootics (1996) Manual of standards for diagnostic tests and vaccines: lists A and B diseases of mammals, birds and bees. Office international des epizooties, Paris, France. Pg no: 723.
- Cassidy JP, Bryson DG, Pollock JM, Evans RT, Forster F, et al. (1999) Lesions in cattle exposed to Mycobacterium bovis-inoculated calves. J Comp Pathol 121: 321-337.
- Kramer F, Modilevsky T, Waliany AR, Leedom JM, Barnes PF (1990) Delayed diagnosis of tuberculosis in patients with human immunodeficiency virus infection. Am J Med 89: 451-456.
- Varello K, Pezzolato M, Mascarino D, Ingravalle F, Caranelli M, et al. (2008) Comparison of histological techniques for the diagnosis of bovine tuberculosis in the framework of eradication programs. J Vet Diagn Invest 20: 164-169.
- Liebana E, Johnson J, Gough J, Durr P, Jahans K, et al. (2008) Pathology of naturally occurring bovine tuberculosis in England and Wales. Vet J 176: 354-360.
- Ojo O, Sheehan S, Corcoran GD, Okker M, Gover K (2008) Mycobacterium bovis strains causing smear-positive human Tuberculosis, Southwest Ireland. Emerg Infect Dis 14: 1931-1934.
- Kaufmann SH, Shaible UE (2005) 100th anniversary of Robert Koch’s Nobel Prize for the discovery of the tubercle bacillus. Trends Microbiol 13: 469-475.
- Vincent V, Gutiérrez MC (2007) Mycobacterium: Laboratory characteristics of slowly growing mycobacteria. American Society for Microbiology Pg no: 573-588.
- Wood P, Jones S (2001) BOVIGAM: an in vitro cellular diagnostic test for bovine tuberculosis.Tuberculosis (Edinb) 81: 147-155.
- Van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD et al. (1993) Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 31: 406-409.
- Michel AL, Muller B, Van Helden PD (2010) Mycobacterium bovis at the animal-human interface: A problem, or not? Vet Microbiol 140: 371-381.
- Anon (2004) Consolidated version of council directive on animal health problems affecting intra- Community trade in bovine animals and swine. O J the European Communities 29: 1977-1985.
- Coad M, Hewinson RG, Clifford D, Vordermeier HM, Whelan AO (2007) Influence of skin testing and blood storage on interferon-gamma production in cattle affected naturally with Mycobacterium bovis. Vet. Rec 160: 660-662.
- Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ (1994) The tuberculin test. Vet Microbiol 40: 111-124.
- Sharma R, Gupta V (2011) Spoligotyping for the detection of Mycobacterium tuberculosis complex bacteria. A J Biochemistry 6: 29-37.
- Radostits O, Gay CC, Hinchcliff KW, Constable PD (2007) Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats, and Horses (10thedn). Elsevier Saunders, Philadelphia USA.
- Rhodes S, Holden T, Clifford D, Dexter I, Brewer J (2012) Evaluation of Gamma Interferon and Antibody Tuberculosis Tests in Alpacas. Clin Vaccine Immunol 19: 1677-1683.
- Van Soolingen D, Bauer J, Ritacco V, Leao SC, Pavlik I, et al. (1998) IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization. J Clin Microbiol 36: 3051-3054.
- Morrison WI, Bourne FJ, Cox DR, Donnelly CA, Gettinby G (2000) Pathogenesis and diagnosis of infections with Mycobacteria bovis in cattle. Vet Rec 146: 236-242.
- OIE (2009) Bovine tuberculosis: Terrestrial manual. Pg no: 1-16.
- Regassa A, Tassew A, Amenu K, Megersa B, Abuna F, et al. (2010) A cross-sectional study on bovine tuberculosis in Hawassa town and its surrounding, Southern Ethiopia. Trop Anim Health Prod 42: 915-920.
- Wards BJ, Collins DM, de Lisle GW (1995) Detection of Mycobacterium bovis in tissues by polymerase chain reaction. Vet Microbiol 43: 227-240.
- Buddle BM, Ryan TJ, Pollock JM, Andersen P, De Lisle GW (2001) Use of ESAT-6 in the interferon-γ test for diagnosis of bovine tuberculosis following skin testing. Vet Microbiol 80: 37-46.
- Hughes MS, Ball NW, McCarroll J, Erskine M, Taylor MJ, et al. (2005) Molecular analyses of mycobacteria other than the tuberculosis complex isolated from Northern Ireland cattle. Vet Microbiol 108: 101-112.
- Wangoo A, Johnson L, Gough J, Ackbar R, Ingult S (2005) Advanced granulomatous lesions in Mycobacterium bovis-infected cattle are associated with increased expression of type I procollagen gammadelta (WC1+) T cells and CD 68+ cells. J Comp Pathol 133: 223-234.
- Lilenbaum W, Fonseca de L (2006) The use of ELISA as a complementary tool for bovine tuberculosis in Brazil. Braz J vet Res anim Sci 43: 256-261.
- Griffen JFT, Cross JP, Chinn DN, Rogers CR, Buchan GS (1994) Diagnosis of tuberculosis due tobovis in New Zealand red deer (Cervuselephus) using a composite blood test (BTB) and antibody assays. N Z Vet J 42: 173-179.
- Lilenbaum W, Marassi DC, Medeiros LS (2011) Use of MPB-70 ELISA as a complementary test for bovine tuberculosis in the field in Brazil. Vet Rec 168: 167-168.
- OIE (2004) Manual of diagnostic tests and vaccines for terrestrial animals (5thedn). World Organization for Animal Health, Paris, France.
- Chan ED, Reves R, Belisle JT, Brennan PJ, Hahn WE (2000) Diagnosis of Tuberculosis by a Visually Detectable Immunoassay for Lipoarabinomannan. Am J Respir Crit Care Med 161: 1713-1719.
- Whelan AO, Hope JC, Howard CJ, Clifford D, Hewinson RG,et al. (2003) Modulation of the bovine delayed-type hypersensitivity responses to defined mycobacterial antigens by a synthetic bacterial lipopeptide. Infect Immun 71: 6420-6425.
- Whipple DL, Palmer MV, Slaughter RE, Jones SL (2001) Comparison of purified protein derivatives and effect of skin testing on results of a commercial gamma interferon assay for diagnosis of tuberculosis in cattle. J Vet Diagn Invest 13: 117-122.
- Miller J, Jenny A, Rhyan J, Saari D, Suarez D (1997) Detection of Mycobacterium bovis in formalin-fixed, paraffin-embedded tissues of cattle and elk by PCR amplification of an IS6110 sequence specific for Mycobacterium tuberculosis complex organisms. J Vet Diagn Invest 9: 244-249.
- World Health Organization (2016) The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. Policy update, World Health Organization, Geneva Switzerland.
- Groenen PM, Bunschoten AE, van Soolingen D, van Embden JD (1993) Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; Application for strain differentiation by a novel method. Mol Microbiol 10: 1057-1065.
- Hermans PW, van Soolingen D, Bik EM, De Haas PE, Dale JW, et al. (1991) The insertion element IS987 from bovis BCG is located in a hot spot integration region for insertion elements in M. tuberculosis complex strains. Infect Immun 59: 2695-2705.
- Rodriguez-Campos S, Smith NH, Boniotti MB, Aranaz A (2014) Overview and phylogeny of Mycobacterium tuberculosis complex organisms: Implications for diagnostics and legislation of bovine tuberculosis. Res Vet Sci 97: 5- 19.
- Mangiapan G, Vokurka M, Schouls L, Cadranel J, Lecossier D, et al. (1996) Sequence capture - PCR improves the detection of mycobacterial DNA in clinical specimens. J Clin Microb 3: 1209-1215
- Iwnetu R, Van Den Hombergh J, Woldeamanuel Y, Asfaw M, Gebrekirstos C et al. (2009) Is tuberculous lymphadenitis over-diagnosed in Ethiopia? Comparative performance of diagnostic tests for mycobacterial lymphadenitis in a high-burden country. Scand J of Infect Dis 41: 462-468.
- Collin DM, De Lisle GW, Collins JD, Costello E (1994) DNA restriction fragment typing of Mycobacterium bovis isolates from cattle and badgers in Ireland. Vet Rec 134: 681-682.
- Aranaz A, Liébana E, Mateos A, Domínguez L, Cousins D (1998) Restriction fragment length polymorphism and spacer oligonucleotide typing: A comparative analysis of fingerprinting strategies for Mycobacterium bovis. Vet Microbiol 61: 311-324.
- Thoen CO, Williams DE (1994) Tuberculosis, tuberculoidoses and other mycobacterial infections. In: Beran GW (2ndedn). CRC Press. Pg no: 41-55.
Citation: Aman E, Dessalegn B, Masrie O, Debalke D, Enbiyale G, et al. (2017) A Review on: Current Diagnostic Techniques of Bovine Tuberculosis. Archiv Zool Stud 1: 001.
Copyright: © 2017 Endris Aman, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

