
Development of Liposomes-in-Hydrogel Formulations Containing Betamethasone for Topical Therapy
*Corresponding Author(s):
Helena FerreiraCespu, Instituto De Investigação E Formação Avançada Em Ciências E Tecnologias Da Saúde, Rua Central De Gandra, 1317, 4585-116 Gandra PRD, Portugal
Tel:+351 224157136,
Fax:+351 224157102
Email:helenaferreira@dep.uminho.pt
Artur Cavaco-Paulo
Centre Of Biological Engineering, University Of Minho, Campus Of Gualtar, 4710-057 Braga, Portugal
Tel:+351 253510271,
Fax:+351 253510293
Email:helenaferreira@dep.uminho.pt
Abstract
In this work, betamethasone was entrapped in unilamellar liposomes that were further dispersed in two different hydrogels to promote a localized effect of the drug with minimal systemic delivery in order to minimize the associated toxicity. The optimized betamethasone-loaded liposomes showed an average particle size of 155.1+4.9 nm, narrow size distribution (polydispersity index-PDI<0.1) and positive surface charge (mean zeta potential of +19.7+2.0 mV). The physico-chemical characterization of these vesicles also demonstrated that they presented spherical shape, good physical stability and drug entrapment efficiency values higher than 80%. Following characterization, the liposome dispersion was in corporated into 1% w/w poly(acrylic acid) gel base or in 2% w/w hydroxypropyl gum guar (Jaguar HP-8®) gel base. The betamethasone entrapment efficiency in liposomes and its incorporation in hydrogel were determined using a validated High Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) method. The two hydrogel formulations demonstrated adequate properties as dermatological nanomedicines, namely pH, viscosity, physical stability and drug content.
Keywords
INTRODUCTION
Betamethasone is a highly effective corticosteroid widely used in the topical treatment of skin diseases, as psoriasis and eczema. Indeed, this synthetic glucocorticoid presents immunosuppressive and anti-inflammatory activities. Unfortunately, due to the topic and systemic side effects that commonly occur, its use is frequently restricted [1]. Optimization of betamethasone-carrier formulations to modulate the release of this steroid over a long-lasting period and reduce its systemic absorption, improving, therefore, its therapeutic effectiveness are of growing interest. Liposomal formulations have been the focus of special attention, since their introduction in the early eighties as skin drug delivery systems. Mezei and Gulasekharam were the first to use liposomes for topical therapy [2]. In vivo studies have demonstrated that liposomes, when compared to other formulations, enhance steroid concentrations in the epidermis and dermis and reduce their systemic absorption [2,3]. These studies were criticized and contradictory results have been published [4-11]. However, it has become evident that liposomes without an edge activator (deformable liposomes), for example, did not present a significant skill for transdermal drug delivery [6,12,13]. Therefore, conventional liposomes can be used to obtain a localized skin effect.
In 1988, a liposomal formulation containing the antimycotic agent econazole was first introduced in the market [9]. Since then, other liposomal formulations have been marketed and many other may follow the same trend. For instance, promising clinical data was obtained by Agarwal et al., [14] with an aqueous gel-based containing dithranol-loaded liposome for the treatment of patients with stable plaque psoriasis. The developed formulation was effective and significantly reduced the adverse effects of dithranol, having, therefore, potential advantages over other available preparations of this drug. In another study, the efficacy of a liposomal formulation of betamethasone dipropionate was compared to that of a conventional gel in patients presenting atopic eczema and psoriasis vulgaris [15]. Although results reflected no improvements in the treatment of psoriasis, in eczema conditions the liposomal formulation led to a higher reduction of the erythema and skin scaling compared with the conventional gel.
Singh et al., reported that the entrapment of the non-steroidal anti-inflammatory drug Nimesulide in Multilamellar Liposomes (MLVs) improved its performance comparatively to the marketed gel and to a Carbopol® hydrogel base formulations [16]. Further studies demonstrated that Carbopol® hydrogel base containing liposomes was also successfully explored as a topical formulation to release an antifungal agent during a considerable period of time [17].
In the present study, betamethasone was entrapped into Large Unilamellar Liposomes (LUVs) to obtain a localized topical effect, without the undesired systemic side effects. LUVs were composed of Egg-yolk Phosphatidylcholine (EPC) and α-tocopherol, which was used to protect liposomes against oxidation. As liposomes were prepared from phospholipids, the main components of cells membranes, they act as non-irritating moisturizing agents and they are biocompatible, biodegradable and nontoxic [18,19]. It has also been reported that liposomes of EPC are biocompatible with human skin fibroblasts showing the potentialities to be applied in the treatment of skin diseases [20]. The physicochemical characterization of the prepared liposomes included the determination of particle size and Poly Dispersity Index (PDI), zeta-potential, morphology, betamethasone entrapment efficiency and physical stability. To produce topical formulations, the liposomes were incorporated in 1% w/w poly (acrylic acid) gel base or in 2% w/w hydroxypropyl guar gum (Jaguar HP-8®) gel base. In contrast with marketed formulations where betamethasone is dispersed in gels or creams, in this work it was prepared liposome’s-in-hydrogel formulations. Liposomes were incorporated in hydrogels to obtain a controlled release of betamethasone and to increase its concentration on the formulation, since that betamethasone has limited solubility in water. Poly (acrylic acid) and hydroxypropyl guar gum are able to retain large quantities of water even when subjected to some pressure [21,22]. These polymers provide, therefore, viscosity to aqueous solutions even at low concentrations and are also compatible with various drugs. Additionally, poly(acrylic acid) and hydroxypropyl guar gum have been shown to be biocompatible in several biomedical applications [23,24], making them a good candidate for the purposed work Hydrogels are often used as skin formulations due to their technological feasibility of controlling their viscosity, providing suitable characteristics to be applied onto the skin [25]. Additionally, the use of liposome’s-in-hydrogel as a delivery system can exhibit favorable texture properties for topical administration and can lead to a sustained drug release throughout their prolonged contact time with a tissue, contributing the high viscosity presented by some hydrogels to liposomes stabilization [26,27].
A High Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD) method was developed and validated for the determination of betamethasone entrapment efficiency in liposomes and its concentration in hydrogel formulation prepared. HPLC-DAD was also used to assess betamethasone stability to thermal, acidic and alkaline stress conditions.
The aim of this work is, therefore, the development and characterization of two novel liposomes-in-hydrogel formulations containing betamethasone with high potential application in the treatment of various topical disorders. Liposomes will allow a controlled release of the entrapped drug through the hydrogel into the skin, in order to achieve a prolonged and localized effect. Additionally, being EPC the main component of liposomes, it is expected a synergistic effect with betamethasone in counteracting inflammation, due to the phospholipid antioxidant activity [28,29].
MATERIALS AND METHODS
Chemical reagents
Preparation of betamethasone-loaded liposomes
Phospholipid concentration determination
LIPOSOMES CHARACTERIZATION
Particle size, Polydispersity Index (PDI) and zeta-potential
Liposomes morphology
Betamethasone entrapment efficiency in liposomes
The corticosteroid concentrations were evaluated by a validated HPLC-DAD method described below. Analyses were performed with a La Chrom Merck Hitachi equipped with a L-7100 pump, a D-7000 interface, a L-7200 auto-sampler and a L-7455 Diode Array Detector. For the chromatographic analyses, a HPLC System Manager HSMD-7000, version 3.0 and a Lichrocart® 250-4 LiChrospher® (100 RP-18, 5 μm; Merck) column preceded by a guard column LiChrospher® 100 RP-18 (5 ?m) LiChrocart® (4x4 i.d.; Merck) were used. The system was operated at room temperature, in the isocratic mode at a flow rate of 0.8 mL/min, with a mixture of acetonitrile/water (50:50, v/v) as mobile phase. The injected volume was 20 μL and the monitorization of betamethasone was carried out at 240 nm.
Prior to HPLC quantification, samples were diluted 10x with ethanol.
HPLC-DAD method validation
A stock standard solution of betamethasone (1 mg/mL) in ethanol was diluted with water/ethanol (1:9, v/v) in order to obtain seven standard solutions of different concentrations (ranging between 5 and 130 ?g/mL).
The specificity of the analytical method was evaluated by comparing the chromatograms of the standard solutions with samples of empty liposomes and samples obtained by the mixture of betamethasone and liposomes (i.e., spiked with empty liposomes).
Linearity was assessed through the construction of calibration curves with a set of seven concentration levels ranging from 5 to 130 μg/mL (5, 20, 40, 60, 80, 100 and 130 μg/mL), prepared in triplicate.
The accuracy of the method was evaluated as the percentage of agreement between the betamethasone concentration expected and the experimentally obtained value. For the determination of this parameter, three Quality Control (QC) standard solutions with concentrations of 10, 50 and 120 μg/mL were prepared and analyzed. The three replicates of QC standard solutions were prepared by diluting the stock standard solution of betamethasone (1 mg/mL) with a mixture of water/ethanol (1:9, v/v).
Recovery was assessed by adding known amounts of betamethasone to the suspension of empty liposomes. The corticosteroid concentrations used, in triplicate, were analogous to the three QC concentrations previously referred and recovery was calculated by comparing the peak area of the samples of liposomes spiked with betamethasone with those of equal drug concentration in a mixture of water/ethanol (1:9, v/v).
Intra- and inter-batch precisions were determined by the analyses of three replicates of the QC standard solutions obtained in the same day and in three different days, respectively. Precision was expressed as the Relative Standard Deviation (%RSD) of the determinations performed in triplicate. DL and QL were determined as detailed in equations 2 and 3:
The specificity of the analytical method was also evaluated in samples of betamethasone submitted to thermal, acidic and alkaline stress conditions. The resistance to thermal degradation was determined by placing a required amount (1 mg) of the compound in an oven at 120 °C during 72h. At specified time points (2, 4, 10, 24, 48 and 72 h), an amount of sample was collected, dissolved in ethanol and analyzed by HPLC-DAD. To evaluate if the acidic and alkaline stress conditions provoked some corticosteroid degradation, 1 mg of the betamethasone was mixed with 25 mL of 1N HCl and with 1N NaOH, respectively. Following determined time points to a maximum of 72h of stirring at room temperature, the pH values of aliquots of the acidic or alkaline solutions were adjusted to water pH with 6M NaOH and HCl 98%, respectively. After that, samples were filtered and submitted to HPLC analysis.
Liposomes stability
Preparation of hydrogels containing liposomes entrapping betamethasone
Hydroxypropyl guar gum was dispersed in the liposomes suspension and to obtain an appropriate viscosity of the gel, the pH of the polymeric dispersion was adjusted to ≈ 6 with lactic acid. In the case of poly (acrylic acid), it was necessary to neutralize the polymeric dispersion to a pH near to 6 with triethanolamine before addition of liposomes, in order to avoid a production of a heterogeneous formulation. Placebo hydrogels using empty liposomes or deionized water were also prepared.
Hydrogels characterization
The stability of the hydrogels, stored at 4°C, over 1 month, was measured in terms of pH, viscosity and centrifugation stability by the previously described procedure.
Statistical analysis
RESULTS
Size distribution, zeta-potential and morphology of liposomes
Figure 1 illustrates that the obtained liposomes are spherical. In addition, the size distribution obtained with STEM was identical to that reported using DLS analyses.
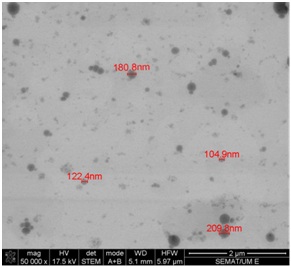
Betamethasone entrapment efficiency
HPLC-DAD method validation
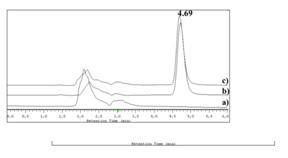
Table 1 contains the results obtained for accuracy and recovery assays and, as observable, they ranged between 100.1-105.4% and 99.7-102.6%, respectively. The intra and inter-day assays, expressed by %RSD, were performed to estimate the precision of the chromatographic method. The %RSD calculated for both parameters presented a maximum value of 2.96% (Table 1), which indicates that the method presents an acceptable precision [34].
| Nominal concentration (μg/mL) | Accuracy (%) | Recovery (%) | %RSD (inter-day) | %RSD (intra-day) |
| 10 | 105.4 | 100.5 | 1.92 | 2.96 |
| 50 | 103.4 | 99.7 | 1.35 | 0.83 |
| 120 | 100.1 | 102.6 | 1.49 | 2.82 |
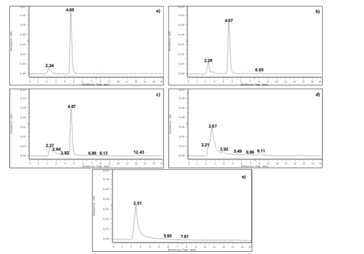
Chromatograms of the samples containing the drug submitted to thermal, acidic and alkaline stress conditions are shown in figure 3. Comparison of the representative chromatogram of betamethasone (Figure 2) with those in figure 3 allows concluding that betamethasone did not suffer any quantifiable degradation under the tested temperature and time conditions (Figure 3A). Additionally, betamethasone presents a higher resistance to acidic degradation than to the considered alkaline conditions. The samples subjected to acidic conditions only showed a small additional peak (retention time approximately at 8.0 min), after 72h of incubation (Figure 3B). On the other hand, in the samples submitted to alkaline stress conditions, after 72h of incubation, almost all betamethasone was degraded (Figure 3E). In fact, after 20 min (Figure 3C) of incubation, additional peaks were found and after 4h (Figure 3D) betamethasone concentration was below the QL. The betamethasone UV-spectrum was the same after submitting the samples to thermal, acidic and alkaline (considering only 20 min of incubation; Figure 3C) conditions and the peak purity tests performed by the HPLC-DAD software revealed that the peaks had a purity of 100%. These results highlight that there were no degradation products eluting simultaneously with betamethasone.
Stability of liposomes
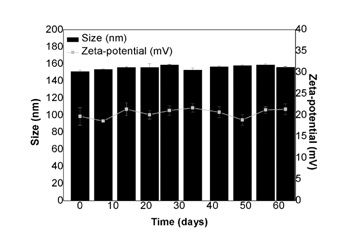
Figure 4: Evaluation of the liposomes stability. Size (nm; ?) and zeta-potential (mV; ?) obtained for LUVs entrapping betamethasone over a period of two months.
Hydrogels characterization
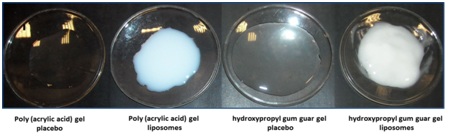
Figure 5: Physical appearance of the hydrogels. Hydrogels based on poly(acrylic acid) or hydroxypropyl gum guar without (placebo) or with liposomes presents, respectively, a translucent or opalescent aspect.
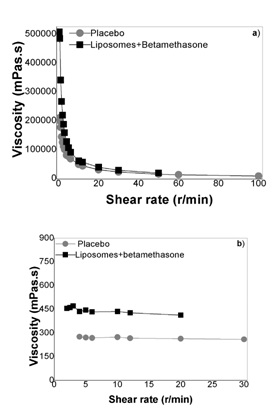

Finally, the determination of pH, viscosity and centrifugation stability, over 1 month, demonstrated that the hydrogels prepared showed good physical stability, once that the data obtained presented approximately the same behavior throughout the time considered.
HPLC-DAD method validation
Figure 8 allows the comparison between the chromatograms obtained for a betamethasone standard solution and for the placebo hydrogels (with or without empty liposomes). As none of the chromatograms exhibits interfering peaks in the betamethasone retention time, the selectivity of the method was verified. A calibration curve with standard solutions that were submitted to the extraction method was also prepared, in order to demonstrate that this process did not induce any modification on the drug. In fact, liposome preparation method and their incorporation into hydrogels did not induce any degradation of the betamethasone. As expected, the assay was linear in the concentration range of 3 to 10 μg/mL, with a correlation coefficient higher than 0.9993 and a %RSD ranging between 0.6-2.5%.
Three QC standard solutions, corresponding to 80%, 100% and 120% of betamethasone in hydrogels, were used to assess accuracy, recovery and precision of the method. In table 2, it is possible to note that accuracy ranged between 98.9-101.0% and 98.8-101.7% and recovery was between 97.7-101.7% and 97.1-99.3% for hydrogels of poly (acrylic acid) and hydroxypropyl gum guar, respectively. Additionally, table 2 shows that the method was precise, taking into account the values of %RSD calculated for intra- and inter day assays.
| Hydrogels | Nominal concentration (mg/g) | Accuracy (%) | Recovery (%) | %RSD (inter-day) | %RSD (intra-day) |
| Poly(acrylic acid) | 0.4 | 98.9 | 97.7 | 1.42 | 1.58 |
| 0.5 | 99.7 | 97.8 | 1.26 | 1.35 | |
| 0.6 | 101 | 101.7 | 1.18 | 2.49 | |
| Hydroxypropyl gum guar | 0.4 | 98.8 | 97.9 | 1.58 | 2.11 |
| 0.5 | 101.7 | 99.3 | 1.25 | 1.41 | |
| 0.6 | 99.9 | 97.1 | 1.45 | 1.97 |
DISCUSSION
The main purpose of this work was the development of topical formulations based in hydrogels presenting in their composition bethametasone-loaded liposomes to avoid the systemic absorption of this corticosteroid, thus contributing to the improvement of betamethasone therapeutic index.
The characterization of the LUVs demonstrated that they are homogeneous in terms of size (PDI < 0.1), presenting a mean diameter around 155 nm. These data were corroborated by STEM analyses, which also allowed concluding that the liposomes were morphologically spherical. To confirm this assumption, it was calculated the sphericity factor [37]. As the values of the sphericity factors were less than 0.15, it is possible to conclude that LUVs are spherical. Besides other factors, this shape presents advantages in the field of drug controlled release [38]. Additionally, LUVs showed a significant positive surface charge (+19.7+2.0 mV), related to the protonation of EPC phosphate group at an acidic medium (water pH ≈ 5.5) [39]. The zeta-potential values gives an indication of the surface charge of liposomes, allowing the prediction of the physical stability of the colloidal dispersion [40-42]. In general, particle aggregation of charged particles is less likely to occur, due to electric repulsion. Taking into account the zeta-potential results, it is possible to conclude that the liposomal suspension is relatively stable, once that its value is in the range of ±10-20 mV [43]. This was corroborated with the stability assays. Indeed, liposomes can suffer destabilization and aggregation over time, particularly in aqueous dispersions. Therefore, particle size was monitored for two months, after LUVs preparation, in order to evaluate their physical stability. The zeta-potential was also determined, since this parameter can change over time, due to chemical and/or physical structural alterations. The stability studies demonstrated that LUVs maintained their properties in terms of size, PDI and surface charge during storage. As previously referred, the positive zeta-potential value obtained for this drug-carrier can produce repulsive interactions between the lipid vesicles dispersed in the water, leading to long-lasting stability [44].
The increase of phospholipid concentration promoted an increment of anti-inflammatory entrapment, since betamethasone, due to its hydrophobic nature, is mainly located between phospholipids in the lipid bilayer. In fact, betamethasone is associated with the lipid bilayer, as demonstrated resorting to differential scanning calorimetry analysis [5]. On the other hand, the inclusion of the betamethasone into the lipid bilayer versus the aqueous compartment through, for example, the use of cyclodextrin complexes, seems to be the best choice as it gives rise to higher entrapment efficiencies [6]. The developed and validated adequate HPLC-DAD method presented specificity, linearity, accuracy, recovery and precision values that allowed performing the determination of the betamethasone concentrations when entrapped or not into the liposomes. Indeed, the method validation was performed to demonstrate that the HPLC-DAD method used in this work accomplishes all the requirements of the analytical applications, ensuring the reliability of the results.
The characterization of the hydrogels based on poly(acrylic acid) or hydroxypropyl gum guar showed that they present adequate pH values for topical formulations, since that the skin pH is ideally slightly acidic [25]. Additionally, the determination of the rheological behavior of the two hydrogels demonstrated that the poly(acrylic acid) gel base is more fluid than the hydroxypropyl gum guar hydrogel, which, consequently, can be suited to the treatment of more extensive areas. Moreover, the addition of liposomes increased the hydrogels viscosity, particularly of the poly(acrylic acid) gel. However, the significant difference of viscosity of the two hydrogels was maintained at the end. The assessment of stability and drug content (quantified by the validated HPLC-DAD method) demonstrated that these formulations preserved their properties and presented a drug concentration similar to the marketed betamethasone topical medications. In this sense, these formulations have potential to promote a prolonged and localized therapeutic effect, allowed and coadjuvated by the presence of liposomes of EPC, which presents antioxidant properties [28,29].
CONCLUSION
This work exploited the potential of liposomes as carriers for topical delivery of betamethasone. The use of liposomes may represent a promising approach in the treatment of inflammatory skin diseases, by increasing betamethasone benefit-risk ratio, which is crucial for this drug presenting severest side effects. In this sense, two hydrogel formulations containing betamethasone-loaded liposomes were developed and characterized, which demonstrated compatible properties with topical application, regarding pH values, physical stability, drug content and rheological behavior. Additionally, the higher fluidity displayed by the poly (acrylic acid) gel can be useful to treat extensive areas. By contrast, the hydroxypropyl gum guar gel, more viscous, may be used in the treatment of smaller areas without considerable risk to drain to healthy skin.
As conclusion, it is possible to state that the hydrogels formulations developed may have potential to control and heal topical inflammatory conditions, as psoriasis and eczema.
ACKNOWLEDGMENT
Helena Ferreira acknowledges Human Potential Operational Programme/European Social Fund (POPH/FSE) for co-financing and Portuguese Foundation for Science and Technology (FCT) for the fellowship SFRH/BPD/38939/2007. The authors acknowledge Cooperativa de Ensino Superior Politécnico Universitário (CESPU) for financial support (04-GCQF-CICS-2011N). This work was also partially supported through national funds provided by FCT/MCTES - Foundation for Science and Technology from the Minister of Science, Technology and Higher Education (PIDDAC) and European Regional Development Fund (ERDF) through the COMPETE - Programa Operacional Factores de Competitividade-(POFC) program, under the Strategic Funding UID/Multi/04423/2013, in the framework of the program PT2020.
REFERENCES
- Nam YS, Kwon IK, Lee KB (2011) Monitoring of clobetasol propionate and betamethasone dipropionate as undeclared steroids in cosmetic products manufactured in Korea. Forensic Sci Int 210: 144-148.
- Mezei M, Gulasekharam V (1980) Liposomes--a selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci 26: 1473-1477.
- Mezei M, Gulasekharam V (1982) Liposomes--a selective drug delivery system for the topical route of administration: gel dosage form. J Pharm Pharmacol 34: 473-474.
- Egbaria K, Ramachandran C, Kittayanond D, Weiner N (1990) Topical delivery of liposomally encapsulated interferon evaluated by in vitro diffusion studies. Antimicrob Agents Ch 34: 107-110.
- Fresta M, Puglisi G (1997) Corticosteroid dermal delivery with skin-lipid liposomes. J Control Release 44: 141-151.
- Gillet A, Lecomte F, Hubert P, Ducat E, Evrard B, et al. (2011) Skin penetration behaviour of liposomes as a function of their composition. Eur J Pharm Biopharm 79: 43-53.
- Hasanovic A, Hollick C, Fischinger K, Valenta C (2010) Improvement in physicochemical parameters of DPPC liposomes and increase in skin permeation of aciclovir and minoxidil by the addition of cationic polymers. Eur J Pharm Biopharm 75: 148-153.
- El Maghraby GM, Barry BW, Williams AC (2008) Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci 34: 203-222.
- Verma DD, Verma S, Blume G, Fahr A (2003) Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm 55: 217-277.
- Vermorken AJ, Hukkelhoven MW, Vermeesch-Marksiag AM, Goos CM, Wirtz P, et al. (1984) The use of liposomes in the topical application of steroids. J Pharm Pharmacol 36: 334-336.
- Yu HY, Liao H-M (1996) Triamcinolone permeation from different liposome formulations through rat skin in vitro. Int J Pharm 127: 1-7.
- Paolino D, Cosco D, Cilurzo F, Trapasso E, Morittu VM, et al. (2012) Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles. J Control Release 162: 143-151.
- Elsayed MMA, Abdallah OY, Naggar VF, Khalafallah NM (2007) Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int J Pharm 332: 1-16.
- Agarwal R, Saraswat A, Kaur I, Katare OP, Kumar B (2002) A Novel Liposomal Formulation of Dithranol for Psoriasis: Preliminary Results. J Dermatol 29: 529-532.
- Korting HC, Zienicke H, Schäfer-Korting M, Braun-Falco O (1990) Liposome encapsulation improves efficacy of betamethasone dipropionate in atopic eczema but not in psoriasis vulgaris. Eur J Clin Pharmacol 39: 349-351.
- Singh B, Mehta G, Kumar R, Bhatia A, Ahuja N, et al. (2005) Design, development and optimization of nimesulide-loaded liposomal systems for topical application. Curr Drug Deliv 2: 143-153.
- Koutsoulas C, Pippa N, Demetzos C, Zabka M (2014) Preparation of liposomal nanoparticles incorporating terbinafine in vitro drug release studies. J Nanosci Nanotechnol 14: 4529-4533.
- Manconi M, Mura S, Sinico C, Fadda AM, Vila AO, et al. (2009) Development and characterization of liposomes containing glycols as carriers for diclofenac. Colloids and Surfaces A: Physicochemical and Engineering Aspects 342: 53-58.
- Watson DS, Endsley AN, Huang L (2012) Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 30: 2256-2272.
- Ferreira H, Matamá T, Silva R, Silva C, Gomes AC, et al. (2013) Functionalization of gauzes with liposomes entrapping an anti-inflammatory drug: A strategy to improve wound healing. React Funct Polym 73: 1328-1334.
- Kono H, Hara H, Hashimoto H, Shimizu Y (2015) Nonionic gelation agents prepared from hydroxypropyl guar gum. Carbohydr Polym 117: 636-643.
- Tang Z, Wu J, Liu Q, Zheng M, Tang Q, et al. (2012) Preparation of poly(acrylic acid)/gelatin/polyaniline gel-electrolyte and its application in quasi-solid-state dye-sensitized solar cells. J Power Sources 203: 282-287.
- Zhao Y, He J, Han X, Tian X, Deng M, et al. (2012) Modification of hydroxypropyl guar gum with ethanolamine. Carbohydr Polym 90: 988-992.
- Zheng LL, Vanchinathan V, Dalal R, Noolandi J, Waters DJ, et al. (2015) Biocompatibility of poly(ethylene glycol) and poly(acrylic acid) interpenetrating network hydrogel by intrastromal implantation in rabbit cornea. J Biomed Mater Res A 103: 3157-3165.
- Ourique AF, Melero A, de Bona da Silva C, Schaefer UF, Pohlmann AR, et al. (2011) Improved photostability and reduced skin permeation of tretinoin: Development of a semisolid nanomedicine. Eur J Pharm Biopharm 79: 95-101.
- Hurler J, Zakelj S, Mravljak J, Pajk S, Albin Kristl, et al. (2013) The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. Int J Pharm 456: 49-57.
- Ruel-Gariépy E, Leclair G, Hildgen P, Gupta A, Leroux JC (2002) Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release 82: 373-383.
- Demirbilek S, Ersoy MO, Demirbilek S, Karaman A, Akin M, et al. (2004) Effects of polyenylphosphatidylcholine on cytokines, nitrite/nitrate levels, antioxidant activity and lipid peroxidation in rats with sepsis. Intensive Care Med 30: 1974-1978.
- Ghyczy M, Torday C, Kaszaki J, Szabo A, Czobel M, et al. (2008) Oral phosphatidylcholine pretreatment decreases ischemia-reperfusion-induced methane generation and the inflammatory response in the small intestine. Shock 30: 596-602.
- Bangham AD, Horne RW (1964) Negative Staining of Phospholipids and their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J Mol Biol 8: 660-668.
- European Comission (1998) Ditectorate general III- industry pharmaceuticals and cosmetics validation of analytical procedures: Methodology the rules governing medicinal products in european union. 3A: 107-117.
- European Comission (1998) Ditectorate general III- industry pharmaceuticals and cosmetics validation of analytical procedures: Definition and terminology the rules governing medicinal products in european union. 3A: 119-125.
- Teixeira M, Afonso CMM, Pinto MM, Barbosa CM (2008) Development and Validation of an HPLC Method for the Quantitation of 1,3-Dihydroxy-2-methylxanthone in Biodegradable Nanoparticles. J Chromatogr Sci 46: 472-478.
- Chandran S, Singh RS (2007) Comparison of various international guidelines for analytical method validation. Pharmazie 62: 4-14.
- Lee CH, Moturi V, Lee Y (2009) Thixotropic property in pharmaceutical formulations. J Control Release 136: 88-98.
- Alves MP, Pohlmann AR, Guterres SS (2005) Semisolid topical formulations containing nimesulide-loaded nanocapsules, nanospheres or nanoemulsion: development and rheological characterization. Pharmazie 60: 900-904.
- Chan ES, Wong SL, Lee PP, Lee JS, Ti TB, et al. (2011) Effects of starch filler on the physical properties of lyophilized calcium-alginate beads and the viability of encapsulated cells. Carbohydr Polym 83: 225-232.
- Bunjes H (2005) Characterization of solid lipid nanoparticles and microparticle. In: Nastruzzi C (ed.). Lipospheres in drug targets and delivery approaches, methods and applications. CRC Press LLC, Boca Raton, Florida, USA, Pg no: 41-66.
- Matos C, de Castro B, Gameiro P, Lima JLFC, Reis S (2004) Zeta-potential measurements as a tool to quantify the effect of charged drugs on the surface potential of egg phosphatidylcholine liposomes. Langmuir 20: 369-377.
- Berg JM, Romoser A, Banerjee N, Zebda R, Sayes CM (2009) The relationship between pH and zeta potential of 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 3: 276-283.
- Mandzy N, Grulke E, Druffel T (2005) Breakage of TiO2 Agglomerates in Electrostatically Stabilized Aqueous Dispersions. Powder Technol 160: 121-126.
- Zhang Y, Yang M, Portney N, Cui D, Budak G, et al. (2008) Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed Microdevices 10: 321-328.
- Bhattacharjee S (2016) DLS and zeta potential - What they are and what they are not? J Control Release 235: 337-351.
- Feng SS, Huang G (2001) Effects of emulsifiers on the controlled release of paclitaxel (Taxol) from nanospheres of biodegradable polymers. J Control Release 71: 53-69.
Citation: Ferreira H, Gonçalves VMF, Silva R, Tiritan ME, Teixeira M, et al. (2017) Development of Liposomes-in-Hydrogel Formulations Containing Betamethasone for Topical Therapy. J Pharmacol Pharmaceut Pharmacovigil 1: 005.
Copyright: © 2017 Helena Ferreira, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

