
Expanding the Boundaries of Oral Probiotic Formulations to Target Atopic Dermatitis
*Corresponding Author(s):
Yoram C Padeh, M.D., FACPDiplomate, American Board Of Allergy And Immunology, Allergy And Immunology Private Practice, Miami, FL, United States
Email:ypadeh@gmail.com
Abstract
Rationale
Some clinical trials already demonstrate statistically significant improvement of AD symptoms with oral probiotics. The addition of regulatory T-cell (Treg)-enhancing vitamins and amino acid cofactors and improved gut delivery of viable probiotic organisms could further increase the efficacy of probiotics in reducing AD symptoms.
Purpose
The aim of this pilot study is to show the efficacy of a novel formulation of probiotics and vitamins specifically targeted for improving Atopic Dermatitis (AD) symptoms.
Methods
A novel Targeted Probiotics and Vitamin Complex (TPVC) was formulated, consisting of prebiotics, 11 probiotic strains, Vitamin D, Vitamin A, and L-tryptophan. It utilizes a patented micro-encapsulation technology which allows it to deliver a higher yield of viable probiotic organisms to the small and large intestines compared to other probiotic formulations such as liquids, powders, or capsules. The TPVC was offered as a free sample (60 pills) to interest AD patients ages 6-years-old and older, with parental consent for minors (ages 6 through 17-years-old). Data on symptoms was collected with the validated Patient Oriented Eczema Measure (POEM) self-assessment survey tool which was completed by patients or parents of minors prior to starting the TPVC as well as after taking it daily for at least two consecutive weeks.
Results
Forty-eight interested patients/parents with AD accepted samples of the TPVC. Seventeen of the 48 patients/parents completed the pre-treatment POEM survey. Of those seventeen, 9 patients/parents completed the post-treatment POEM survey. Pre-treatment POEM scores ranged from 3 to 20. Post-treatment POEM scores ranged from 2 to10. The 9 score changes were all improvements and ranged from 3 to 16 where a decrease of 3.4 or greater has been established as a clinically meaningful improvement. Only 2 patients had a score improvement of 3, while 7 had an improvement of 4 or greater.
Conclusion
The TPVC, a novel probiotic formulation utilizing 11 different probiotic species combined with Vitamins A and D and the amino acid L-tryptophan and designed to deliver a high yield of viable organisms past the stomach, improved the AD symptoms of 7 out of 9 patients to a clinically meaningful degree.
Keywords
Oral Probiotic, Atopic Dermatitis, Allergy and Immunology
INTRODUCTION
By adding the concept of the microbiome as a facet of human physiology, the complexity level grows exponentially. As an example, nutrition not only affects human physiology directly but it also affects the populations and composition of the gut microbiome which in turn further affects human physiology and disease, such as in AD [1]. Therapeutic attempts with probiotics to date have been wholly one-dimensional, utilizing only populations of probiotic bacterial species and their nutritional substrates (prebiotics). However as just noted, many other nutritional factors likely impact the potential effects of these probiotics, which have not been addressed by therapeutic probiotic studies to date.
Possibly one the most important and challenging aspects of oral probiotic therapy is delivering a viable population of probiotic bacterial strains to the target sections of the gut. Colony Forming Units (CFU) describe the number of organisms in a dose of a probiotic product, but the number that finally reaches target site may be far less than what is printed on the label. The CFU can diminish for a number of reasons, including time on the shelf, exposure to higher temperatures, and exposure to gastric acid. Some products attempt to overpower these factors by increasing the CFU to compensate for such losses. Despite this, a recent article in Cell casts doubt on whether oral probiotics can even achieve any significant impact on the indigenous gut microbiome population [2]. Regardless, given that there have been study-proven statistically significant improvements in AD symptoms with simple one-dimensional probiotic therapies, there is a clear therapeutic effect to oral probiotics, even if they remain present in the gut only transiently. Taking these complexities into account, one can begin to imagine that future oral probiotic therapies might begin to include other nutritional factors as well as methods to enhance delivery of viable probiotic organisms to the target sections of the gut. It is possible that such combination products could take oral probiotics to another level of efficacy, perhaps even positively impacting disease symptoms which to date have not improved in clinical trials. As perhaps the first of its kind and as proof-of-concept of this idea, this pilot study utilized a novel Targeted Probiotic and Vitamin Complex (TPVC) designed to improve AD symptoms.
Atopic Dermatitis (AD) is an inflammatory condition affecting 9.6 million children and 18 million adults in the US alone [3,4]. The complex inflammatory milieu of AD skin involves the influx of lymphocytes and liberation of cytokines, most typically lymphocytes of the Th2 and Th22 variety [5]. These lymphocytes and cytokines, whose activation is often triggered by scratching, lead to the typical symptoms and features of the condition. Genetic mutations such as those of filaggrin are currently known to be but a relatively small percentage of all AD cases. Most cases have no currently identifiable specific genetic origin, though a family history of AD is clearly a risk factor.
Analysis of unaffected skin in patients with AD has shown the presence of inflammatory lymphocytes despite the lack of gross skin changes [6]. This suggests that there may be a more generalized and systemic inflammatory milieu, at least affecting the entire skin as a single organ system. Dupilumab, a monoclonal antibody targeting the IL-4 and IL-13 receptors to reduce atopic signaling, has been shown to significantly improve AD symptoms [7,8] further supporting this concept. Studies of infant exposure and colonization with varying types of gut flora suggest that the development of atopy and atopic conditions may stem from gut flora diversity or lack thereof [9]. Many studies have investigated utilizing oral probiotics as possible treatments for atopic conditions, both for primary prevention as well as for secondary improvement of symptoms. To date, most atopic conditions have not shown to benefit from probiotics except for AD [10].
The body of literature on treatment of AD symptoms with probiotics is varied by age groups, probiotic species, and quantities used (number of “colony forming units” or CFU), making its interpretation somewhat complex. While early studies tended not to reach statistical significance with respect to symptom improvement, newer studies have in fact shown statistically significant improvements of AD symptoms with probiotics [11-24]. Meta-analyses are useful to visualize this trend, but the differences of the studies used must be kept in mind. One particular meta-analysis shows a clear overall benefit of probiotics for improving AD symptoms, except in infant populations [23]. It is possible that the lack of improvement in some infants could be due to previous colonization with specific flora, such as Bifidobacterium dentium[24].
On review of the probiotic literature, probiotics have also been shown to be statistically beneficial in the treatment of Ulcerative Colitis (UC), an inflammatory bowel disease [25]. A portion of the GI probiotic literature focuses on the ability of specific bacterial species and strains to promote regulatory T-cell (Treg) activation as well as the increased production of the regulatory cytokine, IL-10 [26,27]. The theory is that some probiotics may exert an immunomodulatory effect through Tregs and IL-10. Given that the inflammation of UC and the area of probiotic colonization are the same, this theory seems logical.
Since most lymphocytes reside in the GI tract and then also patrol and affect other parts of the body, it is possible that local activation of Tregs and production of IL-10 in the gut may also create a remote anti-inflammatory effect, such as in the skin. Some probiotics, as noted above, have been shown to promote Treg activation and proliferation. In the course of their activation, Tregs can migrate to the skin and dampen all T-cell activation in the skin, including Th2 and Th22. Additionally; the production of IL-10 by Tregs can also act remotely and suppress T-cell activation in the skin. Perhaps this is the mechanism by which oral probiotics can improve skin symptoms in AD [28,29]. One study also shows enhanced Treg activation and IL-10 production with the addition of Vitamin A and L-tryptophan in the presence of specific probiotic species [30]. Additionally, some of the literature on Vitamin D and AD has shown benefit in improving AD symptoms [31].
METHODS
The Patient Oriented Eczema Measure (POEM), a validated AD self-assessment survey, was copied with permission from the University of Nottingham website [32]. The survey questions, possible answers, scores, and score interpretations are presented in tables 1-3. The POEM survey includes 7 questions, each with a score from 0 to 4. The total POEM score can range from 0 to 28, with a higher score corresponding to worse symptoms. A POEM score improvement (reduction) of 3.4 or higher has been previously established as the Minimal Clinically Important Difference (MCID) [33]. Therefore a score improvement of over 3.4 was a priori considered to be the cutoff for a significant improvement of symptoms being achieved by the TPVC.
|
1. Over the last week, on how many days has your/your child’s skin been itchy because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
2. Over the last week, on how many nights has your/your child’s sleep been disturbed because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
3. Over the last week, on how many days has your/your child’s skin been bleeding because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
4. Over the last week, on how many days has your/your child’s skin been weeping or oozing clear fluid because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
5. Over the last week, on how many days has your/your child’s skin been cracked because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
6. Over the last week, on how many days has your/your child’s skin been flaking off because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
7. Over the last week, on how many days has your/your child’s skin felt dry or rough because of the eczema? |
||||
|
No days |
1-2 days |
3-4 days |
5-6 days |
Every day |
|
Answer |
Score |
|
No days |
0 |
|
1-2 days |
1 |
|
3-4 days |
2 |
|
5-6 days |
3 |
|
Every day |
4 |
|
Total Score |
Interpretation |
|
0 to 2 |
Clear or almost clear |
|
3 to 7 |
Mild eczema |
|
8 to 16 |
Moderate eczema |
|
17 to 24 |
Severe eczema |
|
25 to 28 |
Very severe eczema |
RESULTS
|
ID |
Pr1 |
Pr2 |
Pr3 |
Pr4 |
Pr5 |
Pr6 |
Pr7 |
PrT |
Po1 |
Po2 |
Po3 |
Po4 |
Po5 |
Po6 |
Po7 |
PoT |
Δ |
# |
MF |
Age |
|
1 |
4 |
4 |
2 |
0 |
2 |
4 |
4 |
20 |
1 |
0 |
0 |
0 |
0 |
1 |
2 |
4 |
-16 |
2 |
F |
27 |
|
2 |
3 |
3 |
1 |
1 |
1 |
0 |
3 |
12 |
1 |
1 |
0 |
0 |
0 |
0 |
1 |
3 |
-9 |
2 |
F |
6 |
|
3 |
4 |
4 |
0 |
0 |
3 |
1 |
4 |
16 |
4 |
1 |
0 |
0 |
1 |
0 |
2 |
8 |
-8 |
2 |
F |
18 |
|
4 |
2 |
0 |
0 |
0 |
0 |
0 |
4 |
6 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
-4 |
2 |
F |
26 |
|
5 |
2 |
2 |
1 |
0 |
1 |
1 |
3 |
10 |
1 |
0 |
0 |
0 |
1 |
2 |
2 |
6 |
-4 |
3 |
F |
21 |
|
6 |
4 |
1 |
2 |
0 |
4 |
4 |
4 |
19 |
1 |
1 |
0 |
0 |
1 |
2 |
1 |
6 |
-13 |
3 |
M |
42 |
|
7 |
3 |
4 |
1 |
0 |
2 |
1 |
2 |
13 |
3 |
1 |
1 |
0 |
2 |
1 |
2 |
10 |
-3 |
2 |
F |
7 |
|
8 |
3 |
3 |
0 |
1 |
1 |
1 |
2 |
11 |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
-9 |
1 |
M |
10 |
|
9 |
4 |
4 |
0 |
0 |
0 |
0 |
4 |
12 |
4 |
1 |
0 |
0 |
0 |
0 |
4 |
9 |
-3 |
2 |
F |
40 |
ID: Patient number
Pr1-7: Pre-treatment POEM question score
PrT: Pre-treatment total POEM score
Po1-7: Post-treatment POEM question score
PoT: Post-treatment total POEM score
Δ: POEM score change (Post-treatment total POEM score minus Pre-treatment total POEM score)
#: Average number of pills taken daily
MF: Male or Female
|
ID |
Age |
M/F |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Total |
|
1 |
27 |
F |
4 |
4 |
2 |
0 |
2 |
4 |
4 |
20 |
|
2 |
6 |
F |
3 |
3 |
1 |
1 |
1 |
0 |
3 |
12 |
|
3 |
18 |
F |
4 |
4 |
0 |
0 |
3 |
1 |
4 |
16 |
|
4 |
26 |
F |
2 |
0 |
0 |
0 |
0 |
0 |
4 |
6 |
|
5 |
21 |
F |
2 |
2 |
1 |
0 |
1 |
1 |
3 |
10 |
|
6 |
42 |
M |
4 |
1 |
2 |
0 |
4 |
4 |
4 |
19 |
|
7 |
7 |
F |
3 |
4 |
1 |
0 |
2 |
1 |
2 |
13 |
|
8 |
10 |
M |
3 |
3 |
0 |
1 |
1 |
1 |
2 |
11 |
|
9 |
40 |
F |
4 |
4 |
0 |
0 |
0 |
0 |
4 |
12 |
ID: Patient number
|
ID |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Total |
Δ |
Pills |
|
1 |
1 |
0 |
0 |
0 |
0 |
1 |
2 |
4 |
-16 |
2 |
|
2 |
1 |
1 |
0 |
0 |
0 |
0 |
1 |
3 |
-9 |
2 |
|
3 |
4 |
1 |
0 |
0 |
1 |
0 |
2 |
8 |
-8 |
2 |
|
4 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
-4 |
2 |
|
5 |
1 |
0 |
0 |
0 |
1 |
2 |
2 |
6 |
-4 |
3 |
|
6 |
1 |
1 |
0 |
0 |
1 |
2 |
1 |
6 |
-13 |
3 |
|
7 |
3 |
1 |
1 |
0 |
2 |
1 |
2 |
10 |
-3 |
2 |
|
8 |
1 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
-9 |
1 |
|
9 |
4 |
1 |
0 |
0 |
0 |
0 |
4 |
9 |
-3 |
2 |
ID: Patient number
Δ: Post-treatment score minus Pre-treatment score (not shown in this table)
|
ID |
Age |
M/F |
Pills Daily |
Pre Total |
Post Total |
Δ |
|
1 |
27 |
F |
2 |
20 |
4 |
-16 |
|
2 |
6 |
F |
2 |
12 |
3 |
-9 |
|
3 |
18 |
F |
2 |
16 |
8 |
-8 |
|
4 |
26 |
F |
2 |
6 |
2 |
-4 |
|
5 |
21 |
F |
3 |
10 |
6 |
-4 |
|
6 |
42 |
M |
3 |
19 |
6 |
-13 |
|
7 |
7 |
F |
2 |
13 |
10 |
-3 |
|
8 |
10 |
M |
1 |
11 |
2 |
-9 |
|
9 |
40 |
F |
2 |
12 |
9 |
-3 |
ID: Patient number
Pre Total: Pre-treatment POEM score
Post Total: Post-treatment POEM score
Δ: Post Total minus Pre Total
Two patients, one and six, had symptoms categorized as “severe eczema” based on the POEM severity scale. These two patients had the highest POEM score changes on the TPVC, 16 and 13 respectively. Patient four had AD symptoms categorized as “mild eczema”, while the remaining six patients had “moderate eczema”. Patients seven and nine, both of whom had “moderate eczema”, did not achieve a clinically meaningful improvement of their AD symptoms on the TPVC, with a POEM score decrease of 3 each. The other seven patients had POEM score decreases ranging from 4 to 16, above the POEM MCID of 3.4 (Figure 1).
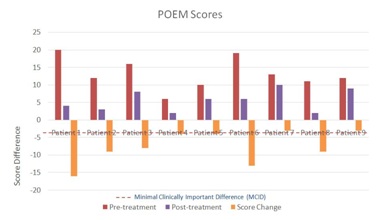 Figure 1: POEM Scores and Changes.
Figure 1: POEM Scores and Changes.Patient 1 volunteered the following written comments by email about her experience while taking the TPVC: “I had the sample you gave me for a month, taking two a day. I would say after a week or so I completely stopped itching. Towards the end of the month it seemed as though the hyper-pigmentation I had caused from scratching was lightening. I hadn't had any breakouts.” Other patients did not volunteer any written comments about their symptoms while taking the TPVC.
Attempts were made via email and phone to reach the 31 patients who completed neither survey as well as the 8 patients who never completed the post-treatment questionnaires. The data available for the 8 patients who completed only the pre-treatment questionnaire are provided in table 8. These 39 patients, none of whom reported worsening symptoms, were similarly distributed in age and AD symptom severity to the 9 with complete data. While some admitted to being inconsistent with treatment, none offered specific reasons why.
|
ID |
Age |
M/F |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Total |
|
10 |
7 |
F |
4 |
0 |
0 |
0 |
4 |
0 |
4 |
12 |
|
11 |
35 |
F |
4 |
3 |
2 |
2 |
4 |
0 |
4 |
19 |
|
12 |
11 |
M |
4 |
2 |
2 |
1 |
2 |
2 |
4 |
17 |
|
13 |
50 |
F |
0 |
0 |
1 |
0 |
0 |
1 |
1 |
3 |
|
14 |
38 |
F |
2 |
1 |
1 |
0 |
1 |
1 |
1 |
7 |
|
15 |
12 |
F |
4 |
0 |
1 |
0 |
4 |
4 |
4 |
17 |
|
16 |
16 |
F |
4 |
3 |
1 |
0 |
4 |
4 |
4 |
20 |
|
17 |
8 |
F |
4 |
0 |
0 |
0 |
0 |
0 |
4 |
8 |
DISCUSSION
The TPVC is the first probiotic specifically formulated for the treatment of AD symptoms which utilizes 11 different probiotic species. By incorporating a diverse group of 11 probiotic species shown in studies to improve AD symptoms in a statistically significant way, it is possible that the TPVC increases the probability of improvement and absolute efficacy because some patients may respond better to some probiotic species while other patients may respond better to other probiotic species. The inclusion of additional probiotic species in the future could therefore further enhance the probiotic diversity as well as the efficacy of the treatment in larger numbers of AD patients.
Additionally, Vitamin A, Vitamin D, and L-tryptophan were added with a specific intent of activating Tregs and increasing IL-10 production to enhance the potential anti-inflammatory effects promoted by the specific probiotic species. Future studies would benefit from measurements of IL-10 levels as a marker of Treg activation. A correlation of an increase in IL-10 production with improvement of symptom scores would further support the theory that oral probiotics improve AD symptoms by enhancing the anti-inflammatory effects of Treg cells in the gut.
The limitations of this pilot study include small sample size, selection bias, and no control for a possible placebo effect. As a pilot study, the goal was proof of concept that a novel formulation of diverse probiotics could in fact improve AD symptoms. Though the sample size was small, this goal was achieved. Additionally, patients who tolerate the product and have mild to modest symptom improvements could then titrate up the daily pill dose to attempt to achieve a more robust clinical effect. With further study, optimization of pill contents to achieve the highest possible clinical response can reduce the pill burden to 1 or 2 pills daily.
With a total of 48 patients who accepted a sample of the TPVC and 9 who completed both surveys, a selection bias of patients who had improvement on the product being the ones motivated to complete the surveys cannot be excluded. Even if this were in fact the case, the bias does not detract from the clinically meaningful improvements of 7 of the 9 patients who completed both surveys. Though there are numerous studies showing statistically significant improvements of AD symptoms with specific probiotics, the recommendations in the conclusions of the studies have remained cautious and skeptical on recommending probiotics for patients with eczema without further studies. With rare exception, probiotic supplementation has very little risk of minor side effects and exceptionally minimal risk of major side effects. In simple terms, there is very little downside to recommending probiotic supplementation and potentially a high upside with improvement of AD symptoms.
On a mechanistic level, this study also lacks any data regarding Treg activation including Treg activation markers and IL-10 levels pre- and post-treatment. While these data would be useful, they are beyond the scope of this pilot study. Future studies attempting to elucidate the mechanism by which probiotics effect an improvement of AD symptoms should include such data.
CONCLUSION
REFERENCES
- Lee MJ, Kang MJ, Lee SY, Lee E, Kim K, et al. (2018) Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J Allergy Clin Immunol 141: 1310-1319.
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, et al. (2018) Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 174: 1388-1405.
- Silverberg JI (2017) Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clin 35: 283-289.
- Silverberg JI, Simpson EL (2014) Associations of childhood eczema severity: a US population-based study. Dermatitis 25: 107-114.
- Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, et al. (2012) Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 130: 1344-1354.
- Suárez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, et al. (2011) Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 127: 954-964.
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, et al. (2014) Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N Engl J Med 371: 130-139.
- Mennini M, Dahdah L, Fiocchi A (2017) Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 376: 1090.
- Mennini M, Dahdah L, Artesani MC, Fiocchi A, Martelli A (2017) Probiotics in Asthma and Allergy Prevention. Front Pediatr 5: 165.
- Toh ZQ, Anzela A, Tang ML, Licciardi PV (2012) Probiotic therapy as a novel approach for allergic disease. Front Pharmacol 3: 171.
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, et al. (2003) Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 111: 389-395.
- Passeron T, Lacour JP, Fontas E, Ortonne JP (2006) Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy 61: 431-437.
- Roessler A, Friedrich U, Vogelsang H, Bauer A, et al. (2008) The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy 38: 93-102.
- Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, et al. (2010) Clinical Effects of Probiotic Bifidobacterium breve Supplementation in Adult Patients with Atopic Dermatitis. Yonago Acta Med 53: 37-45.
- Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, et al. (2006) Is the effect of probiotics on atopic dermatitis confined to food sensitized children? Clin Exp Allergy 36: 629-633.
- Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI (2010) Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol 11: 351-361.
- Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS (2010) Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol 104: 343-348.
- Wu KG, Li TH, Peng HJ (2012) Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br J Dermatol 166: 129-136.
- Drago L, Iemoli E, Rodighiero V, Nicola L, De Vecchi E, et al. (2011) Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int J Immunopathol Pharmacol 24: 1037-1048.
- Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, et al. (2012) Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol 46: 33-40.
- Han Y, Kim B, Ban J, Lee J, Kim BJ, et al. (2012) A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 23: 667-673.
- Ye?ilova Y, Çalka Ö, Akdeniz N, Berkta? M (2012) Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol 224: 189-193.
- Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, et al. (2014) Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol 113: 217-226.
- Avershina E, Cabrera Rubio R, Lundgård K, Perez Martinez G, Collado MC, et al. (2017) Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J Allergy Clin Immunol 139: 1399-1402.
- Ganji-Arjenaki M, Rafieian-Kopaei M (2018) Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J Cell Physiol 233: 2091-2103.
- Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, et al. (2012) Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog 8: 1002714.
- Zuo L, Yuan KT, Yu L, Meng QH, Chung PC, et l. (2014) Bifidobacterium infantis attenuates colitis by regulating T cell subset responses. World J Gastroenterol 20: 18316-18329.
- Kim HJ, Kim YJ, Lee SH, Yu J, Jeong SK, et al. (2014) Effects of Lactobacillus rhamnosus on allergic march model by suppressing Th2, Th17, and TSLP responses via CD4(+)CD25(+)Foxp3(+) Tregs. Clin Immunol 153: 178-186.
- Yoshida T, Fujiwara W, Enomoto M, Nakayama S, Matsuda H, et al. (2013) An increased number of CD4+CD25+ cells induced by an oral administration of Lactobacillus plantarum NRIC0380 are involved in antiallergic activity. Int Arch Allergy Immunol. 162: 283-299.
- Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, et al. (2012) Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 61: 354-366.
- Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ (2016) Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 8: 789.
- University of Nottingham (2017) Centre of Evidence Based Dermatology. England, UK.
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, et al. (2012) EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 67: 99-106.
Citation: Padeh YC (2019) Expanding the Boundaries of Oral Probiotic Formulations to Target Atopic Dermatitis. J Allergy Disord Ther 5: 010.
Copyright: © 2019 Yoram C Padeh, M.D., FACP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

