ABSTRACT
Birt-Hogg-Dubé Syndrome (BHDS) is a hereditary condition associated with skin fibrofolliculomas, multiple lung cysts, and kidney tumors. We report a case of Birt-Hogg-Dubé Syndrome confirmed by folliculin (FLCN) genetic testing in a 45-year-old femalepatient with spontaneous and recurrent pneumothorax during recovery after laparoscopic-assisted partial nephrectomy.
Keywords
Birt-Hogg-Dubé Syndrome; Folliculin; Pneumothorax
Introduction
Birt-Hogg-Dubé Syndrome (BHDS) is an inherited autosomal dominant disorder associated with 3 clinical manifestations, including skin fibrofolliculomas, multiple lung cysts and kidney tumors. These symptoms generally do not appear till adult. BHDS can be suggested by clinical manifestations but is definitively confirmed by molecular genetic testing to detect mutations in the FLCN gene [1-4].
The pulmonary manifestations of BHDS are common findings and mainly related to multiple lung cysts and development of spontaneous pneumothorax. In the rate of occurrence of pneumothorax, patients with BHDS have a 50-fold increase after adjusting for age. The management of pulmonary manifestations of BHDS largely focused on the approach to treatment of pneumothorax [1].
Postoperative respiratory complications have diverse etiologies and commonly occur in the Post-Anesthesia Care Unit (PACU) and hospital wards. Sometimes they are potentially life-threatening complications of anesthesia. Appropriate response to respiratory complications involves early intervention, development of a differential diagnosis, and an organized approach to respiratory support and patient disposition [2].
Therefore, we present a case of BHDS with spontaneous and recurrent pneumothorax during recovery after laparoscopic partial nephrectomy.
Case Report
One month prior to presentation, a 45-year-old woman had medical check-up in private clinic and was referred for evaluation and treatment of right renal mass. She was a non-smoker with no previous episodes of pneumothorax, and had been treated with CO2 laser and dermatologic ointment for multiple round shaped papules on face and neck but with little effect. She received Laparoscopically Assisted Vaginal Hysterectomy (LAVH) for uterine leiomyoma 3 years ago. Her family history was nonspecific and not notable.
A Chest X-ray (CXR) was nonspecific (Figure 1A) and abdominopelvic Computed Tomography (CT) showed multiple hepatic hemangioma and about 1 cm sized solid mass on Rt. lower pole at right kidney. She underwent laparoscopic partial nephrectomy for the solid mass. Carbon dioxide is insufflated into the peritoneal cavity at a rate of 4-6 liter/min to a pressure of 12-16 mm Hg. Anesthetic managements were applied to the patient; Tidal volume 12-15 ml/kg, respiratory rate 10-12 breaths/min to maintain end tidal CO2 35-40 mmHg and PEEP 5 cm H2O. The patient’s vital signs were not eventful during surgery. The patient was given intravenous analgesics using a PCA pump containing morphine (60 mg), ketorolac (150 mg), and ramosetron (0.6 mg) in a total volume of 100 ml of saline.
She complained chest pain and short of breath after moving to PACU. The patient had normal vital signs and diminished breathing sound of both lungs on auscultation. CXR and Electrocardiography (EKG) were taken and Arterial Blood Gas Analysis (ABGA) was examined. EKG was normal and CXR showed both pneumothoraxes (Figure 1B). ABGA revealed pH 7.41, PCO2 36.1, PO2 90.6, and HCO3 22.5. The patient was consulted to cardiothoracic surgeon regarding the pneumothorax.
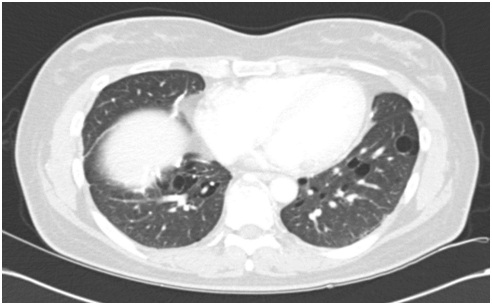
After bilateral tube thoracostomy, chest CT (Figure 2A) was taken and showed multiple, variable shaped cystic lesions with random distribution, and moderate to large amount of pneumothorax on both lung and sub segmental atelectasis on right lung. Pulmonary function test (% predicted) revealed FVC 72, FEV1 77, FEV1/FVC 78 and DLCO 68, which were restrictive pattern in this patient.
One week later, she underwent Video-Assisted Thoracoscopic Surgery (VATS). Lymphangioleiomyomatosis (LAM) affects predominantly women with reproductive age and is characterized by multiple cysts scattered throughout both lung fields. Given medical history with leiomyoma, multiple lung cysts and pneumothorax, at first, LAM was suspected. However, skin lesions including several flesh-colored, dome-shaped papules on physical examination, fibrofolliculomas on skin biopsy and oncocytoma finding on permanent section of right partial nephrectomy strongly suggested BHDS. It was diagnosed and confirmed by FLCN gene mutation on genetic examination. She discharged home after recovering from laparoscopic partial nephrectomy and VATS. One week later, she admitted to hospital again owing to dyspnea and chest pain. ABGA showed pH 7.44, PCO2 34, PO2 135, HCO3 23.1. CXR revealed the right pneumothorax (Figure 1C). Anesthetic managements including no PEEP, tidal volume 3-4 ml/kg, respirate rate 15-18 breaths/min to maintain end-tidal CO2 35-40 mmHg, and peak airway pressure 35-40 mmHg or manual ventilation to prevent over-expand bullae were applied to the patient during VATS. Following appropriate catheter insertion, an initial bolus dose with local anesthetics was given and a continuous epidural infusion was started with a mixture of bupivacaine 2.5 mg/ml and morphine 0.04 mg/ml. An i.v. dose of ketorolac 30 mg was administered if patients reported a VAS score ≥ 40, and 15 mg ketorolac was added as needed during PACU stay.
Cyst rupture during induction and subsequent release of contents into a tracheobronchial tree were not shown. Six months later, her chest CT (Figure 2B) showed resolved pneumothorax, and no significant interval change of multiple variable sized cystic lesions with random distribution in both lungs.
Discussion
In present case, the patient had no symptoms and signs of BHDS except skin lesions. The patient knew renal mass incidentally on medical checkup performed in private clinic. Chest CT for recurrent pneumothorax after surgery, permanent section finding of partial nephrectomy, skin biopsy and genetic study confirmed BHDS in this patient. Although BHDS is an inherited autosomal dominant disorder, her family history was nonspecific. Like this case, the pattern of mutations and spectrum of symptoms are heterogeneous between individuals. Therefore the diagnosis is sometimes delayed because of variability in its expression and unfamiliarity to many physicians.
The multiple lung cysts in BHDS do not cause problems with breathing and but increase risk of spontaneous pneumothorax, which may result in a collapsed lung. A Korean study with BHDS reported that most of the patients had 91.7-100% lung cysts. Pneumothorax developed in 66.7% and 75% was recurrent. However, LAM, pulmonary Langerhans cell histiocytosis, lymphocytic interstitial pneumonia/follicular bronchiolitis, and amyloidosis should be considered in patients with multiple lung cysts [5].
In present case, chest CT showed that multiple lung cysts, pneumothorax, and atelectasis, after spontaneous pneumothorax during recovery after surgery. Multiple lung cysts and uterine leiomyoma strongly suspected LAM among cystic lung diseases, before skin biopsy and gene study were performed. PFT in most of the patients with BHDS show percentages of normal predicted values, although mild restrictive pattern in some patients is detected [3,6,7]. Our patient had restrictive pattern in PFT with dyspnea after first pneumothorax occurred.
The first spontaneous pneumothorax on both lungs in this patient might be triggered from laparoscopic partial nephrectomy and conventional anesthetic management. Laparoscopy induced pneumoperitoneum, long duration of surgery and Positive End-Expiratory Pressure (PEEP) applied during surgery might cause rupture of multiple large bullae on both lungs. However, based on uneventful vital signs and airway pressure during surgery, postoperative rupture of the cysts was suspected. Our authors should have paid scrupulous attention to anesthetic management for the patient during the surgery, if we’d known the patient was BHDS.
When the second pneumothorax on right lung in the patients occurred, BHDS was diagnosed earlier. Although treatment in a patient who have pneumothorax with BHDS is similar for sporadic primary pneumothorax [2,6], the careful anesthetic managements including small tidal volume, no PEEP, lower peak airway pressure (20-25 mmHg), or manual ventilation to avoid application of excessive pressure at any point during a respiratory cycle and active pain management with epidural analgesia were applied to our patient.
In conclusion, BHDS with multiple intrathoracic cysts carry the risk of rupture peri-anesthetic period. When a patient with BHDS complains chest pain or dyspnea in perioperative period, pneumothorax may be first thing anesthesiologists should consider. The scrupulous anesthetic management and active pain management in patients with BHDS should be required to prevent postoperative pneumothorax.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Figures
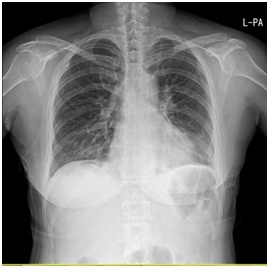
Figure 1A: Chest x-ray on admission show normal.
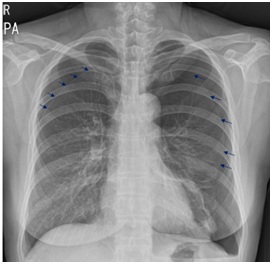
Figure 1B: Chest x-ray in PACU after laparoscopic partial nephrectomy shows both pneumothorax (small arrows).
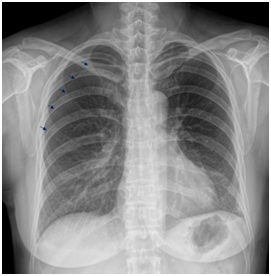
Figure 1C: Chest x-ray 1 week later shows right pneumothorax (small arrow).
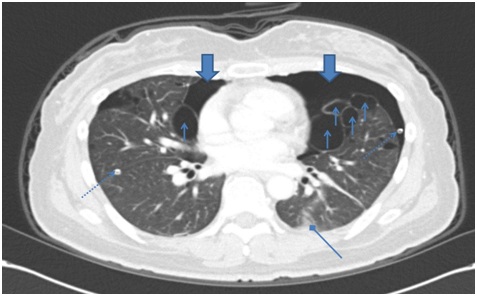
Figure 2A: Chest CT after thoracotomy shows improving state of pneumothorax in both hemithorax (big arrow), multiple variable sized cystic lesion with random distribution in lung field (small arrow), malposition of Rt. chest tube with tip in the lung parenchyme and Lt. chest tube (dotted line arrow) and sub segmental atelectasis (diamond arrow).
Figure 2B: Chest CT after 6 month’s shows resolved pneumothorax.