
Journal of Angiology & Vascular Surgery Category: Medical
Type: Case Report
Management of an Aortic Graft Infection after Endovascular Aneurysm Repair
*Corresponding Author(s):
Moustafa ELFEKYVascular Surgery In Agaplesion Hospital Academic Hospital Of The University Of Hamburg, Hamburg, Germany
Tel:+49 15758738150,
Email:dr.mostafa.elfeky@gmail.com
Received Date: Feb 26, 2019
Accepted Date: Mar 21, 2019
Published Date: Mar 28, 2019
Abstract
Introduction
Endovascular Aneurysm Repair (EVAR) is currently considered the predominant treatment for Abdominal Aortic Aneurysm (AAAs), especially in a high-risk patient, providing a low early morbidity and mortality compared with open repair. A very low number of EVAR patients suffer complications, ranging from 0.7% related to local-vascular or implant-related complications to 11% for systemic complications. Endograft infection after EVAR represents one of the most serious complications. Although the incidence of Endograft infection is rare and below 1% it is often associated with high mortality and morbidity rates.
The therapeutic options are limited and should be individualized based on patient’s general condition and comorbidities. The common clinical presentations are acute or chronic sepsis. The treatment options of Endograft infections classified either to remove the graft and it is considered the gold Standard approach, or bridging therapy and conservative treatment for patients which not suitable for surgery. Till now there is no evidence to prove the superiority of a treatment method over the other.
The in-situ arterial reconstruction is preferred because of the low mortality and morbidity rate in comparison with extra anatomical bypass like axillo-bifemoral bypass.
We report a case of infected Aortic graft after EVAR. The sequence of events in this case sheds light upon the importance of synchronization between conservative treatment, endovascular intervention and surgical intervention to achieve better results in terms of mortality and morbidity. The patient provided written informed consent to the publication
Objective
The sequence of events in this case report sheds light upon the importance of synchronization between conservative treatment, endovascular intervention and surgical intervention demonstration better prognostic outcomes in terms of mortality and morbidity.
Results
The endograft Infection and its complication is managed and with 3 years survivalrate.
Conclusion
In situ reconstruction for aortic graft infection with either autologous femoral vein or Bovine path remains the treatment of choice with acceptable rates of mortality and morbidity.
Endovascular Aneurysm Repair (EVAR) is currently considered the predominant treatment for Abdominal Aortic Aneurysm (AAAs), especially in a high-risk patient, providing a low early morbidity and mortality compared with open repair. A very low number of EVAR patients suffer complications, ranging from 0.7% related to local-vascular or implant-related complications to 11% for systemic complications. Endograft infection after EVAR represents one of the most serious complications. Although the incidence of Endograft infection is rare and below 1% it is often associated with high mortality and morbidity rates.
The therapeutic options are limited and should be individualized based on patient’s general condition and comorbidities. The common clinical presentations are acute or chronic sepsis. The treatment options of Endograft infections classified either to remove the graft and it is considered the gold Standard approach, or bridging therapy and conservative treatment for patients which not suitable for surgery. Till now there is no evidence to prove the superiority of a treatment method over the other.
The in-situ arterial reconstruction is preferred because of the low mortality and morbidity rate in comparison with extra anatomical bypass like axillo-bifemoral bypass.
We report a case of infected Aortic graft after EVAR. The sequence of events in this case sheds light upon the importance of synchronization between conservative treatment, endovascular intervention and surgical intervention to achieve better results in terms of mortality and morbidity. The patient provided written informed consent to the publication
Objective
The sequence of events in this case report sheds light upon the importance of synchronization between conservative treatment, endovascular intervention and surgical intervention demonstration better prognostic outcomes in terms of mortality and morbidity.
Results
The endograft Infection and its complication is managed and with 3 years survivalrate.
Conclusion
In situ reconstruction for aortic graft infection with either autologous femoral vein or Bovine path remains the treatment of choice with acceptable rates of mortality and morbidity.
Keywords
Aortic graft infection; EVAR
CASE PRESENTATION
A 77-years-old hypertensive patient who underwent EVAR for an enlarging 7-cm Abdominal Aortic Aneurysm (AAA) Using a Lombard Aorfix Graft. Protective embolization of the patient inferior mesenteric artery.
The post-operative CT Angiography confirmed a successful treatment of the aneurysm without need of further intervention. The patient attends for follow up controls afterward. Twenty-one months after the EVAR, the patient admitted due to abdominal pain and elevated infection parameters for further investigations.
An abdominal CT scan is done and demonstrated a remarkable contrast enhancement of the aneurysm wall and periaortic fluid collections (Figure 1).
The post-operative CT Angiography confirmed a successful treatment of the aneurysm without need of further intervention. The patient attends for follow up controls afterward. Twenty-one months after the EVAR, the patient admitted due to abdominal pain and elevated infection parameters for further investigations.
An abdominal CT scan is done and demonstrated a remarkable contrast enhancement of the aneurysm wall and periaortic fluid collections (Figure 1).
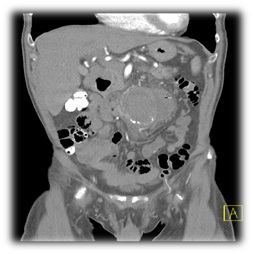
Figure 1: Periaortic fluid collections.
An empirical antibiotic therapy was started with Meropenem. The following days started the patients to develop high fever. The blood cultures revealed infection with Staphauereus; accordingly the antibiotic was changed to Linezolid.
For both diagnostic and potentially therapeutic purposes, CT guided drainage of the periaortic fluid collections was done (Figure 2).
For both diagnostic and potentially therapeutic purposes, CT guided drainage of the periaortic fluid collections was done (Figure 2).
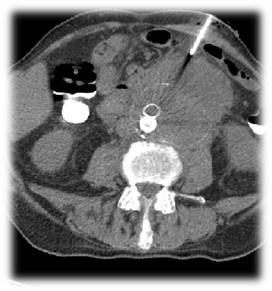
Figure 2: CT guided drainage of the periaortic fluid collections.
Two days later we decided to operate the patient. A complete surgical excision of the infected aortic stent was done. The intraoperative findings were remarkable for inflamed aneurysmal sac with thick purulent exudates surrounding the loosely incorporated graft (Figure 3).
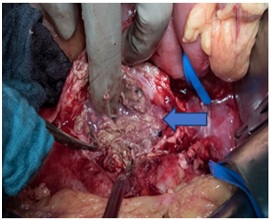
Figure 3: Inflamed aneurysmal sac with thick purulent exudates.
The arterial reconstruction is done through an aorto-unifemoral bypass at the right femoral artery using the right femoral vein (Figure 4). Then cross over femro-femoral bypass from the right side to the left side using biologic ovine graft.
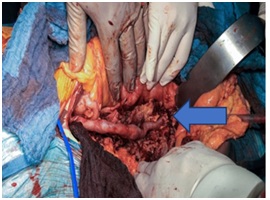
Figure 4: Aorto-unifemoral bypass using the right femoral vein.
One month later the patient developed abdominal pain. An abdominal CT showed a perforated sigmoid colons and fistula between the Sigmoid colon and the infected retroperitoneal abscess. The patient underwent rectum and sigmoid colon resection with terminal Colostomy. Then patient stayed in the intensive care from for one month under observation, then transferred to the ward and discharged from our hospital in a very stable and good general condition.
4 Months later the patient complained again of abdominal pain. A CT angiography was done and showed active contrast agent leakage from the native right common iliac artery and a Hematoma around it about 4.7 x 4.5 cm (Figure 5).
4 Months later the patient complained again of abdominal pain. A CT angiography was done and showed active contrast agent leakage from the native right common iliac artery and a Hematoma around it about 4.7 x 4.5 cm (Figure 5).
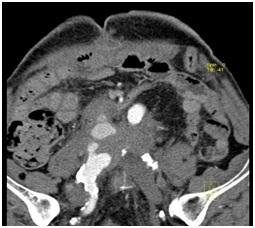
Figure 5: Bleeding from the native right common iliac artery.
The bleeding point was successfully controlled and embolized through an implantation of a 22 mm Amplatzer vascular Plug (Figures 6a and 6b).
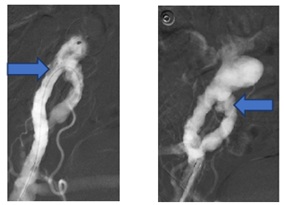
Figure 6: a) Bleeding from The right common iliac artery. b) Controlled by amtz plug.
The following day a CT Abdomen is done and revealed a successful result without leakage of the contrast agent (Figure 6c). The patient is discharged after 1-week stable and good condition.
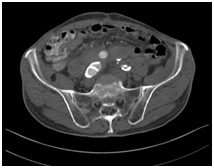 Figure 6c: Successful embolization of the native. Right common iliac artery.
Figure 6c: Successful embolization of the native. Right common iliac artery.One-month later patient presented again in the emergency room with rectal bleeding.
Ct abdomen does not show exactly where the bleeding point is however, it shows a fistula between the venous graft and the rectal stump. A conventional angiography was carried out and it shows diffuse oozing from the venous graft (Figure 7a).
Ct abdomen does not show exactly where the bleeding point is however, it shows a fistula between the venous graft and the rectal stump. A conventional angiography was carried out and it shows diffuse oozing from the venous graft (Figure 7a).
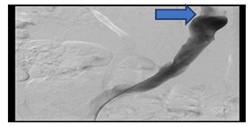
Figure 7a: Diffuse oozing from venous graft.
As a Bridging step, the bleeding was controlled in the same session by implantation of a Viabahn stent (Figure 7b).
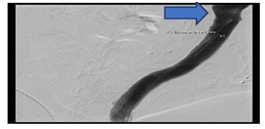 Figure 7b: Successful implantation of Viabahn stent in the venous graft.
Figure 7b: Successful implantation of Viabahn stent in the venous graft.After stabilization of the patient condition, we decided to re-operate the patient for another time; Intraoperative we prepared the suprarenal aorta and then identification of the coeliac trunk origin. Then carefully preparation and removal of the infected Venous graft.
The wall of the native right common iliac artery was eroded with diffuse bloody oozing around it, so that we had to prepare a good segment from it and then it was ligated.
An Anterior rectum resection was done with complete separation of the stump from the surrounding vessels.
At the same time a bovine graft cylinder about 12x25cms was made so that to try to shortens the operation time and then implanted followed by repeatedly peritoneal lavage (Figure 8). The postoperative course was remarkably uneventful; the patient discharged very stable and good general condition.
The wall of the native right common iliac artery was eroded with diffuse bloody oozing around it, so that we had to prepare a good segment from it and then it was ligated.
An Anterior rectum resection was done with complete separation of the stump from the surrounding vessels.
At the same time a bovine graft cylinder about 12x25cms was made so that to try to shortens the operation time and then implanted followed by repeatedly peritoneal lavage (Figure 8). The postoperative course was remarkably uneventful; the patient discharged very stable and good general condition.
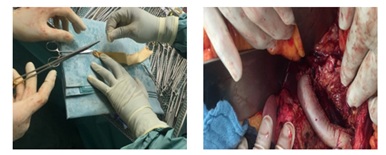 Figure 8: Preparing and implanting a Bovine graft cylinder to replace the infected venous Graft.
Figure 8: Preparing and implanting a Bovine graft cylinder to replace the infected venous Graft.DISCUSSION
Although the incidence of endograft infection is rare and below 1% [1]. However, once occurred it is always accompanied with high mortality rate of about 25-60% and limb loss of about 25%.
The clear etiology of the occurrence of endograft infection until now is not clear, however there are multiple theories that can explain that, with many Risk factors that can lead to Graft infection, like preforming an urgent EVAR, post EVAR interventional vascular procedure or perioperative infection [2]. Treatment of an Endoleak or another nonvascular operation like urological or spinal interventions before the diagnosis of the graft infection can be a Spreading cause of infection for the patient who underwent EVAR [3]. The most common micro bacterial finding in a case of graft Infection is Staphylococcus [4]. Accordingly, we suggested that the route of infection in this case was hematogenous spread, as our patient underwent TURP 2 months before the incidence of the graft infection and blood culture that was done 2 months before the graft infection shows infection with Staphylococcus.
The diagnosis of infected graft depending on the clinical findings, radiological evidence and microbial cultures according to Management of Aortic collaboration Consensus (MAGIC) [5]. The CT angiography should be done in all patients. The CT scan has a very high sensitivity and specificity, it is also being performed quickly and always available considered a gold standard in Cases of Graft infection.
In our case, a CT was done and revealed a peri graft air, intrasac collections, fluid accumulation, and soft tissue attenuation.
If the CT was not helpful in the Diagnosis of a suspected Graft infection, MRI and PET CT should be taken in consideration [6].
Now a days there is no internationally evidence-based guidelines in the management of Endograft infection. In Patients with much comorbidity with many risk factors, a conservative management is recommended [7]. However, it still recommended by a Suitable patient to remove the Graft. These recommendations are accompanied with high survival rate compared with those who managed by conservative treatment only. Lyons et al., reported that 30-day mortality after removal of infected graft is about 30%, but without removal lead to death of all patient with endograft infection [8]. The treatment decision very difficult and depend on the opinion of the operators.
Surgical treatment includes two steps, the graft removal and arterial reconstructions. Although the Extra Anatomical Bypass (EAB) was the Gold standard in management of an Endograft infection for years, however it is accompanied with a lot of complications like the risk of aortic stump blow out of about 25% in patients with persistent retroperitoneal sepsis, the risk of amputation reaches about 29%, also the risk of renal artery occlusion in case aortic stump closure [6]. That’s why is the in-situ reconstruction nowadays has surpassed the extra anatomical bypass EAB regarding the (mortality, Patency, amputation rate) [9]. The usage of Autogenous superficial femoral-popliteal veins graft in the treatment of Endograft infection and as a material of aorto-iliac reconstruction represents the most common method to be used. This technique of reconstruction called neoaortoiliac procedure [10].
In This case the patient was fit to the operation. We decided to remove the graft. In order to remove the infected graft the proximal portion of the graft is dislodged without any occurrence of dissection of the pararenal aorta [11]. Then we used the right femoral vein making the aortofemoral bypass on the right side and to save the time of operation and extended surgical Trauma. We decided to do femro-femoral bypass using Omni flow graft.
The endovascular intervention plays a very important a significant role especially in a case of bleeding situation without clear indication to do surgery. It’s a bridging or staging solution till stabilization of the patient condition and identifying an indication for the operation, like in our case. However bridging therapy is not a curative option [12].
Although the rate of Reinfection of the Vein Graft is less 2% [13]. Our patient developed again reinfection the venous graft is removed and replaced with Bovine tube graft without any signs of reinfection till now. Our argument also by the usage of Bovine patch is the low rate of reinfection and biocompatibility and that’s proved in the Replacement of Infected bypass below the knee [14].
Our patient is discharged and the postoperative follow up in our outpatient clinic.
The clear etiology of the occurrence of endograft infection until now is not clear, however there are multiple theories that can explain that, with many Risk factors that can lead to Graft infection, like preforming an urgent EVAR, post EVAR interventional vascular procedure or perioperative infection [2]. Treatment of an Endoleak or another nonvascular operation like urological or spinal interventions before the diagnosis of the graft infection can be a Spreading cause of infection for the patient who underwent EVAR [3]. The most common micro bacterial finding in a case of graft Infection is Staphylococcus [4]. Accordingly, we suggested that the route of infection in this case was hematogenous spread, as our patient underwent TURP 2 months before the incidence of the graft infection and blood culture that was done 2 months before the graft infection shows infection with Staphylococcus.
The diagnosis of infected graft depending on the clinical findings, radiological evidence and microbial cultures according to Management of Aortic collaboration Consensus (MAGIC) [5]. The CT angiography should be done in all patients. The CT scan has a very high sensitivity and specificity, it is also being performed quickly and always available considered a gold standard in Cases of Graft infection.
In our case, a CT was done and revealed a peri graft air, intrasac collections, fluid accumulation, and soft tissue attenuation.
If the CT was not helpful in the Diagnosis of a suspected Graft infection, MRI and PET CT should be taken in consideration [6].
Now a days there is no internationally evidence-based guidelines in the management of Endograft infection. In Patients with much comorbidity with many risk factors, a conservative management is recommended [7]. However, it still recommended by a Suitable patient to remove the Graft. These recommendations are accompanied with high survival rate compared with those who managed by conservative treatment only. Lyons et al., reported that 30-day mortality after removal of infected graft is about 30%, but without removal lead to death of all patient with endograft infection [8]. The treatment decision very difficult and depend on the opinion of the operators.
Surgical treatment includes two steps, the graft removal and arterial reconstructions. Although the Extra Anatomical Bypass (EAB) was the Gold standard in management of an Endograft infection for years, however it is accompanied with a lot of complications like the risk of aortic stump blow out of about 25% in patients with persistent retroperitoneal sepsis, the risk of amputation reaches about 29%, also the risk of renal artery occlusion in case aortic stump closure [6]. That’s why is the in-situ reconstruction nowadays has surpassed the extra anatomical bypass EAB regarding the (mortality, Patency, amputation rate) [9]. The usage of Autogenous superficial femoral-popliteal veins graft in the treatment of Endograft infection and as a material of aorto-iliac reconstruction represents the most common method to be used. This technique of reconstruction called neoaortoiliac procedure [10].
In This case the patient was fit to the operation. We decided to remove the graft. In order to remove the infected graft the proximal portion of the graft is dislodged without any occurrence of dissection of the pararenal aorta [11]. Then we used the right femoral vein making the aortofemoral bypass on the right side and to save the time of operation and extended surgical Trauma. We decided to do femro-femoral bypass using Omni flow graft.
The endovascular intervention plays a very important a significant role especially in a case of bleeding situation without clear indication to do surgery. It’s a bridging or staging solution till stabilization of the patient condition and identifying an indication for the operation, like in our case. However bridging therapy is not a curative option [12].
Although the rate of Reinfection of the Vein Graft is less 2% [13]. Our patient developed again reinfection the venous graft is removed and replaced with Bovine tube graft without any signs of reinfection till now. Our argument also by the usage of Bovine patch is the low rate of reinfection and biocompatibility and that’s proved in the Replacement of Infected bypass below the knee [14].
Our patient is discharged and the postoperative follow up in our outpatient clinic.
CONCLUSION
Infected EVAR is a very complex und devastating complication accompanied with high mortality and morbidity; however a regular follow up for patients undergoing EVAR will help in the early diagnosis of graft infection. A good combination between conservative and surgical intervention when possible with immediate or staged aortic reconstruction can lead to a better prognostic outcome.
REFERENCES
- Flora HS, Chaloner EJ, Sweeney A, Brookes J, Raphael MJ, et al. (2003) Secondary intervention following endovascular repair of abdominal aortic aneurysm: A single centre Experience. Eur J Vasc Endovasc Surg 26: 287-292.
- Chaufour X, Gaudric J, Goueffic Y, Khodja RH, Feugier P, et al. (2017) A multicenter experience with infected abdominal aortic endograft explantation. J Vasc Surg 65: 372-380.
- Smeds MR, Duncan AA, Harlander-Locke MP, Lawrence PF, Lyden S, et al. (2016) Treatment and outcomes of aortic endograft infection. J Vasc Surg 63: 332-340.
- Dosluoglu HH, Curl GR, Doerr RJ, Painton F, Shenoy S (2001) Stent-ralated iliac arteryand iliac vein infections: two unreported presentations and review of literature. J Endovasc Ther 8: 202-209.
- Lyons OT, Baguneid M, Barwick TD, Bell RE, Foster N, et al. (2016) Diagnosis of aortic graft infection: a case de?nition by the management of aortic graft infection collaboration (MAGIC). Eur J Vasc Endovasc Surg 52: 758-763.
- Capoccia L, Mestres G, Riambau V (2014) Current technology for the treatment of infection following abdominal aortic aneurysm (AAA) ?xation by endovascular repair (EVAR). J Cardiovasc Surg (Torino) 55: 381-389.
- Fatima J, Duncan AA, de Grandis E, Oderich GS, Kalra M, et al. (2013) Treatment strategies and outcomes in patients with infected aortic endografts. J Vasc Surg 58: 371-379.
- Lyons OT, Patel AS, Saha P, Clough RE, Price N, et al. (2013) A 14-year experience withaortic endograft infection: management and results. Eur J Vasc Endovasc Surg 46: 306-313.
- Berger P, Moll FL (2011) Aortic graft infections: is there still a role for axillobifemoral reconstruction? Semin Vasc Surg 24: 205-210.
- Ducasse E, Calisti A, Speziale F, Rizzo L, Misuraca M, et al. (2004) Aortoiliac stent graft infection: current problems and management. Ann Vasc Surg 18: 521-526.
- Setacci C, De Donato G, Setacci F, Chisci E, Perulli A, et al. (2010) Management of abdominal endograft infection. J Cardiovasc Surg (Torino) 51: 33-41.
- Haidar GM, Hicks TD, Strosberg DS, El-Sayed HF, Davies MG (2017) "In situ" endografting in the treatment of arterial and graft infections. J Vasc Surg 65: 1824-1829.
- Chung J, Clagett GP (2011) Neoaortoiliac System (NAIS) Procedure for the treatment of the infected aortic graft. Semin Vasc Surg 24: 220-226.
- Töpel I, Betz T, Uhl C, Wiesner M, Bröckner S, et al. (2012) Use of biosynthetic prosthesis (Omniflow II®) to replace infected infrainguinal prosthetic grafts--first results. Vasa 41: 215-220.
Citation: ELFEKY M, Strietzel M, Feldmann med M (2019) Management of an Aortic Graft Infection after Endovascular Aneurysm Repair. J Angiol Vasc Surg 4: 020.
Copyright: © 2019 Moustafa ELFEKY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2024, Copyrights Herald Scholarly Open Access. All Rights Reserved!
