
Venous Pressure Gradient: It’s Role in Diagnosis and Treatment of Steno-Obstructions of Deep Venous System
*Corresponding Author(s):
Sergio PetronelliDepartment Of Interventional Radiology And Vascular Surgery, Surgical Department, “Miulli” General Hospital, Acquaviva Delle Fonti, BA, Italy
Tel:+39 0803054246,
Email:spetro@tiscali.it
Abstract
Objectives: To show a simple endovascular technique both to do the diagnosis of the steno-obstructions of caval iliac femoral veins, in chronic venous hypertensions and to evaluate the results of angioplasty and stent treatment.
Materials and methods: A total of 90 Patients (Pt) in CEAP clinical class 3-6 (according to CEAP classification-clinical, etiologic, anatomic, pathophysiologic for chronic venous disease) were evaluated for steno-obstructions of caval iliac femoral veins with a new technique of measurement of Venous Pressure Gradient (VPG) and with a morphological evaluation of these deep veins; we considered as indicator of venous hypertension a pressure gradient ≥ 3mmHg /400 Pa.
In the first experience we analyzed 60 Pt; we considered eligible for the treatment of venous iliac Percutaneous Transluminal Angioplasty/Stenting (PTA/Stent). Only the patients with the presence of pressure gradient associated to a morphological stenosis >50%, measured through retrograde Digital Phleboscopy (DPh).We named Pt DPh this court of patients.
In the second experience with the remaining patients, we considered the patients with positivity to the pressure gradient are eligible for the treatment. In this case we used a new morphological technique Intra-Venous Ultrasound (IVUS), only to have a few details about neointima (Pt Iv).
Technical success will be considered after the PTA /stent value of VPG is equal to 0mmHg/Pa. Clinical and Ultrasound (US) evaluation was performed before and after the treatment; in case of stenting we performed Computerized Tomography (CT) evaluation during follow up.
Results: We have totally evaluated 90 Pt; in the first experience with 60 consecutive Pt ranked as CEAP III-VI. In 15 Pt we found VPG ≥ 3mmHg (≥ 400 Pa) and only 5 Pt with stenosis ≥ 50% by DPh.
In these 5 Pt we performed iliac venous 4 PTA and 1 stenting.
In the second experience with the remaining 30 consecutive Pt ranked as CEAP III-VI. In 6 Pt we found VPG ≥ 3mmHg (≥ 400 Pa). In all these Pt IVUS confirmed a morphological wall thickening without morphological stenosis to DPh. We performed iliac venous 4 PTA and 2 Stenting.
We obtained the technical success after angioplasty/stenting of iliac vein steno-obstructions in all cases. No other complications is observed during the procedure.
F.W. at 12 months (range: 3-24 months) showed a clinical down-staging in all patients with no occlusion in the site of the treatment.
Conclusion: Our endovascular technique allows the measurement of the pressure gradient and the discover of the venous chronic hypertension in caval iliac femoral veins.
The measurement of VPG provides the finding of iliac steno-obstructions without the evidence of stenosis by DPh. Moreover it is possible that the minimal changes in neointima can alter wall characteristics and its capacitance and increase the venous pressure. The VPG equal to 0mmHg/Pa after iliac venous PTA/stenting together with good clinical outcome could be used to show the effectiveness of the treatment of venous chronic hypertension.
Keywords
ABBREVIATIONS
VCSS: Venous Clinical Severity Score
DVS: Deep Venous System
CVI: Chronic Venous Insufficiency
CFV: Common Femoral Vein
ECD: Eco-Color Doppler
IVUS : Intra-Venous Ultrasound
VPG: Venus Pressure Gradient
Pt DPh: First cohort of Pt with Digital Phleboscophy
Pt Iv: Second cohort of Pt with IVUS
PTA: Percutaneous Transluminal Angioplasty
US: Ultrasound
CT: Computerized Tomography
DVT: Deep Venous Thrombosis
DPh: Digital Phleboscopia
INTRODUCTION
Chronic venous outflow obstructions more than the reflux are important in venous hypertension; it is known that they usually occur months to years after an initial Deep Venous Thrombosis (DVT). In symptomatic patients, recanalisation of thrombosis veins is incomplete and the collateral circulation is inadequate resulting in distal venous hypertension with lower extremity swelling, pain worsened after ambulation, venous ulcers and others clinical manifestations of post thrombotic syndrome. Although venous outflow obstructions of the lower extremity may involve the entire venous system, iliocaval venous steno-obstructions, more than peripheral obstructions, play an important role in determining the most severe symptoms of venous insufficiency [1-6].
Related to diagnostic difficulties, steno-obstructions have been ignored whereas emphasis was placed only on reflux [3]. Pelvic Eco Color Doppler examination (ECD) indeed does not provide us the possibility to evaluate exactly the anatomy of iliac veins. On the other hand, second-level imaging examinations, such as Angiography Computed Tomography (Angio-CT) or Magnetic Resonance Imaging (MRI) is too expensive and do not provide hemodynamic data.
Moreover in the last decade a few authors describe the much broaded disease profile that emerged with the use of IVUS for diagnosis, finding the incidence of no thrombotic iliac vein outflow obstructions to be very high in symptomatic CVI cases; it has been known that the etiology of venous steno-obstructions can be primary (nonthrombotic) or secondary (post-thrombotic) with equal prevalence estimated in patients with chronic venous disease [2-7]. It was already known that primary forms are related to compression of the left iliac common vein, anatomically near to the hypogastric artery bifurcation, with presence of webs or membranes resulting from traumatic injury caused by pulsations of the artery [7,8]. Morever, with IVUS has emerged that the primary disease is dominant but not exclusive to left lower limb; so any patient should be excluded from consideration of these lesions based on age, sex, bilaterality, or involvement of the right side [9].
A combination of reflux and obstruction is commonly present in either aetiology [10]. Regard the treatment of limbs with CVI, while the interventions on the superficial venous system and perforator veins is more or less effective both with surgical and innovative technique, that on deep venous system, has pointed on the treatment of the deep reflux even if with poor results. Moreover, in the past open surgical treatment of iliac steno-obstructions failed for the low success rates and for the high invasiveness.
Recently, the treatment options on deep venous system have changed dramatically with a great interest for chronic deep venous iliac steno-obstructions. Endovascular treatment involving the use of iliac venous PTA and stenting has become gold standard treatment [1-11].
But in literature there is not a universal and recognized parameter able to select patients with caval iliac femoral out-flow obstructions responsible of venous hypertension and CVI; the limitations of diagnostic tests are related to the absence of a uniform definition of hemodynamically significant venous stenosis. Currently there is not a direct correlation between the morphological degree of stenosis and the venous pressure gradient. Small changes in the vessel wall, for example as outcomes of previous inflammation, could provide compliance’s reduction and as a consequence significant hypertension and CVI .The absence of a “gold standard” is a major obstacle to the evaluation of the chronic outflow obstructions, the selection of the limb to be treated and the monitoring of technical success of endovascular treatment with PTA and stenting [1].
We would like to find a simple and standardized endovascular technique to assess caval-iliac-femoral out-flow obstructions using hemodynamic rather than morphological criteria.
The aim of this report is to demonstrate the use of venous pressure gradient in the assessment of iliac-femoral-cava venous hypertension so as to provide both a better selection of patients for endovascular treatment and a standard evaluation of the treatment’s effectiveness.
MATERIALS AND METHODS
Following scientific literature data and our previous clinical experience, we considered as normal a pressure gradient value equal to zero. We considered as an indicator of venous hypertension a pressure gradient ≥ 3mmHg/400 Pa.
Our technique consists of a contralateral inguinal puncture of the side to be evaluated [12]. After skin disinfection of the groin we proceed with a puncture and catheterise of Common Femoral Vein (CFV) using a 6Fr introducer. After iliac cross-over, using a stiff guidewire 0, 35 of 260 cm in length and a multipurpose catheter 5Fr of 90 cm in length we catheterize the CFV of the other side and we proceed with the measurement of the venous gradient between femoral-iliac veins and caval veins and then of the side of the puncture (Figure 1). For the measurement of the venous pressure we put the transducer at the cardiac level and after zero we connect it through a catheter connection to the vein; by moving the catheter we measure the pressure at different points along the femoral – iliac and caval venous axis. We carry out measurements with the patient at the same stage of breathing .Then we measure the gradient between the highest value and the lowest measured value.
In the first experience (Pt DPh) with 60 Pt we performed retrograde DPh of the femoral iliac caval venous system to confirm the catheter tip position and to have a morphological evaluation of the venous axis; we used 1-2 injections of approx 20ml of visipaque 270 contrast solution with Medrad Mark V digital automatic injector using Philip Allura X angiographic machine; the use of digital phleboscopy reduces radiations to the patient. In this court of patients we considered for treatment the presence of a stenosis of a diameter vessel reduction ≥50% and a pressure gradient ≥ 3mmHg/400Pa.
In the second experience (Pt Iv) with 30 Pt we only considered for treatment the presence of a pressure gradient ≥ 3mmHg/400Pa; in these cases we performed IVUS, even if there was not a stenosis with DPh so as to evaluate changes in vein wall. Technical success will be considered if VPG is equal to zero after PTA/stent treatment.
In case of angioplasty, we used balloon FOX ABBOTT with diameter 9-12 mm and pressure 8-10 atmosphere. In case of stent, we used stent SMART CORDIS (14x80mm).
Clinical and ecodoppler follow-up was carried out at 12 months (range: 3-24 months) in all treated patients. Clinical outcome was evaluated with Venous Clinical Severity Score (VCSS) before and after treatment.
The team involved in the present study consisted of a vascular surgeon and an interventional radiologist with experience in venous disease and endovascular techniques on the superficial and deep venous system.
RESULTS
| VPG measurement | Positive >3mmHg | Negative | Not Treated | Treated |
| n.60 (Ph) | 15 | 45 | 10 | 5 |
| n.30 (Iv) | 6 | 24 | 0 | 6 |
| Total n.90 | 21 | 69 | 10 | 11 |
In our first experience with a number of 60 Pt (Pt Ph) we carried out the evaluation of VPG and morphological parameter of steno-obstruction with DPh: we had in 15 procedures VPG ≥ 3mmHg/400 Pa and only in 5 cases of these 15 procedures we had a stenosis ≥50%. We performed 4 PTA and 1 Stent. In conclusion, we performed angioplasty/stenting treatment in 5 cases classified as CEAP III-VI.
About 5 cases were treated with angioplasty/stenting: three of all regarding iliac left veins alone, one regarding left iliac and carrefour iliac vein and one stenting left iliac vein (Figure 2) (Table 2).
 Figure 2: Phleboscopic steno-obstruction images of carrefour and left common iliac vein a) the steno-obstruction b-c) the balloon angioplasty of these segments d) the phleboscopic final control post-angioplasty.
Figure 2: Phleboscopic steno-obstruction images of carrefour and left common iliac vein a) the steno-obstruction b-c) the balloon angioplasty of these segments d) the phleboscopic final control post-angioplasty.
Phpt |
PTA | PTA |
| Left iliac vein | 3 | 3 |
| Left iliac vein +Iliac carrefour | 1 | 1 |
| Total | 4 | 4 |
In second experience with a number of 30 Pt (Pt Iv), we carried out the evaluation of pressure gradient without morphological stenosis with DPh. In 6 procedures VPG was ≥ 3mmHg/400Pa and in all these cases IVUS confirmed thickening of the venous wall. We performed 4 angioplasty and 2 stenting.
In conclusion, we performed angioplasty/stenting treatment in 6 cases classified as CEAP III-VI.
About 6 cases treated with angioplastic/stenting: two of all regarding iliac left veins alone, one left femoral and left iliac vein, one right iliac vein, one stenting of left iliac vein after unsuccessful angioplasty and one stenting for right iliac vein occlusion (Figures 3,4) (Table 3).
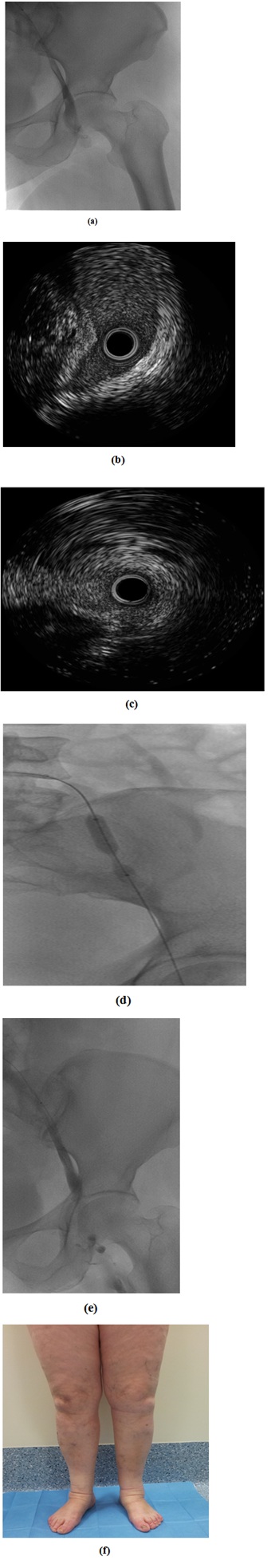
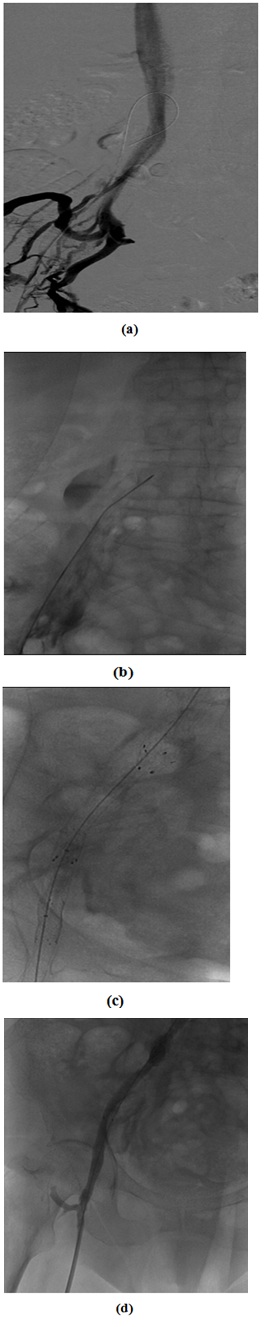 Figure 4: Pt with phlebography images with right iliac vein obstruction and collateral pathway and VPG > 3mmHg (Pt Ph) a) Phlebography image it shows right iliac vein obstruction and collateral pathway b) catheterism VPG > 3mmHg after the guide crossing c) primary stenting of iliac vein d) Phleboscopic re-canalization of iliac vein and disappearance of collateral pathway and catheterism VPG = 0 mmHg.
Figure 4: Pt with phlebography images with right iliac vein obstruction and collateral pathway and VPG > 3mmHg (Pt Ph) a) Phlebography image it shows right iliac vein obstruction and collateral pathway b) catheterism VPG > 3mmHg after the guide crossing c) primary stenting of iliac vein d) Phleboscopic re-canalization of iliac vein and disappearance of collateral pathway and catheterism VPG = 0 mmHg. | Iv Pz | PTA | STENT |
| Left iliac vein | 2 | 1 |
| Left iliac vein + femoral vein | 1 | |
| Right iliac vein | 1 | 1 |
| Total | 4 | 2 |
Technical success of angioplasty/stenting of iliac vein steno-obstructions with resolution pressure gradient (P=0mmHg) was equal to 100% of cases. No early and later complications occurred in our experience.
The early clinical improvement after angioplasty/stenting of iliac vein was demonstrated by reduction of edema and symptoms of heaviness and pain on the inferior limbs suddenly after the treatment; all Pt had a clinical down staging during follow-up. ECD follow-up demonstrated no occlusions and computed tomography follow-up showed pervious stents without evidence re-stenosis or fractures.
DISCUSSION
Recently endovascular treatment involving the use of PTA and stenting has become gold standard treatment of caval iliac and femoral outflow obstructions because it is a minimally invasive approach, has a high technical success rate and an acceptable complication profile [1-11].
Iliac vein PTA/Stenting is an extension of arterial endovascular technology; the two share some technical similarities and much of the hardware, however the indications for and the purpose of iliac vein PTA/Stenting are fundamentally different.
Current diagnostic modalities do not allow a definitive assessment of critic hemodynamic venous obstructions to be treated. The diagnosis is based both on clinical signs and symptoms and on radiological assessment of morphological venous outflow such as trans-femoral phlebography, Intravascular Ultrasound (IVUS), CT and RM venography [5,11,13].
Moreover, does not help the history of a previous thrombosis to selecting patients; from an etiological point of view, the incidence of primary form is higher than the secondary type. In our series, only 3 Pt were previously diagnosed with venous deep thrombosis.
On the base of previous results published in literature, the incidence of outflow steno-obstructions is higher in the most several clinical classes (CEAP 5-6) with 37% of ilio-caval steno-obstructions =50% and 23% of cases greater than 80%, on average [5]. Only in case of obstructions greater than 80% the association with reflux, female gender and history of deep vein thrombosis was significant and only in these cases authors suggest the performance of CT or MR venography routinely.
Imaging diagnosis cannot be related to US alone. The visualization of the iliac veins in pelvis, in fact, could be difficult due to pelvic organs and bowel gas. In addition, Doppler wave forms in the common femoral veins can display normal spontaneous flow and respiratory variation due to large collateral vessels around the site of proximal obstruction [15]. Nowadays, there is not a gold standard for selection of patients who need treatment of iliac outflow obstruction; on one hand the anterograde phlebography can result in false negative images, on the other hand IVUS is very helpful but could be used only in a small number of patients [15].
Recently, the introduction of IVUS allowed advances in knowledge of venous stenosis because it could provide us the possibility to evaluate intra-luminal details such as wall thickness and neo-intimal hyperplasia in patients with venous steno-obstructions, usually underestimated by phlebography [11]. It is possible that this intra-luminal alterations resulting from inflammatory processes are correlated with increase in pressure gradient, such as in other venous districts (e.g., superior vena cava syndrome, stenosis in A-V fistulas, etc.,) [16-18]. From this consideration it is clear that not always a venous hypertension caused by reduction of wall compliance may be morphologically related to a critical stenosis.
On the other hand, the venous hypertension has been correlated with clinical manifestations of chronic venous disease (edema, erythema, ulcer, dyschromia) determining inflammation. As a consequence, flogosis cause vein wall changes, increasing venous hypertension [17].
In our study, we found that the parameter of iliac-femoral pressure gradient could be correlated with venous hypertension. Our data show, in fact, that the pressure gradient equal to zero has a high sensitivity for the absence of venous hypertension. Starting from this parameter, despite the small number of cases (21/90 cases), we believe that an increase of pressure gradient >3mmHg/400 Pa could be indicative of venous hypertension. In literature there are not studies about pressure gradient along venous stenosis. Few authors described an increase of pressure in the femoral vein before and after the exercise or compared to the opposite side; but this pressure value concern the whole leg instead of segmental venous axis [14,15,19].
Moreover the use of gradient pressure is easy method because it is a scalar magnitude that does not suffer from other variables because it is a pressure difference.
Furthermore, we found that pressure gradients >3mmHg/400Pa is also important when morphological images do not show signs of venous stenosis because, after treatment of axis femoral iliac involved, the pressure gradient become normal.
More evidences in the literature showed that mild degrees of stenosis could be responsible of venous hypertension and, as a consequence, of chronic venous disease [1,20-22].
For this reason we believe that this parameter can be used as a predictor of chronic venous hypertension even in absence of evident morphological stenosis [18]. In our second experience we use only VPG for the evaluation of patients. Following this parameter, we increased the number of patients treated (6/30 vs 5/60 treated in the first experience). The measurement of VPG, in our experience, is a more sensible parameter in the diagnosis of venous steno-obstructions confirmed by IVUS. In fact, when we used as parameter to treat the presence of morphological stenosis with DPh, we missed 47% of patients with VPG ≥ 3 (Table 4).
| Patient with VPG>3mmHg | Without endovascular treatment | Endovascular treatment |
| 21 | 10*/21 (47%) | (5*+6**) 11/21 (52%) |
In patients without morphological evidence of steno-obstructions the pressure gradient normalization post-PTA leds necessarily to a further investigations with other techniques such as IVUS before and after dilatation.
CONCLUSION
REFERENCES
- Mahnken AH, Thomson K, de Haan M, O'Sullivan GJ (2014) CIRSE Standards of Practice Guidelines on Iliocaval Stenting . Cardiovasc Intervent Radiol 37: 889-897.
- Hartung O, Otero A, Boufi M, De Caridi G, Barthelemy P, et al. (2005) Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg 42: 1138-1144.
- Neglén P (2008) Chronic deep venous obstruction: definition, prevalence, diagnosis, management. Phlebology 23: 149-157.
- Titus JM, Moise MA, Bena J, Lyden SP, Clair DG (2011) Iliofemoral stenting for venous occlusive disease. J Vasc Surg 53: 706-712.
- Marston W, Fish D, Unger J, Keagy B (2011) Incidence of and risk factors for iliocaval venous obstruction in patients with active or healed venous leg ulcers. J Vasc Surg 53: 1303-1308.
- Neglén P, Raju S (2002) Proximal lower extremity chronic venous outflow obstruction: recognition and treatment. Semin Vasc Surg 15: 57-64.
- Almeida JI, Boatright C (2011) Iliocaval stenting for advanced chronic venous disease. Endovascular Today 10: 62-64.
- Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, et al. (2004) Iliac vein compression in an asymptomatic patient population. J Vasc Surg 39: 937-943.
- Raju S, Neglen P (2006) High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg 44: 136-143.
- Raju S, Darcey R, Neglén P (2010) Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg 51: 401-408.
- Raju S (2013) Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg 57: 1163-1169.
- Neglén P (2007) Chronic venous obstruction: diagnostic considerations and therapeutic role of percutaneous iliac stenting. Vascular 15: 273-280.
- Mussa FF, Peden EK, Zhou W, Lin PH, Lumsden AB, et al. (2007) Iliac vein stenting for chronic venous insufficiency. Tex Heart Inst J 34: 60-66.
- Neglén P, Thrasher TL, Raju S (2003) Venous outflow obstruction: An underestimated contributor to chronic venous disease. J Vasc Surg 38: 879-885.
- Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, et al. (2006) Chronic venous disease. N Engl J Med 355: 488-498.
- Costa A,Veneziani A,Vergara C (2005-2006) Modellazione Numerica dei distretti venosi a partire da modelli matematici monodimensionali Tesi di Laurea Facoltà di Ingegneria Biomedica matricola 632389 Politecnico di Milano. 32-49.
- Raju S (2015) Treatment of iliac-caval outflow obstruction. Semin Vasc Surg 28: 47-53.
- Neglèn P, Raju S (1993) Detection of outflow obstruction in Chronic venous insufficiency. J Vasc Surg 17: 583-589.
- Nayak L, Hildebolt CF, Vedantham S (2012) Postthrombotic syndrome: feasibility of a strategy of imaging-guided endovascular intervention. J Vasc Interv Radiol 23: 1165-1173.
- Wen-da W, Yu Z, Yue-Xin C (2016) Stenting for chronic obstructive venous disease: A current comprehensive meta-analysis and systematic review. Phlebology 31: 376-389.
- Alhalbouni S, Hingorani A, Shiferson A, Gopal K, Jung D, et al. (2012) Iliac-femoral venous stenting for lower extremity venous stasis symptoms. Ann Vasc Surg 26: 185-189.
- Petronelli S, Prudenzano R, Mariano L, Violante F (2006) Endovenous laser therapy of the incompetent great saphenous vein. Radiol Med 111: 85-92.
Citation: Petronelli S, Zurlo MT, Guglielmi G (2017) Venous Pressure Gradient: It’s Role in Diagnosis and Treatment of Steno-Obstructions of Deep Venous System. J Angiol Vasc Surg 2: 009.
Copyright: © 2017 Sergio Petronelli, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

