
Novel Ex Vivo Stricture Model for Evaluation of Gastrointestinal Stent Migration
*Corresponding Author(s):
Miroslav P. PeevDepartment Of Surgery, New York University Langone Medical Center & NYU School Of Medicine, New York, United States
Tel:+1 6174709195,
Email:Miroslav.Peev@nyumc.org
Abstract
Background
The development of novel, high performance colonic stents requires in depth understanding of migration and perforation properties. The aim of this study is to establish a porcine ex vivo stricture model that could reliably assess stent migration and could guide the design and development of the next generation gastrointestinal implantable devices.
Methods
A simple ex vivo porcine stricture model was created and subsequently integrated into a newly designed dynamic testing apparatus that simulates gastrointestinal migration. We investigated the migration characteristics of six of the most commonly used colonic stents (WallFlex, Ultraflex, Ella, Choostent, Niti-S-D-type, Niti-S-ComVi). A total of thirty-six experiments were performed and the maximal pullout resistance force, the total migration energy, the migration initiation force and the time to migrate were recorded. All colon specimens were assessed for injury at the end of the experiments.
Results
Ultra flex achieved the highest maximal pullout resistance force (7.9±0.9N) and also required the largest amount of energy to migrate (168.3±30.5J). Alternatively WallFlex generated the least pullout energy (52.7±10J), but due to its elongation ability, it required the longest time to completely migrate (80.6±1.8sec). The non-covered Niti-S D-Type and the partially covered Niti-S-ComVi reached the highest force before the migration occurred (4.2N and 3.7N) and were associated with a subsequent colon perforation.
Conclusions
This is the first dynamic ex vivo animal model that closely simulates human colonic stricture and tests stent migration. This model could be a valuable tool to help understanding the process of migration and steer the development of modern stent technology.
Keywords
Colon stricture; Ex vivo porcine model; Stent migration
INTRODUCTION
Colorectal Cancer (CRC) continue to be among the most common malignant diseases in the United States with over 134,000 new cases diagnosed annually including more than 95,000 colon and 39,000 rectal cancers [1]. Despite the progressive advancement in screening and therapy, colorectal cancer still remain the third most common cause of cancer related death in the US with approximately 50,000 deaths each year [1,2].
Over time, 7-29% of the patients with CRC will present with near complete or complete bowel obstruction that require rapid intervention such as emergency surgery with or without creation of colostomy. Unfortunately such invasive procedures result in an unacceptably high rate of morbidity (40 - 78%) and mortality (12 - 24%) [3]. In addition, colostomy in the elderly and frail patients has adverse effect on the quality of life with ostomy reversal in only half of the patients [4,5].
Self-Expandable Colonic Stents (SECS) as an alternative treatment for malignant colonic obstruction was initially described by Dohomoto in 1991 [6]. Within the last two decades colonic stenting gained popularity and was mainly used with two indications: as a Bridge to Surgery (BTS) or definitive therapy of palliative malignant strictures. Currently, only 12.1% of all colonic obstructions are treated with SECS, leaving the majority of the patients with only invasive surgical alternatives [7].
One of the main reasons for such low penetration into clinical practice is the underwhelming performance profile of the available colonic stents. Unacceptably high migration rates (10 - 11.8%) [3,8], excessive stiffness and lack of flexibility resulting in dangerous perforations (7.2-10.9%) are responsible for the high failure rates and justified skepticism among clinicians [9].
In order to develop novel colonic stents that can overcome the deficiencies of the currently available SECS, there is an acute need to develop testing models that could reliably evaluate and compare key stent characteristics.
The aim of this study is to establish a simple dynamic ex vivo colon stricture model that could be used for in depth investigation of the migration properties of SECS in the early phase of development. Better understanding of the various pullout forces associated with migration would help to guide the design of the next generation gastrointestinal implantable devices.
MATERIALS AND METHODS
Commercially available stents
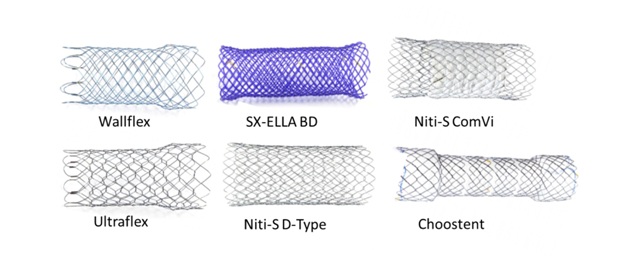 Figure 1: Photographic overview of the colonic stents tested in the study. Wallflex, Ultraflex and Niti-S D-Type are non-covered self expandable nitinol stents. Niti-S ComVi is partially covered with ePTFE and the Choostent is a segmented fully covered with silicone stent. SX-ELLA BD is a self expandable, biodegradable stent.
Figure 1: Photographic overview of the colonic stents tested in the study. Wallflex, Ultraflex and Niti-S D-Type are non-covered self expandable nitinol stents. Niti-S ComVi is partially covered with ePTFE and the Choostent is a segmented fully covered with silicone stent. SX-ELLA BD is a self expandable, biodegradable stent.Ex vivo stricture model and testing apparatus
After a 30mm long red rubber stricture was created, it was attached to a custom-designed, 3D-printed plastic base that was later integrated into the fixture of an automated shuttle system used for the migration experiments as shown on Figures 2 (A and B). This fixture features a proximal collar designed to secure the proximal end of the tissue and an attachment point for the base with the stricture at the distal end. The easily removable base with the attached red rubber stricture allowed for easy tissue exchange after each set of experiments. The tissue fixture was secured to a shuttle that rides on an aluminum rail. This shuttle was driven at a rate of 0.777mm/s by rotating a threaded shaft via a high-torque stepper motor and microcontroller (Arduino Uno R3).
Next, a 130mm previously isolated segment of distal fresh porcine colon (24-48h between testing and tissue extraction) was introduced through the stricture and attached to the proximal end of the shuttle box as shown on Figure 2 C. The length of the bowel segment and the stricture was selected in order to allow each stent to extent at least 15mm beyond the proximal and distal end of the stricture once deployed.
The various stents were deployed, so that the stricture was located in the middle of the stent body. The distal end of each stent was secured to a 3D-printed plastic extension of the force gauge that remained static throughout the experiments. In such a way, by slowly and continuously advancing the shuttle with the attached stricture (along the aluminum rail), the various stents were “pulled out” from the stricture and the generated forces have been recorded. For maximal precision, the forces were measured using a high-accuracy, high-resolution force gauge (M5-20, Mark-10 Corporation, Copiague, New York, USA). In addition, software was used to collect real-time force measurements (MESURTM Lite, Mark-10 Corporation, Copiague, New York, USA) at a pre-selected rate of 10 times per second. The comprehensive data was exported to Microsoft Excel (Microsoft Corporation, Redmond, WA) for further analysis.
Testing details
The recorded forces
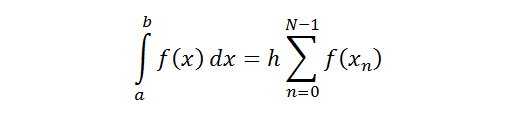
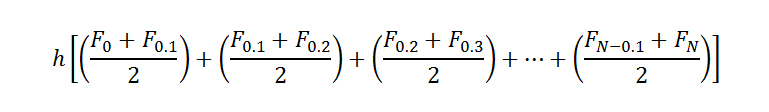
We reported the migration initiation force, which is the maximal force that was reached before the onset of migration. The migration initiation force was determined by finding an inflection point in the average pullout response curve for each stent. Each of these points represented the displacement and the force at which the initial increase of force changed from a largely linear response. It is hypothesized that this change indicates a transition from measurement of static friction force to kinetic friction force, which denotes the point at which the stent begins to migrate.
The inflection point for each stent was estimated by calculating the coefficient of determination, or R2value, for a linear regression of each response. The point at which this coefficient fell below 0.97 was considered the point at which the linear regression no longer fit the response sufficiently and was thus chosen as the inflection point.
Data from all 36 experiments was recorded and exported to Microsoft Excel (Microsoft Corporation, Redmond, WA) for further analysis. Data were summarized as means ± Standard Deviations, if normally distributed, as medians with interquartiles, if abnormally distributed, or as frequencies (%) when appropriate.
RESULTS
Maximum pullout resistance force
Total migration energy
Migration initiation force
Table 4 shows at what distance and at what force the “inflection point” for each stent was reached. Despite Ultraflex having the highest maximal pullout resistance force and also requiring the highest total energy to migrate, the force and the distance to reach the inflection point (2.3N/4.4mm) was lower when compared to the Taewong Medical stents Niti-S D-Type and Niti-S-Comvi stents (4.2N/14.1mm and 3.7N/8.2mm respectfully). Wallflex and the biodegradable Ella stent quickly reached the inflection point and started migrating (0.5N/0.9mm and 0.9N/0.4mm respectfully).
Migration time
Tissue evaluation
DISCUSSION
Stent migration is among the most commonly encountered complications in patients with GI obstruction. The reported migration rates vary significantly depending on the stent design and mechanical characteristics (1-12.5%) [10-15]. In this study, we established a novel porcine model of colonic obstruction that could be used to assess the migration properties and test the performance of novel GI stents. The ability to perform such testing especially early in the development will allow for timely design alterations with subsequent significant cost savings. To our knowledge, this is the first ex vivo model that dynamically tests the anti-migration features of SECS. In addition, we investigated the migration profile of the most commonly used colonic stents in Europe, Asia and the United States.
The colonic stricture models introduced to date involve mostly small animals such as rabbits, rats or sheep fetus used to evaluate bowel obstruction and atresia [16-24]. Those models mostly focus on the pathophysiology involved in colonic obstruction rather than evaluation of the mechanical properties of the various stents. Recently published literature presented in vivo colonic stricture model tested in mongrel dogs over several weeks [25,26]. The authors used non-absorbable synthetic mesh and rubber bands to wrap the descending colon of the animals in order to simulate the disease process. Using a canine model, Park et al., compared the migration rates of two covered colonic stents currently available for use only in Asia and Europe [25]. Such animal model could be a valuable tool in the assessment of the mechanical characteristics of SECS, however the time and costs invested in planning and performance of those experiments is substantial.
The ability to reliably guide the stent design, select the most optimal materials and test the migration properties early in the development is a “must” on the path of designing a novel high-performance gastrointestinal stent. Large animal studies should be reserved for late in the process, once the stent structure has been adequately validated in a reliable ex vivo model.
The significant advantage of an in vitro/ex vivo migration models has been proven in the development of vascular stents [27-30]. Such models were used to develop and pioneer abdominal aortic aneurysm stent grafts more than two decades ago. Secure proximal fixation of aortic stents is the key for long-term success. Failure to provide adequate fixation and the potential for graft migration are associated with catastrophic events. While the world of vascular stent grafts significantly evolved over the years, the development of sophisticated, high performance colonic stents is still in its early phase.
In order to test the validity of our novel colonic stricture model, we used a wide variety of currently available colonic stents used in human clinical practice. We investigated the migration properties of non-covered nitinol stents (WallFlex, Ultraflex, Niti-S D-Type), partially covered (Niti-S ComVi), completely covered (Choostent) and a biodegradable stents (Ella BD).
Generally, there are two main types of forces responsible for the adequate fixation of colonic stent to the wall of the intestine: the outward radial force and the friction of the stent wall towards the wall of the colon. The various stent designs address and alternate one or both of those characteristics. The results of our study illustrate how the stent design differences translate into higher or lower probability of the stent to migrate.
The stent behavior could be characterized based on their recorded force response curves (Figure 3). For instance, the WallFlex (Boston Scientific, USA) and the SX-ELLA (ELLA-CS, Czech Republic) stents are similar in their full-length braided constructions. Their responses during migration were similar as well, with a short increase to a plateau region followed by a marked increase in resistance force as the proximal stent end passed through the stricture. The structural similarity of WallFlex and SX-Ella is also responsible for the elongation capability of those stents resulting in the highest times required to migrate the stents (80.6s and 70.2s respectively).
Alternatively, the Niti-S D-Type and Niti-S ComViCOMVI Colonic Stents (Taewoong Medical, South Korea) have similar unfixed cell structures with weaving constructions and their force response curves are also comparable to one another. The close physical dimensions of those stents also contributed to their similar force responses with consecutive effect on the tissue (more pronounced than the WallFlex and SX-ELLA).
Among all six stents Ultraflex (Boston Scientific) reached the highest maximal pull out resistance force (7.9±0.9 N) and also required the highest total energy to migrate (168.3±30.5 J). Based on the results of our testing, this is the least likely stent to migrate. However, once the migration process began, it took the least amount of time for complete displacement. Those results were expected considering that Ultraflex colonic stent is relatively stiff and does not elongate due to the type of chain link of the nitinol wires. As such, it is non-compressible and exhibits the highest outward radial force among the tested stents. This is also evident based on the stent’s delivery system; In order to load Ultraflex, the manufacturer folds the stent within the large (24 Fr) delivery sheath. In addition, Ultraflex has large gaps between the stiff nitinol wires that allow the colonic mucosa to prolapse and further fixate the stent to the colon wall. While the high outward radial force does stabilize the stent and helps to prevent migration, it is a risk factor for perforation.
On the other side of the spectrum, Wallflex achieved the least maximal pullout resistance (1.7±0.5 N) and required the lowest total energy for complete migration (57.2±10 N). It is an easily compressible stent resulting in significant elongation. Among all six stents, it required the highest time to completely migrate (80.6±1.8s).
In addition to the outward radial force provided by the nitinol wires, the length of the stent, in particular the size and the type of the surface are key in defining the ability of a stent to resist migration. Based on our results, the maximum pullout resistance force was not necessarily related to the total energy required for full migration. The Choostent (M.I. Tech, South Korea) with a tested length of 100mm required much higher total energy to pullout when compared to the Niti-S ComVi (testing length 60mm) despite having quite similar maximum pullout resistance forces.
The flare is an important feature that helps for proper stent positioning during the deployment. In addition, the flange contributes to the anti-migration features of the various SECS. Figure 3 shows how after the pullout resistance force reached an initial plateau, later in the migration process there is a local maximum corresponding to the flare. Those are most pronounced in the curves of the Wallflex and the SX-Ella stents. Further testing is needed in order to better quantify the contribution of the flare as an anti-migration stent feature.
The post experimental assessment of the tissue identified micro/macro perforations caused by the non-covered Niti-S D -Type (Figure 5C) and the partially covered Niti-S -Comvi (Figure 5E). Those two stents reached the highest forces before the onset of the migration (Table 4). The migration initiation force at the previously described inflection point was 3.7N for the Niti-S ComVi and 4.2N for the Niti-S D-Type stent. This force is an important measure since it represents the resistance that the stent has to overcome in order to start migrating. The higher the migration initiation force, the higher the pressure generated in the colonic wall and respectfully the higher the likelihood for injury and perforation. As such, both the Niti-S ComVi and the Niti-S D-Type caused a perforation during the experiments. Based on our observations we do not claim causation but rather a possible correlation that has to be taken into account during the development of new stents. Further and more detailed experiments will be needed in order to better understand the relationship between tissue injury and maximal force reached at the time when the migration begins.
A number of limitations mitigate the power of our conclusions. There are differences in the function and physiologic response when testing in vivo colon and ex vivo colonic model. The lack of motility and muscle tone in addition to the lack of obstruction physiology of an ex vivo model will likely affect the migration of the various stents. However, our intent was not to substitute an in vivo colonic stricture model, but rather to develop a simple, reliable and easy to assemble in vitro tool that could help better understanding migration and efficiently guide the early GI stent development. The ease and accessibility of fresh porcine tissue makes our novel-testing model an attractive alternative that could be used repeatedly to test various stent characteristics and result in significant decrease of developmental time and costs.
In conclusion, our stricture model is the first dynamic ex vivo animal model that closely simulates human colonic stricture and tests stent migration. This model could be a valuable tool to help understanding the process of migration and steer the development of modern stent technology.
AUTHORS CONTRIBUTIONS
All of the listed authors contributed substantially in the design, development and execution of the experiments. Dr. Miroslav Peev designed the study, performed the experiments and wrote the manuscript. Russell Corwin, Andrew Harvey, Matthew Baird were closely involved in the design and development of the testing apparatus and also in the execution of the experiments. Dr. Jeffrey Milsom and Dr. Frederick Cornhill supervised the performance of all experiments and also the completion of the manuscript.
REFERENCES
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics. CA Cancer J Clin 66: 7-30.
- Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, et al. (2016) Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 122: 1312-1337.
- Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99: 2051-2057.
- Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD (1999) Quality of life in stoma patients. Dis Colon Rectum 42: 1569-1574.
- Wong RW, Rappaport WD, Witzke DB, Putnam CW, Hunter GC (1994) Factors influencing the safety of colostomy closure in the elderly. J Surg Res 57: 289-292.
- Dohmoto M (1991) New Method: Endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig.
- Mabardy A, Miller P, Goldstein R, Coury J, Hackford A, et al. (2015) Stenting for obstructing colon cancer: fewer complications and colostomies. JSLS 19 : 2014.00254.
- Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89: 1096-1102.
- van Halsema EE, van Hooft JE, Small AJ, Baron TH, García-Cano J, et al. (2014) Perforation in colorectal stenting: a meta-analysis and a search for risk factors. Gastrointest Endosc 79: 970-982.
- Young CJ, Suen MK, Young J, Solomon MJ (2011) Stenting large bowel obstruction avoids a stoma: consecutive series of 100 patients. Colorectal Dis 13: 1138-1141.
- Di Mitri R, Mocciaro F, Traina M, Montalbano LM, Familiari L, et al. (2014) Self-expandable metal stents for malignant colonic obstruction: data from a retrospective regional SIED-AIGO study. Dig Liver Dis 46: 279-282.
- Meisner S, Gonzalez-Huix F, Vandervoort JG, Repici A, Xinopoulos D, et al. (2012) Self-Expanding Metal Stenting for Palliation of Patients with Malignant Colonic Obstruction: Effectiveness and Efficacy on 255 Patients with 12-Month's Follow-up. Gastroenterol Res Pract 2012: 296347.
- Gianotti L, Tamini N, Nespoli L, Rota M, Bolzonaro E, et al. (2013) A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc 27: 832-842.
- Zhao XD, Cai BB, Cao RS, Shi RH (2013) Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol 19: 5565-5574.
- Yoon JY, Jung YS, Hong SP, Kim TI, Kim WH, et al. (2011) Clinical outcomes and risk factors for technical and clinical failures of self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointestinal endoscopy 74: 858-868.
- Rehn M, Agren MS, Syk I (2011) Collagen levels are normalized after decompression of experimentally obstructed colon. Colorectal Dis 13: e165-169.
- Ozbek E, Ilbey YO, Cekmen M, Sim?ek A, Tekerekoglu M, et al. (2009) Bacterial translocation to kidney in rats with intestinal obstruction and the role of nitric oxide. Arch Ital Urol Androl 81: 56-58.
- Gurleyik G, Ozturk E, Adaleti R, Gunes P, Guran M, et al. (2004) Effects of prostaglandin E1 and E2 analogues on mucosal injury-induced, and on bacterial translocation promoted by, experimental intestinal obstruction. J Invest Surg 17: 127-134.
- Syk I, Mirastschijski U, Jeppsson BW, Agren MS (2003) Experimental colonic obstruction increases collagen degradation by matrix metalloproteinases in the bowel wall. Dis Colon Rectum 46: 1251-1259.
- Kocdor MA, Kocdor H, Gulay Z, Gokce O (2002) The effects of pentoxifylline on bacterial translocation after intestinal obstruction. Shock 18: 148-151.
- Baglaj SM, Czernik J, Kuryszko J, Kuropka P (2001) Natural history of experimental intestinal atresia: morphologic and ultrastructural study. J Pediatr Surg 36: 1428-1434.
- Patricolo M, Noia G, Rossi L, Zangari A, Pomini F, et al. (1998) An experimental animal model of intestinal obstruction to simulate in utero therapy for jejunoileal atresia. Fetal Diagn Ther 13: 298-301.
- Morel P, Alexander-Williams J, Rohner A (1990) Relation between flow-pressure-diameter studies in experimental stenosis of rabbit and human small bowel. Gut 31: 875-878.
- Stone HH, Wilkinson AW (1983) Experimental production of rectal stenosis and atresia in the rabbit. J Pediatr Surg18: 89-90.
- Park HS, Choo IW, Seo S, Hyun D, Lim S, et al. (2015) A novel, ring-connected stent versus conventional GI stents: comparative study of physical properties and migration rates in a canine colon obstruction model. Gastrointest Endosc 81: 1433-1438.
- Hyun D, Park HS, Seo S, Choo IW, Lim S, et al. (2014) A novel animal model of gastrointestinal obstruction for the development of stent. J Surg Res 187: 445-449.
- Resch T, Malina M, Lindblad B, Malina J, Brunkwall J, et al. (2000) The impact of stent design on proximal stent-graft fixation in the abdominal aorta: an experimental study. Eur J Vasc Endovasc Surg 20: 190-195.
- Malina M, Lindblad B, Ivancev K, Lindh M, Malina J, et al. (1998) Endovascular AAA exclusion: will stents with hooks and barbs prevent stent-graft migration? J Endovasc Surg 5: 310-317.
- Johnston CR, Lee K, Flewitt J, Moore R, Dobson GM, et al. (2010) The mechanical properties of endovascular stents: an in vitro assessment. Cardiovasc Eng 10: 128-135.
- Flueckiger F, Sternthal H, Klein GE, Aschauer M, Szolar D, et al. (1994) Strength, elasticity, and plasticity of expandable metal stents: in vitro studies with three types of stress. J Vasc Interv Radiol 5: 745-750.
Citation: Peev MP, Corwin R, Harvey A, Baird M, Cornhill F, et al. (2019) Novel Ex Vivo Stricture Model for Evaluation of Gastrointestinal Stent Migration. Archiv Surg S Educ 1: 002.
Copyright: © 2019 Miroslav P. Peev, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

