
Effect of Exogenic H202 on the Content of Endogenic H202, the Activity of Catalase, Hydrolases, and on the Ultrastructure of Cells in Tobacco Leaves
*Corresponding Author(s):
Reunov AVGb Elyakov Pacific Institute Of Bioorganic Chemistry, Far-East Branch Of The Russian Academy Of Sciences, Vladivostok, Russian Federation
Tel:+7 4232318410,
Fax:+7 4232314050
Email:antreunov@mail.ru
Abstract
This study aimed to examine the influence of exogenic ?2?2 on the content of endogenic ?2?2, the activity of catalase, hydrolases (acid phosphatase, protease, RNase) and on the cell ultrastructure in leaves of Nicotiana tabacum L. cv. Samsun to characterize the cell reactions and to evaluate their significance in the development of oxidative stress. It was shown that the treatment of the leaves with ?2?2 at nontoxic concentration (100 mM) substantially increased their content of ?2?2 and the activity of catalase, as well as activity of the hydrolases, in comparison with untreated leaves. Using ultrastructural analysis, we established that some cells in the treated leaves underwent severe abnormal changes (destruction of tonoplast and plasmalemma, condensation of nuclear chromatin, collapse of cytoplasm, etc.), which may be regarded as a manifestation of Programmed Cell Death (PCD). At the same time, many cells demonstrated signs of organelle activation and did not undergo PCD. It is suggested that cells subjected to H2O2-mediated PCD are a source of signal molecules inducing protective mechanisms against oxidative stress in cells not showing signs of PCD. Such mechanisms supposedly are the stimulation of the protein-synthesizing apparatus (nucleolus dimension and amount of both mitochondria and rough endoplasmic reticulum membranes increased) and the lytic compartment (an increase in the activity of hydrolases and enhanced production of dictyosomes, smooth endoplasmic reticulum elements, and cytoplasmic vacuoles) as well as the proliferation of peroxisomes with crystalloids (known to contain catalase).
Keywords
ABBREVIATIONS
ER: Endoplasmic Reticulum PCD: Programmed Cell Death
INTRODUCTION
Aerobic type cell energetics realizing during respiration and photosynthesis is accompanied by the formation of toxic reactive oxygen species, among which ?2?2 plays the most important role in the life of a cell. Under normal physiological conditions, ?2?2 production in cell compartments (peroxisomes, mitochondria, chloroplasts) is controlled by antioxidant enzymes such as catalase and enzymes of ascorbate-glutathione cycle; as a result, a balance between the generation and destruction of ?2?2 is established. Under conditions of oxidative stress, when cell homeostasis is disturbed, the production of ?2?2 increases in cell compartments. There is evidence that ?2?2 acts as a signaling molecule, participating in signaling cascades and inducing the expression of protective genes [1,2]. Comparatively low concentrations of ?2?2 may lead to the adaptation of plants to stress, while higher ones cause the oxidative damage of proteins and other cellular components (lipids, carbohydrates, DNA) which ends in cell death [2-5].
It was reported that, under oxidative stress, cells have increased proteolytic activity and their oxidatively modified proteins are most effectively split by proteases [6-8]. There are data showing that degradation of the oxidized proteins may be carried out by 20S proteasomes [9] and autophagy [5]. These data may testify to the involvement of the lytic compartment in removing oxidized cellular components and thereby in cell defense against oxidative stress. However, a role for this compartment in the anti-oxidative defense of cells is yet not well understood.
The purpose of this work was to study the influence of the exogenic ?2?2 on the content of endogenic ?2?2, catalase and hydrolase (acid phosphatase, protease, RNase) acivities as well as on the cell ultrastructure, including the lytic compartment, in healthy leaves of N. tabacum L. cv. Samsun.
MATERIALS AND METHODS
Plant source
Studies were performed on 4-week-old plants of N. tabacum L. cv. Samsun grown in pots filled with a mixture of garden soil, peat, and sand (1:1:1) in a glasshouse (28ºC/22ºC day/night temperature, 16 h photoperiod, 16000 lux, 70% relative humidity). The leaves from the middle plant tier were detached, cut in half along the main vein and dusted with carborundum. The left (test) leaf halves were rubbed with ?2?2 solution prepared in distilled water, while the right ones (control) were rubbed with distilled water.
?2?2 was tested in various concentrations: 1.0, 10, 50, 100, 200 and 300 mM. The leaf halves treated with ?2?2 at concentrations of 1.0-100 mM did not show any toxic symptomes and looked similar to the control leaf halves. High concentrations of ?2?2 (200, 300 mM) could produce phytotoxic effects. Therefore, in all experiments, the test leaf halves were treated with ?2?2 at 100 mM concentration. The treated leaves were washed with water and placed in a humid chamber that was then transferred to a constant environment room (24ºC, 16 h photoperiod, 16000 lux, 70% relative humidity).
Evaluation of ?2?2 content in leaves
For assay of ?2?2 levels in the leaves, we prepared leaf extracts as described previously [10]. All procedures were executed at 0-4ºC. Leaves (0.4 g) were homogenized in 1.2 ml 25 mM HCl. The homogenates were filtered through two nylon layers and mixed with 15 mg of charcoal (Sigma-Aldrich, St Louis, USA) for removing of the pigments. The pigment-containing charcoal was separated by centrifugation at 5000 g for 5 min, and the supernatants were clarified by filtration through a 0.22-μm filter unit. The pH of leaf extracts was adjusted to 7.0 with NaOH and these extracts were used to measure the ?2?2 concentration. The reaction mixture contained 0.9 ml 50 mM Hepes buffer, pH 7.6, 0.1 ml orthophenylenediamine (0.04%), and 0.1 ml of leaf extract. The reaction was started by adding 40 μM horseradish peroxidase. The optical density was measured in a spectrophotometer μQuant (Bio-Tek instruments, Inc., Winooski, VT, USA) at 492 nm. The ?2?2 concentration was determined from a calibration curve of ?2?2 (Merck, Darmstadt, Germany) in the range 0.5-80 μM.
Determination of catalase activity in leaves
Catalase activity in the leaves was determined spectrophotometrically by measuring the rate of H2O2disappearance in the enzyme reaction mixture according to Aebi [11]. Leaf tissues (0.1 g) were homogenized on ice in 0.4 ml of the extraction buffer (50 mM phosphate buffer, pH 7.0, and 1% Triton X-100). The homogenates were centrifuged at 12000 g for 20 min at 4ºC. Then, 50 μg of the supernatant was added to 0.9 ml of the buffered substrate (50 mM phosphate buffer, pH 7.0, and 20 mM ?2?2) and the optical absorbance of the mixture was measured at 240 nm.
Determination of hydrolase activities in leaves
The activities of hydrolases (acid phosphatase, proteases, RNases) were determined in crude extracts obtained from 50 leaf disks (4 mm in diameter), as described previously [12] and were expressed in terms of optical density.
Electron microscopy
For electron microscopy, small leaf pieces from the processed leaf halves were fixed first for 3 h in 6,5% glutaraldehyde (Sigma, St Louis, USA) prepared in phosphate buffer (pH 7,4) and then for 2 h in 1% osmium tetroxide. The samples were dehydrated in a graded alcohol and acetone series and embedded in Araldite (Fluka AG, Buchs SG, Switzerland). The leaf sections were cut with a glass knife using a LKB-III (LKB, Bromma, Sweden) ultramicrotome. Three blocks were used for each experimental variant. The sections were mounted on slot grids coated with formvar film stabilized with carbon. Twenty mesophyll cells on sections from each block were examined, i.e., 60 cells were analized for each experimental variant. The sections were stained with uranyl acetate and lead citrate and were examined with a LIBRA-120 electron microscope (Carl Zeiss, Jena, Germany). Morphometric analysis was performed according to Weibel [13].
Statistical analysis of the results
The results of the experiments were analysed statistically using Student’s t-test.
RESULTS
Treatment of tobacco leaves with 100 mM ?2?2 increased the content of endogenic ?2?2 in them. The highest excess of ?2?2 levels (by 59.4 %) in the treated leaves in comparison with untreated leaves was registered on the 3rd day of the experiment (Table 1). Catalase activity in ?2?2-treated leaves increased in comparison with control during 3 days from 39.6 to 71.9 % (Table 2).
Every value is the average of ten replicates (± standard error). The differences are statistically significant relative to controls at P
?2?2-treatment had a marked influence on the leaf activity of hydrolases. One day after treatment, the level of acid phosphatase in the test leaves substantially increased (by 45.7 %) in comparison with untreated leaves and remained enhanced for 3 days (Table 3). Protease activity in test samples increased compared to the control during 3 days, from 53.3 to 80 % (Table 3). RNase activity in the leaves one day after ?2?2-treatment considerably increased (by 68.9%) in comparison with the control and then gradually declined (Table 3).
Activities of hydrolases in leaves are expressed in terms of optical density. Every value is the average of ten replicates (± standard error). The differences are statistically significant relative to controls at *P
Ultrastructural studies showed that basic organelles of mesophyll cells in leaves kept for 3 days in a humid chamber usually retained the typical structure. Nuclear chromatin was presented as loose clumps spread in the light nucleoplasm (Figure 1a). Nucleoli were relatively small (Figure 1a). Mitochondria contained a small number of cristae merged into a moderately dense matrix (Figure 1b). Chloroplasts had a rather developed system of photosynthetic membranes (Figure 1b). Cisternae of rough (Figure 1c) and smooth (Figure 1d) Endoplasmic Reticulum (ER), dictyosomes (Figure 1e), peroxisomes (Figure 1d), and cytoplasmic vacuoles (Figure 1c) were found in a comparatively small number.
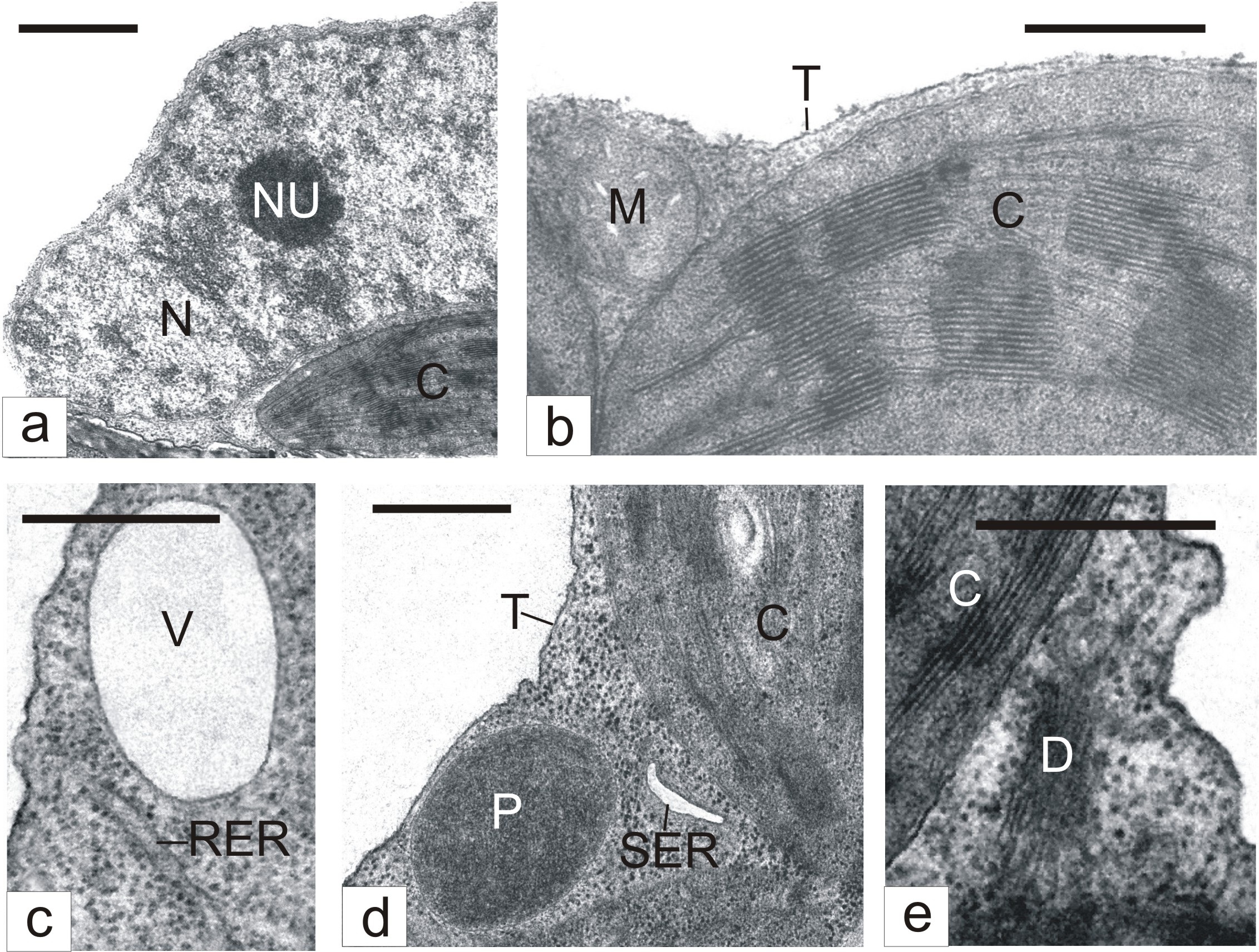 Figure 1: The ultrastructure of mesophyll cells in detached tobacco leaves.
Figure 1: The ultrastructure of mesophyll cells in detached tobacco leaves.
C - Chloroplast; D - Dictyosome; M - Mitochondrion; N - Nucleus; NU - Nucleolus; P - Peroxisome; RER - Rough Endoplasmic Reticulum; SER - Smooth Endoplasmic Reticulum; T – Tonoplast; V – Vacuole; Bar: A - 1 μm; B-E - 500 nm
Some mesophyll cells in ?2?2-treated leaves demonstrated evident features of destruction. The tonoplast in them was not infrequently ruptured, cytoplasmic areas lost ribosomes and became electron-transparent (Figure 2a). Usually, in such areas, we observed lysing membrane structures (Figure 2a). Envelopes of nuclei, mitochondria (Figure 2a) and chloroplasts (Figure 2b) were disturbed. Often, nuclear chromatin became electron dense and condensed (Figure 2c). Chloroplasts demonstrated the sticking of thylakoids and grana transformation into dense amorphous formations (Figure 2b). Mitochondria might lose cristae and became electron-transparent in appearance (Figure 2d). Sometimes, areas of collapsed cytoplasm with destroyed organelles were seen (Figure 2d). The cell periphery frequently had electron-transparent areas with degenerating membrane structures (Figure 2b). In such areas, plasmatic membrane might undergo destruction (Figure 2b).
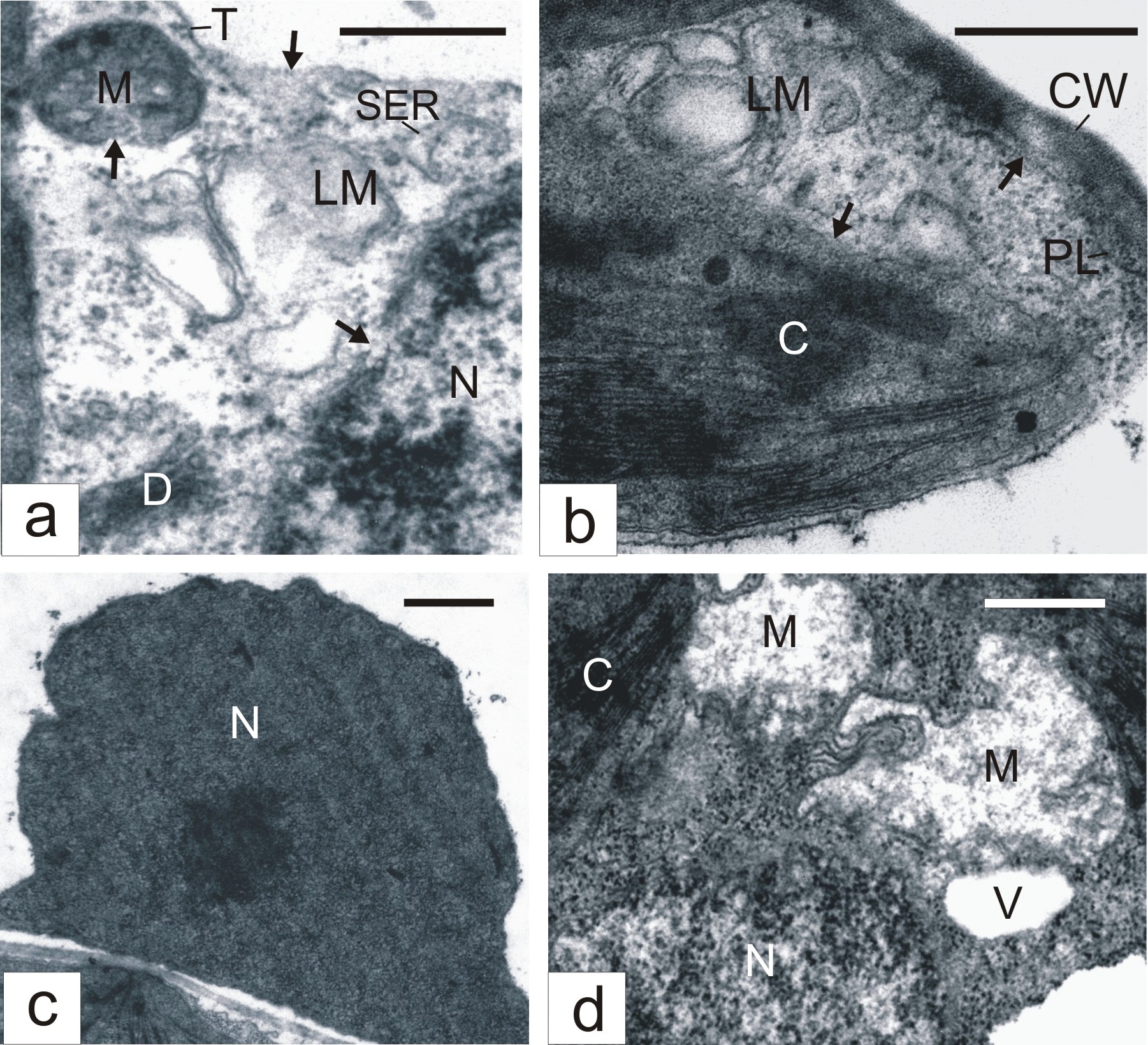
Figure 2: Destructive changes in mesophyll cells of H2O2-treated tobacco leaves. Arrows show tonoplast, mitochondrion, nucleus (A) chloroplast and plasmalemma (B) desintegrations.CW - Cell Wall; LM - Lysing Membranes; PL - Plasmalemma; C - Chloroplast; D - Dictyosome; M - Mitochondrion; N - Nucleus; NU - Nucleolus; P - Peroxisome RER - Rough Endoplasmic Reticulum; SER - Smooth Endoplasmic Reticulum; T - Tonoplast; V - Vacuole; Bar: A, B, D - 500 nm; C - 1 μm
At the same time, many mesophyll cells of ?2?2-treated leaves underwent changes that pointed to organelle activation. Nucleoli (Figure 3a) in such cells were larger than in cells of untreated leaves. The number of mitochondria and the cristae in them (Figure 3b) notably increased. The amount of dictyosomes (Figure 3c), the rough (Figure 3b) and smooth (Figure 3a) ER membranes, and cytoplasmic vacuoles (Figure 3d) were also enhanced. ER smooth elements on sections usually appeared swollen (Figure 3a). A characteristic of the cells was production of microbodies with crystalloids (Figure 3e). Ultrastructure-morphometric analysis showed that the action of exogenic ?2?2 causes an increase in such cells, in comparison with control, of nucleolar volume density, amount and volume density of mitochondria, peroxisomes with crystalloids, and vacuoles, as well as of the surface area of rough and smooth ER membranes and dictyosomes (Table 4).CR - Crystalloid; C - Chloroplast; D - Dictyosome; M – Mitochondrion; N - Nucleus; NU - Nucleolus; P - Peroxisome; RER - Rough Endoplasmic Reticulum; SER - Smooth Endoplasmic Reticulum; T - Tonoplast; V - Vacuole; Bar: A - 1 μm; B-E - 500 nm.
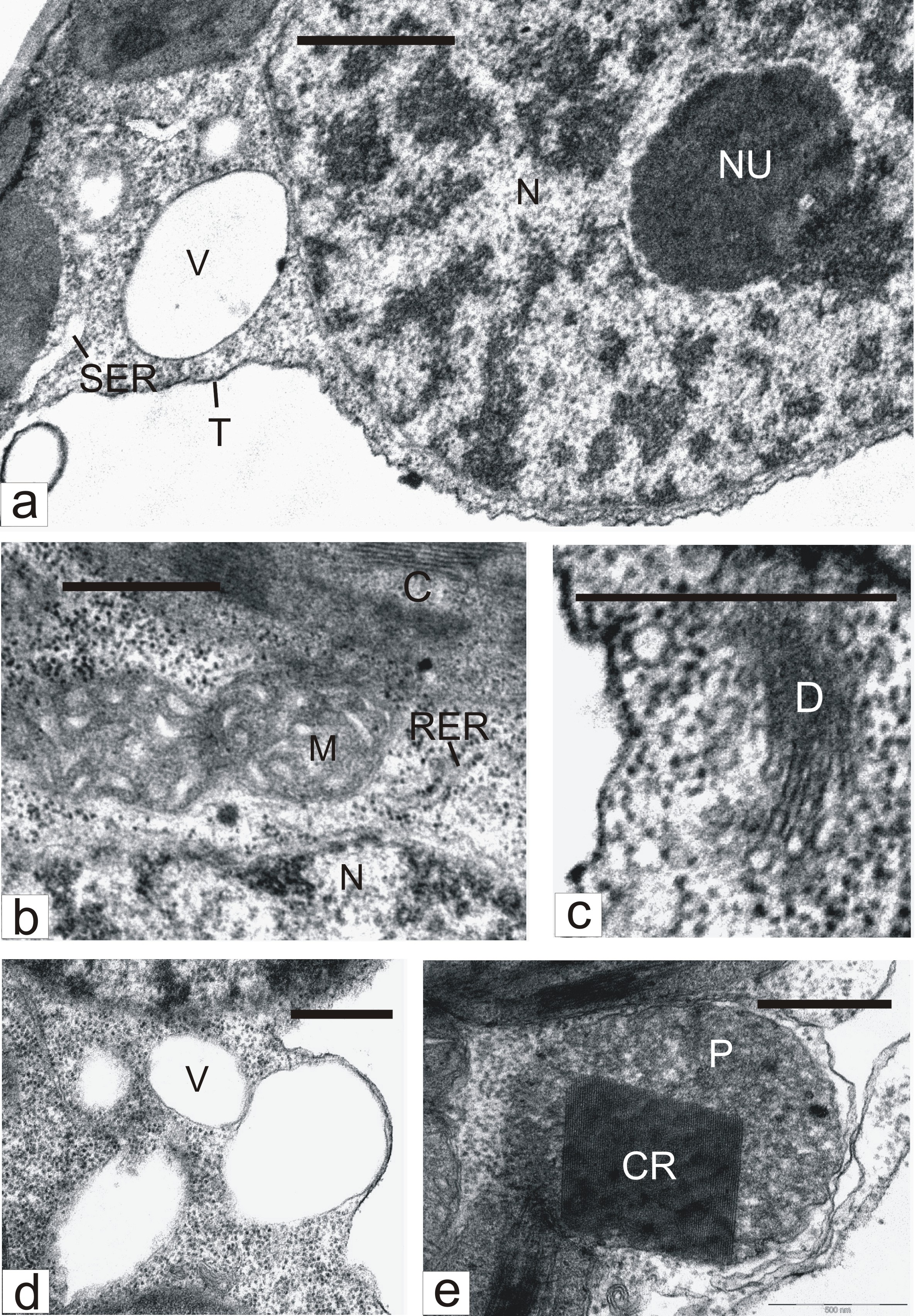 Figure 3: Parts of mesophyll cells in H2O2-treated tobacco leaves. CR - Crystalloid; C - Chloroplast; D - Dictyosome; M – Mitochondrion; N - Nucleus; NU - Nucleolus; P - Peroxisome; RER - Rough Endoplasmic Reticulum; SER - Smooth Endoplasmic Reticulum; T - Tonoplast; V - Vacuole; Bar: A - 1 μm; B-E - 500 nm
Figure 3: Parts of mesophyll cells in H2O2-treated tobacco leaves. CR - Crystalloid; C - Chloroplast; D - Dictyosome; M – Mitochondrion; N - Nucleus; NU - Nucleolus; P - Peroxisome; RER - Rough Endoplasmic Reticulum; SER - Smooth Endoplasmic Reticulum; T - Tonoplast; V - Vacuole; Bar: A - 1 μm; B-E - 500 nm
DISCUSSION
In the present study, we indicated that the treatment of leaves of N. tabacum with H2O2 increases the content of endogenic H2O2 and catalase activity in them compared to those in untreated leaves. These results support the data obtained recently by Yang et al., [14]. Increased catalase activity in parallel with the accumulation of endogenic H2O2 in the leaves, in accordance with existing representations [1], may testify to a signaling role of H2O2 in the induction of catalase.
Our ultrastructure assays showed that the treatment of tobacco leaves with H2O2 causes various modifications in mesophyll cells. Some cells in the treated leaves demonstrated severe abnormal changes (destruction of tonoplast and plasmalemma, condensation of nuclear chromatin, collapse of cytoplasm, etc.), which may be regarded as a manifestation of PCD [15].
At the same time, the ultrastructure-morphometric analysis showed that treatment of the leaves with ?2?2 causes activation of different organelles in many cells, which is manifested in an increase of the corresponding morphometric parameters. In such cells, we found increased nucleoli size, as well as enhanced amount of mitochondria and rough ER membranes in particular. These changes in cell structure point to the stimulation of the protein-synthesizing apparatus, which enhances cell possibilities for defensive responses to unfavourable factors [16]. In addition, H2O2-treatment activated the lytic compartment that is expressed, in particular, in stimulation of the production of dictyosomes, smooth ER elements and cytoplasmic vacuoles, which, as previously reported [17-19], are prominent constituents of this compartment. The observed swelling of cisternae of the smooth ER is probably caused by abnormal changes in the reticular membranes and, as a consequence, via the disturbance of their barrier properties. Such changes in ER can lead to a release of hydrolases from the reticular cavity into the cytoplasm [16]. The hydrolases released from the ER cisternae seem to cause the development of lytic processes, resulting in the degradation of tonoplasts, organelles, and in the formation of electron-light cytoplasmic areas with lysing material in them.
Ultrastructural examinations testifying to the activation of the lytic compartment and lytic processes in cells of H2O2-treated leaves are supported by our biochemical data on the H2O2-mediated increase in the activity of hydrolases (acid phosphatase, RNAase, proteases) in leaves.
The observed variety of structural modifications of cells in H2O2-treated leaves is probably conditioned by different intracellular concentrations of the agent accumulated during leaf treatment.
We suppose that high concentrations of intracellular H2O2 led to oxidative damage of proteins and other cellular components which resulted in cell death. It is known that PCD in plants is caused by the activation of cysteine proteases, which are similar to animal caspases [20,21]. According to some data [22-24] an important role in the induction of plant PCD is played by caspase-like vacuolar processing enzymes. There is evidence that PCD in plants can be mediated not only by caspase-like proteases, but also by other proteolytic enzymes [25,26]. Thus, H2O2-induced protease activation in tobacco leaves revealed here is in line with ultrastructural PCD features observed in some cells.
The cells that have taken comparatively low amount of the agent or have not taken it at all, during leaf rubbing with H2O2, are apparently capable of resisting oxidative stress and, therefore, do not undergo PCD. There is evidence that H2O2 can be involved in initiating cell death-protective responses in the neighboring cells that surround the sites of the hypersensitive response to pathogens and triggers systemic acquired resistance in distant tissues [27,28]. It was reported [29] that H2O2 may function as a diffusible signal for the induction in adjacent cells of genes encoding cellular protectants. We think that cells subjected to H2O2-mediated PCD are a source of signal molecules inducing protective mechanisms against oxidative stress in cells not showing sings of PCD. It seems that one of such mechanisms is the activation of the lytic compartment and lytic processes. Such representation is in accordance with data [5] suggesting that the cellular defense against oxidative stress requires the removal of oxidatively modified cell proteins by degradative process. Another possible cell death-protective mechanism is the proliferation of peroxisomes with crystalloid inclusions, which correlates with the enhanced catalase activity in H2O2-treated leaves. It is known [30] that such inclusions are composed of metabolically active catalase, which is able to destroy toxic H2O2. Thereby, the production of crystalloid-containing peroxisomes in cells probably means that such peroxisomes may limit the redundant accumulation of intracellular H2O2, preventing membrane lipid peroxidation and PCD development.
CONCLUSION
The treatment of the tobacco leaves with H2O2 at nontoxic concentration (100 mM) substantially increases their content of H2O2 and the activity of catalase, as well as of the hydrolases (acid phosphatase, protease, RNase) in comparison with untreated leaves. Ultrastructural analysis showed that such treatment causes various modifications in cells. Some cells in the H2O2-treated leaves undergo severe abnormal changes (destruction of tonoplast and plasmalemma, condensation of nuclear chromatin, collapse of cytoplasm, etc.), which may be regarded as a manifestation of PCD. At the same time, many cells demonstrate signs of organelle activation and do not undergo PCD. The observed differences in H2O2-induced structural modifications of cells seem to be conditioned by different intracellular concentrations of the agent accumulated during leaf treatment. High concentrations of H2O2 apparently cause oxidative damage of the cells which resultes in cell death. The cells that have taken comparatively low amount of the agent or have not taken it at all, are probably able of resisting oxidative stress and do not undergo PCD. We suggest that cells subjected to H2O2-mediated PCD are a source of signal molecules inducing protective mechanisms against oxidative stress in cells not showing signs of PCD. It may be conclused that such mechanisms are the stimulation of the protein-synthesing apparatus (nucleolus dimension and amount of both mitochondria and rough ER membranes increased) and the lytic compartment (an increase in the activity of hydrolases and enhanced production of dictyosomes, smooth ER elements, and cytoplasmic vacuoles) as well as the proliferation of peroxisomes with crystalloids (known to contain catalase).
REFERENCES
- Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168: 17-20.
- Nyathi Y, Baker A (2006) Plant peroxisomes as a source of signalling molecules. Biochim Biophys Acta 1763: 1478-1495.
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405-410.
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373-399.
- Xiong Y, Contento AL, Nguyen PQ, Bassham DC (2007) Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 143: 291-299.
- Grune T, Reinheckel T, Davies KJ (1997) Degradation of oxidized proteins in mammalian cells. FASEB J 11: 526-534.
- Palma JM, Sandalio LM, Corpas FJ, Romero-Puertas MC, McCarthy I, et al. (2002) Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiology and Biochemistry 40: 521-530.
- Romero-Puertas MC, Palma JM, Gómez M, del Rio LA, Sandalio LM (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant, Cell & Environment 25: 677-686.
- Basset G, Raymond P, Malek L, Brouquisse R (2002) Changes in the expression and the enzymic properties of the 20S proteasome in sugar-starved maize roots. evidence for an in vivo oxidation of the proteasome. Plant Physiol 128: 1149-1162.
- Vanacker H, Sandalio L, Jiménez A, Palma JM, Corpas FJ, et al. (2006) Roles for redox regulation in leaf senescence of pea plants grown on different sources of nitrogen nutrition. J Exp Bot 57: 1735-1745.
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121-126.
- Lapshina LA, Nagorskaya VP, Reunov AV, Barabanova AO, Shevchenko NM, et al. (2011) Correlation between influence of polysaccharides on hydrolase activity and their antiviral effect in tobacco leaves. Biochemistry (Mosc) 76: 462-466.
- Weibel ER (1969) Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol 26: 235-302.
- Yang YL, Zhang YY, Lu J, Zhang H, Liu Y, et al. (2012) Exogenous H2O2 increased catalase and peroxidase activities and proline content in Nitraria tangutorum callus. Biol Plant 44: 330-336.
- Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5: 305-315.
- Lapshina LA, Reunov AV, Nagorskaia VP, Zviagintseva TN, Shevchenko NM (2009) [Effect of fucoidan on the ultrastructure of mesophyll cells of Datura stramonium L. and accumulation of potato virus X in them]. Tsitologiia 51: 484-489.
- Matile P (1975) The lytic compartment of plant cells. Springer-Verlag Wien, Austria
- Marty F (1999) Plant vacuoles. Plant Cell 11: 587-600.
- del Pozo O, Lam E (1998) Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr Biol 8: 1129-1132.
- Chichkova NV, Kim SH, Titova ES, Kalkum M, Morozov VS, et al. (2004) A plant caspase-like protease activated during the hypersensitive response. Plant Cell 16: 157-171.
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, et al. (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305: 855-858.
- Rojo E, Martín R, Carter C, Zouhar J, Pan S, et al. (2004) VPEgamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr Biol 14: 1897-1906.
- Misas-Villamil JC, Toenges G, Kolodziejek I, Sadaghiani AM, Kaschani F, et al. (2013) Activity profiling of vacuolar processing enzymes reveals a role for VPE during oomycete infection. Plant J 73: 689-700.
- Beers EP, Woffenden BJ, Zhao C (2000) Plant proteolytic enzymes: possible roles during programmed cell death. Plant Mol Biol 44: 399-415.
- He X, Kermode AR (2003) Proteases associated with programmed cell death of megagametophyte cells after germination of white spruce (Picea glauca) seeds. Plant Mol Biol 52: 729-744.
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, et al. (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773-784.
- Torres MA, Jones JD, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130-1134.
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583-593.
- Tenberge KB, Ruholl C, Heinze M, Eising R (1997) Purification and immuno-electron microscopical characterization of crystalline inclusions from plant peroxisomes. Protoplasma 196: 142-154.
Citation: Lapshina LA, Reunov AV, Nagorskaya VP (2015) Effect of Exogenic ?2?2 on the Content of Endogenic ?2?2, the Activity of Catalase, Hydrolases, and on the Ultrastructure of Cells in Tobacco Leaves. J Cell Biol Cell Metab 2: 007.
Copyright: © 2015 Lapshina LA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

