
The Emerging Role of miRNAs in Tumor Acidosis
*Corresponding Author(s):
Ajay KumarDepartment Of Zoology, Banaras Hindu University, Varanasi, India
Tel:+91 9455470317,
Email:ajaysastri37@gmail.com; ajayzoo@bhu.ac.in
Abstract
Tumor acidosis, an ‘emerging hallmark’ of cancer, promotes unhindered progression of cancer by inducing invasion and metastasis, immunosuppression, apoptosis of surrounding normal cells, and chemo and radio resistivity. pH regulator molecules such as Monocarboxylate Transporters (MCTs), V-type H+ATPases (V-ATPase), Carbonic Anhydrase (CA), Na+/H+ Exchanger (NHE) and Cl-/HCO3- anion exchanger 2 play a critical role in maintaining the pH homeostasis. pH homeostasis is essentially required for various cellular processes including cell proliferation, apoptosis and migration. Interestingly, the expression and/or activity of pH regulators are usually found up-regulated in several cancerous cells to counteract the deleterious effect of intracellular acidification caused by huge production of acidic metabolites. microRNAs (miRNAs) are small non-coding RNAs which tightly control various cellular processes such as proliferation, differentiation and apoptosis via directly or indirectly regulating the expression of various biological pathways. Therefore, a role of altered expression of various miRNAs involved in biological processes is reported in cancer progression. Recent reports indicate the potent role of miRNAs in the regulation of tumor acidosis. miRNAs such as miR-34a, miR-24, miR-224 are reported to play a crucial role in cancer progression via regulating the expression of their target pH regulatory molecules CAIX, Cl-/HCO3- anion exchanger 1 and SLC4A4 (a Na+-coupled HCO−3 transporter), respectively. Further, miR-34a, miR-34c, miR-34b/c, miR-369-3p, miR-374a, miR-4524a/b and miR-449a may play a key role in the regulation of tumor acidosis via targeting a crucial glycolytic emzyme, LDHA. This review discusses the contribution of miRNAs in the acidic tumor microenvironment.
INTRODUCTION
Cancerous cells usually show dependency on glycolysis and/or glutaminolysis due to their high metabolic demand, which is required for their survival and rapid proliferation. The metabolic switch provides rapid energy and precursor molecules for the macromolecular synthesis to the highly proliferative cancerous cells. This reprogrammed tumor cell metabolism produces H+ ions and acidic metabolites, which are transported outside the cell with the help of pH regulators, namely, MCTs, V-ATPase, CA, NHE and Cl-/HCO3- anion exchanger 2 [1,2]. The export of H+ ions and metabolic acids is essentially required for the survival of tumor cells, otherwise their accumulation will cause intracellular acidification which may lead to the death of tumor cells [1]. Therefore, the intracellular pH (pHi) of cancer cells is usually observed >7.4 which is higher than the pHi of normal cells (pHi of ~7.2) whereas a lower extracellular pH (pHe of ~ 6.7-7.1) as compare to normal cells (pHe of ~7.4). This reverse pH gradient supports cancer progression through several ways such as increased pHi of cancer cells inhibits tumor cell apoptosis while acidic pHe promotes immunosuppression, apoptosis of normal cells and invasion and metastasis of tumor cells [2]. Acidic tumor microenvironment is usually observed in most of cancers, therefore, ‘tumor acidosis’ has been emerging as a hallmark feature of cancer [2-5]. Further, pH regulators along with metabolic enzymes such as lactate dehydrogenase play a critical role in the development of tumor acidosis.
MicroRNAs (miRNAs) are small non-coding RNAs, 18 to 25 nucleotides in length, which play a crucial role in the regulation of gene expression at the post-transcriptional level via primarily binding with 3’ untranslated region of mRNA transcribed from target genes either through mRNA degradation or inhibition of translation. miRNAs are reported to control various biological processes including development, differentiation, proliferation and apoptosis [6-8]. Further, the role of altered expression of miRNAs is also reported in progression of various types of cancers including, prostate, thyroid, lung, gastric, pancreatic, colorectal and breast [9-15]. The alteration in the expression of miRNA in cancerous cells occurs either due to mutations, chromosomal alterations and promoter methylation or transcription factor activation [16]. The altered expression of miRNAs promotes cancer progression via modulating the expression of several key regulatory molecules which control various processes such as proliferation, survival, apoptosis, angiogenesis, and invasion and metastasis [17,18]. The miRNA dysregulation in cancer was first observed through mapping of chromosome 13 in Chronic Lymphocytic Leukemia (CLL) in 2002 [19]. In CLL, 13q14 region was found to be frequently deleted in more than 50% of the cases which was further identified as a site for two miRNAs, miR-15 and miRNA-16 [19]. Further, these two miRNAs were identified as tumor suppressor genes due to their negative regulatory action on the expression of an antiapoptotic target, BCL2 [20]. A miRNA can be oncogenic in a tumor while tumor suppressor in other tumors. For example, miR-29 has oncogenic function in breast cancer while tumor suppressive functions in lung cancer [21,22]. Emerging evidence indicate the role of miRNAs in the development and progression of cancer via modulating the expression pattern of cell survival regulatory molecules such as p53, Bcl2, Myc, cyclins, CDKs and MDM2 [23,24]. Further, miRNAs such as miR-21, miR-10b and miR-125 are implicated in providing the metastatic and invasive properties to cancerous cells [24]. Recently, the role of miRNAs has also been identified in the regulation of tumor acidosis. This review is an attempt to understand the role of miRNAs in the regulation of acidic tumor microenvironment.
MICRORNA BIOGENESIS AND MECHANISM OF ACTION
The biogenesis of miRNA is basically two steps process which initially occurs in nucleus followed by cytoplasm. During this process, firstly, the miRNA gene is transcribed by RNA polymerase II to primary miRNA transcript which is further cleaved by a protein complex of RNase III endonuclease Drosha and DGCR8 into an ?60 - 70 bp hairpin intermediate form called as precursor miRNA (pre-miRNA) in the nucleus. This precursor miRNA is transported by exportin 5 from nucleus to cytoplasm where it is further processed by TRBP-associated Dicer to mature double stranded miRNA duplex, ?22 nucleotides in length. Mature miRNA duplex is double stranded structure of passenger and guide strand of miRNA. Argonaute (AGO2) protein, an important component of RISC unwinds the miRNA duplex and promotes incorporation of guide strand into the RISC; this newly formed complex is known as miRNA-associated RNA-induced silencing complex (miRISC). Further, miRNA-associate RISC targets mRNAs for silencing either via RNA degradation or translational inhibition depending on the complementarity between the miRNA and the targeted mRNA (Figure 1) [25].
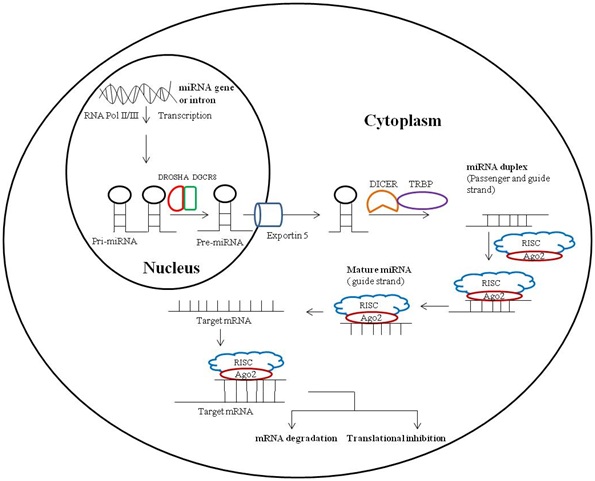
MICRORNAS AND CELLULAR PH REGULATORY MOLECULES
A number of evidence indicates the involvement of several miRNAs in the regulation of pH homeostasis via controlling the expression of various pH regulatory and acid metabolite generating molecules during pathological and non-pathological conditions (Table 1). Further, miRNAs regulate the expression of pH regulatory molecules directly or indirectly through modulating the expression of their transcription factors. There are only few reports which indicate the role of miRNAs in the regulation of pH regulators and acid metabolite generating molecules. However, very little information is known about the contribution of known pH regulatory miRNAs in cancer progression and tumor acidosis (Figure 2).
|
ph regulators/Enzymes |
Physiological role |
miRNA |
References |
|
Monocarboxylate Transporter (MCT1)
|
Transport of L-lactate, pyruvate and ketone bodies, maintenance of pH homeostasis |
miR-124 miR-29a miR-29b |
[26] |
|
Lactate dehydrogenase A |
Reduction of Pyruvate into L-Lactate and Regeneration of NAD+ from NADH |
miR-34a, miR-34c, miR-34b/c, miR-369-3p miR-374a miR-4524a/b, miR-449a |
[27-29] |
|
Carbonic anhydrase IX |
Reversible hydration of carbondioxide to bicarbonate and a proton, maintenance of pH homeostasis, water and ionic equilibrium |
miR-34a, miR-210 |
[30-32] |
|
V-ATPase |
Proton transportation, receptor-mediated endocytosis, intracellular membrane traffic, pro-hormone processing, bone resorption, renal pH homeostasis and sperm maturation |
miR-637 miR-1 |
[30,33] |
|
Bicarbonate transporters (Such as AE1, AE2, SLC26A3 and SLC4A4) |
Transportation of HCO3− across the plasma membranes, removal of waste CO2, regulation of pH homeostasis, fluid movement and acid/base secretion |
miR-24, miR-506, miR-494, miR-224 |
[34-37] |
|
Na+/H+ exchanger (NHE1) |
Regulation of pH homeostasis, cell volume, cytoskeletal organization and cell growth and proliferation |
miR-185 |
[38] |
Table 1: miRNA-mediated regulation of pH regulatory and acidic metabolite producing molecules.
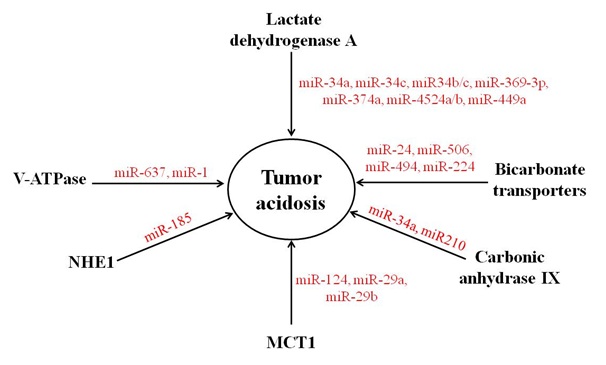
Figure 2: Regulation of pH regulators and acidic metabolite generating molecules by miRNAs. The expression of MCT1, LDHA, CAIX, V-ATPase, bicarbonate transporters and NHE1 is directly or indirectly regulated by several miRNAs.
MONOCARBOXYLATE TRANSPORTER AND THEIR REGULATION BY MIRNAS
Monocarboxylate transporters belong to the solute carrier 16 gene family, consisting of 14 members. Out of fourteen, four members of MCTs, MCT1, MCT2, MCT3 and MCT4 are mainly involved in the proton-linked transport of monocarboxylate metabolites such as L-lactate, pyruvate and the ketone bodies across the plasma membrane. They play a key role in the regulation of pH homeostasis in cancerous cells by exporting excess lactate, produced as an end product of glycolysis, outside the cell. Therefore, MCT1 - 4 play a crucial role in the development and progression of cancer by two ways, increasing intracellular pH (pHi) of cancerous cells and creating tumor supporting acidic microenvironment by lowering the extracellular pH (pHe) [2,3,39,40]. The expression of MCT1, MCT2 and MCT4 are found elevated in several cancers like colon, breast and lung [41]. Recently, several studies have indicated that miRNAs tightly control the expression of monocarboxylate transporters. In cancer, a down-regulated expression of MCT regulatory miRNAs has been observed which lead to a higher expression of MCT and thus promote tumor acidosis. In an investigation, Pullen et al., identified MCT1 as a potential target of miR-124, miR-29a and miR-29b in pancreatic beta cell [26]. These miRNAs inhibit the expression of MCT1 by binding to 3’ UTR of human and mouse MCT1. The report suggested MCT1 as a direct target of these miRNAs because the inhibitory effects of these miRNAs is abrogated via mutation of the cognate miR-29 or miR-124 binding sites. Out of three, miR-124, and miR-29b function as tumor suppressor due to their ability to target cancer promoting genes [40-52]. However, miR-29a differentially regulates cancer progression depending on their tissue origin [43,53-57]. However, there is no report which suggests the role of miR-124, miR-29a and miR-29b in tumor acidosis. Therefore, studies are needed to establish their role in tumor acidosis.
ROLE OF MIRNAS IN LACTATE DEHYDROGENASE A REGULATION
Cancer cells show many types of metabolic adaptations which help them in proliferation, survival and invasion and metastasis. One of the metabolic adaptations is glycolytic switch; cancerous cells usually switch their metabolism from oxidative phosphorylation to glycolysis as glycolysis is the bioenergetic source along with provider of intermediate molecules of macromolecular synthesis for rapidly dividing cells [58,59]. Thus, they generate a large amount of lactate, as an end product of glycolysis by the help of Lactate Dehydrogenase (LDH) in a reversible manner. Further, lactate is passively exported out of the cells with help of MCTs as its intracellular accumulation can lead to intracellular acidification which is fatal for their survival [60,61]. The extruded lactate promotes cancer progression via causing acidosis, promoting angiogenesis and immunosuppression, and supplying metabolic fuel to oxidative tumor cells [62,63]. LDH is a tetrameric enzyme composed of two major subunits, A and/or B, which are encoded by Ldh-A and Ldh-B, respectively. LDHA has a higher affinity for pyruvate than LDHB and thus more efficiently catalyzes the conversion of pyruvate into lactate whereas LDHB catalyzes the conversion of lactate to pyruvate. An up-regulated expression of LDHA is frequently observed in various cancers [64,65]. Several recent studies have suggested the potential role of miRNAs in the regulation of tumorigenesis through modulation of metabolism including carbohydrate, lipid and amino acid metabolism [66,67]. Several miRNAs such as miR-375, miR-143, miR-14 and miR-29b play a crucial role in the altered cancer cell metabolism through modulating the expression of various genes which directly or indirectly modulate the expression of enzymes of metabolic pathways [66]. But, there are only few studies which report the regulation of LDH via miRNAs. These studies suggest that miR-34a, miR-34c, miR-34b/c, miR-369-3p, miR-374a, miR-4524a/b and miR-449a regulate glycolysis in cancer cells by targeting LDHA [27-29]. Wang et al., cloned the 3′ UTR of LDHA gene into a dual-luciferase UTR vector and observed that miR-34a and miR-34c along with other miRNAs such as miR-369-3p, miR-374a and miR-452 a/b binds to the 3′UTR of LDHA and inhibited LDHA [27]. They have also confirmed that LDHA is the main target of miR-34a and miR-34c by comparing the expression level of 22 target genes of miR-34 in colorectal and pancreatic cancer cell lines. Further, miR-34a is also involved in the regulation of glycolysis and tumor growth metabolism in breast cancer [29]. Moreover, Xiao et al., has also identified LDHA as a key target for miR-34a by performing luciferase reporter assay [29].
REGULATION OF CARBONIC ANHYDRASE BY MIRNAS
Carbonic anhydrase (CA) is a zinc containing metaloenzyme which catalyzes the reversible hydration of carbondioxide to bicarbonate and a proton (CO2 + H2O H+ + HCO3-). It plays an important role in the maintenance of pH homeostasis, water and ionic equilibrium. There are 14 isoforms of human CA. They show variations in their activity, tissue distribution, and cellular and subcellular localization [68]. Further, the isoforms of CA are mainly classified into four categories, namely cytosolic (CAs I, II, III, VII); membrane associated (CAs IV, IX, XII, XIV); mitochondrial (CAV); and secreted (CAVI) isoenzymes [68]. Reports indicate that membrane associated CA isoforms, namely CAIX and CAXII are involved in the tumor progression [33,68].
Recent evidence suggests that miRNAs directly or indirectly regulate the expression of CAIX [30-32]. It has been reported that CAIX is a direct target of miR-34a and thus 3′UTR polymorphism of CAIX play a critical role in the regulation of CAIX expression and cancer progression as it affects the targeting efficiency of miR-34a [30,31]. Further, the expression of CAIX is also indirectly regulated via hypoxia-induced miR-210 as CAIX is a HIF-1α target gene [32,69]. But there is no evidence which suggests the miRNA-mediated regulation of CAXII.
MIRNA-MEDIATED REGULATION OF V-ATPASE
V-ATPase belongs to ATP-dependent proton pump family and basically present on the membrane of cellular organelles like endosome, lysosome and a variety of vesicles. Moreover, V-ATPase is also localized on the plasma membrane of some cells such as osteoclasts [70]. V-ATPase transports proton either into intracellular compartments or across the plasma membrane depending on their localization and thus maintains a relatively neutral intracellular pH, an acidic luminal pH and an acidic extracellular pH [71]. It is a large, multi-subunit complex made up of integral VO and peripheral V1 domains. VO is composed of five subunits (a,c,c”,d,e) and is responsible for proton translocation whereas V1 is composed of eight subunit (A- H) and contains catalytic core which hydrolyses ATP [72]. Intracellular V-ATPases regulates various cellular processes including receptor-mediated endocytosis, intracellular membrane traffic, pro-hormone processing, protein degradation and the coupled uptake of small molecules, such as neurotransmitters. However, plasma membrane-associated V-ATPases play a key role in physiological processes such as bone resorption, renal pH homeostasis and sperm maturation [70]. Further, plasma membrane-associated V-ATPases are also involved in the development of various diseases including renal tubular acidosis, and osteoporosis promoting function, and tumor metastasis and invasion [70]. Moreover, an up-regulated expression of plasma membrane-associated V-ATPase has been observed in various invasive cancers like breast, lung, liver, prostate and pancreatic cancers [72-74]. There is only one study which suggests that miR-1 and miR-637 may be involved in the regulation of V-ATPase expression based on the sequence-specific prediction [75,76]. Therefore, wet-lab experiments are needed to validate this prediction.
REGULATION OF BICARBONATE TRANSPORTERS AND NA+/H+ EXCHANGER BY MIRNAS
Apart from MCT, CAIX, LDH and V-ATPase regulation by miRNAs, the expression of other pH regulators such as bicarbonate transporters (namely, Cl−/HCO−3 exchangers and Na+-coupled HCO−3 transporters) and Na+/H+ exchanger is also tightly controlled by miRNAs. Bicarbonate transporters facilitate the movement of HCO3− across the plasma membranes. They help the removal of waste CO2 and play an important role in the regulation of pH homeostasis, fluid movement and acid/base secretion [77]. An investigation by Wu et al., indicates the regulation of Cl-/HCO3- anion exchanger 1 (AE-1) expression by miR-24 in the gastric carcinogenesis [34]. Further, the expression of Cl-/HCO3- Anion Exchanger 2 (AE2) is found to be regulated by miR-506 and thus alteration in the expression of miR-506 is associated with primary biliary cirrhosis [35]. MiR-506 inhibits the expression of Cl-/HCO3- Anion Exchanger 2 (AE2) by binding to 3′UTR region of AE2 mRNA and inhibiting its expression at translational level [35]. Further, the expression of another Cl-/HCO3- exchanger, SLC26A3, is found to be dependent on the level of miR-494 in intestinal epithelial cells [36]. Moreover, a role of down regulated expression of miR-224 is observed in the development of methotrexate-resistant colorectal cancer via relaxing its inhibitory action on SLC4A4 (NBCe1), a Na+-coupled HCO-3 transporter [37]. Therefore, miRNAs also play a vital role in the regulation of bicarbonate transporters. Further, several reports indicate an altered expression of bicarbonate transporters such as AE1, AE2, SLC26A3 and SLC4A4 in various cancers including hepatocellular carcinoma, colorectal, breast and gastric cancers [78-83]. Thus, miRNAs targeting the bicarbonate transporters may be future potential therapeutic targets for various cancers.
Na+/H+ exchanger controls numerous physiological processes such as pH homeostasis, cell volume, cytoskeletal organization and cell growth and proliferation [84,85]. A recent study by Kim et al., suggests the miRNA-mediated regulation of NHE-1 via miR-185 [38]. In this investigation, they showed that miR-185 protects endoplasmic reticulum stress induced apoptosis in cardiomyocytes by directly targeting the 3′-untranslated region of NHE-1. Moreover, the role of NHE-1 is reported in the progression of various cancers via cellular transformation, and invasion and metastasis [86,87].
CONCLUSION
miRNAs play a crucial role in the regulation of pH homeostasis through controlling the expression of pH regulators. In cancer, pH regulators and metabolic acid generating molecules (such as lactate dehydrogenase) are the main culprit for the creation of tumor acidic microenvironment. Tumor acidosis favors cancer progression through modulating several crucial parameters such as cell survival, apoptosis, immunosuppression, invasion and metastasis. Thus, tumor acidosis is one of the key regulatory players of tumor progression. Therefore, miRNAs targeting pH regulatory and metabolic acid generating molecules could be exploited as diagnostic tool or future therapeutic targets for various cancers. But, more studies are still required to explore the role of miRNAs targeting pH regulators in tumor acidosis, whose role is known in normal cells.
ACKNOWLEDGEMENT
This work was supported by start-up grant and Early Career Award from the University Grants Commission and Department of Science & Technology, New Delhi, respectively. This work was also supported by University Grants Commission, Government of India, for providing Centre of Advance Studies to Department of Zoology, Banaras Hindu University, India. Pradip Jaiswara expresses gratitude to UGC, New Delhi, for his fellowship support (1002/(SC) ( CSIR UGC NET DEC.2016).
REFERENCES
- Corbet C, Feron O (2017) Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochim Biophys Acta 1868: 7-15.
- Webb BA, Chimenti M, Jacobson MP, Barber DL (2011). Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11: 671-677.
- Corbet C, Feron O (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17: 577-593.
- Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, et al. (2013) Acidic extracellular microenvironment and cancer. Cancer Cell Int 13: 89.
- Chiche J, Brahimi-Horn MC, Pouysségur J (2010) Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 14: 771-794.
- Wienholds E, Plasterk RH (2005) MicroRNA function in animal development. FEBS Lett 579: 5911-5922.
- Hwang HW, Mendell JT (2006) MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 94: 776-780.
- Ivey KN, Srivastava D (2010) MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 7: 36-41.
- Lo U-G, Yang D, Hsieh J-T (2013) The role of microRNAs in prostate cancer progression. Transl Androl Urol 2: 228-241.
- Forte S, La Rosa C, Pecce V, Rosignolo F, Memeo L (2015) The role of microRNAs in thyroid carcinomas. Anticancer Res 35: 2037-2047.
- Castro D, Moreira M, Gouveia AM, Pozza DH, De Mello RA (2017) MicroRNAs in lung cancer. Oncotarget 8: 81679-81685.
- Zhang Z, Li Z, Li Y, Zang A (2014) MicroRNA and signaling pathways in gastric cancer. Cancer Gene Ther 21: 305-316.
- Yonemori K, Kurahara H, Maemura K, Natsugoe S (2017) MicroRNA in pancreatic cancer. J Hum Genet 62: 33-40.
- Mohammadi A, Mansoori B, Baradaran B (2016) The role of microRNAs in colorectal cancer. Biomed Pharmacother 84: 705-713.
- Takahashi R-U, Miyazaki H, Ochiya T (2015) The Roles of MicroRNAs in Breast Cancer. Cancers (Basel) 7: 598-616.
- Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20: 460-469.
- Seven M, Karatas OF, Duz MB, Ozen M (2014) The role of miRNAs in cancer: from pathogenesis to therapeutic implications. Future Oncol 10: 1027-1048.
- Peng Y, Croce CM (2016) The role of MicroRNAs in human cancer. Signal Transduct Target Ther 1: 15004.
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, et al. (2002) Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 99: 15524-15529.
- Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, et al. (2008) MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA 105: 5166-5171.
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, et al. (2007) MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 104: 15805-15810.
- Gebeshuber CA, Zatloukal K, Martinez J (2009) miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep 10: 400-405.
- Jansson MD, Lund AH (2012) MicroRNA and cancer. Mol Oncol 6: 590-610.
- Lee YS, Dutta A (2009) MicroRNAs in cancer. Annu Rev Pathol 4: 199-227.
- Macfarlane L-A, Murphy PR (2010) MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics 11: 537-561.
- Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA (2011) miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of Monocarboxylate transporter 1 (Mct1). Mol Cell Biol 31: 3182-3194.
- Wang J, Wang H, Liu A, Fang C, Hao J, et al. (2015) Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget 6: 19456-19468.
- Li H, Li X, Ge X, Jia L, Zhang Z, et al. (2016) MiR-34b-3 and miR-449a inhibit malignant progression of nasopharyngeal carcinoma by targeting lactate dehydrogenase A. Oncotarget 7: 54838-54851.
- Xiao X, Huang X, Ye F, Chen B, Song C, et al. (2016) The miR-34a-LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci Rep 6: 21735.
- Hua K-T, Liu Y-F, Hsu C-L, Cheng T-Y, Yang C-Y, et al. (2017) 3?UTR polymorphisms of carbonic anhydrase IX determine the miR-34a targeting efficiency and prognosis of hepatocellular carcinoma. Sci Rep 7: 4466.
- Yang S-F, Liu Y-F, Cheng C-W, Yang W-E, Lin W-L, et al. (2017) Impact of microRNA-34a and polymorphism of its target gene CA9 on susceptibility to uterine cervical cancer. Oncotarget 8: 77860-77871.
- Quero L, Dubois L, Lieuwes NG, Hennequin C, Lambin P (2011) miR-210 as a marker of chronic hypoxia, but not a therapeutic target in prostate cancer. Radiother Oncol 101: 203-208.
- Nógrádi A (1998) The role of carbonic anhydrases in tumors. Am J Pathol 153: 1-4.
- Wu J, Zhang YC, Suo WH, Liu XB, Shen WW, et al. (2010) Induction of anion exchanger-1 translation and its opposite roles in the carcinogenesis of gastric cancer cells and differentiation of K562 cells. Oncogene 29: 1987-1996.
- Banales JM, Sáez E, Uriz M, Sarvide S, Urribarri AD, et al. (2012) Up-regulation of microRNA 506 leads to decreased Cl-/HCO3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 56: 687-697.
- Anbazhagan AN, Priyamvada S, Kumar A, Maher DB, Borthakur A, et al. (2014) Translational repression of SLC26A3 by miR-494 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 306: 123-131.
- Mencia N, Selga E, Noé V, Ciudad CJ (2011) Underexpression of miR-224 in methotrexate resistant human colon cancer cells. Biochem Pharmacol 82: 1572-1582.
- Kim JO, Kwon EJ, Song DW, Lee JS, Kim DH (2016) miR-185 inhibits endoplasmic reticulum stress-induced apoptosis by targeting Na+/H+ exchanger-1 in the heart. BMB Rep 49: 208-213.
- Kumar A, Kant S, Singh SM (2013) Targeting monocarboxylate transporter by α-cyano-4-hydroxycinnamate modulates apoptosis and cisplatin resistance of Colo205 cells: implication of altered cell survival regulation. Apoptosis 18: 1574-1585.
- Kumar A, Kant S, Singh SM (2013) α-Cyano-4-hydroxycinnamate induces apoptosis in Dalton's lymphoma cells: role of altered cell survival-regulatory mechanisms. Anticancer Drugs 24: 158-171.
- Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, et al. (2010) Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol 2010: 427694.
- Yan B, Guo Q, Fu F-J, Wang Z, Yin Z, et al. (2015) The role of miR-29b in cancer: regulation, function, and signaling. Onco Targets Ther 8: 539-548.
- Jiang H, Zhang G, Wu JH, Jiang CP (2014) Diverse roles of miR-29 in cancer (review). Oncol Rep 31: 1509-1516.
- Wang Y, Chen L, Wu Z, Wang M, Jin F, et al. (2016) miR-124-3p functions as a tumor suppressor in breast cancer by targeting CBL. BMC Cancer 16: 826.
- Ru P, Steele R, Newhall P, Phillips NJ, Toth K, et al. (2012) miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther 11: 1166-1173.
- Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, et al. (2013) Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene 32: 4130-4138.
- Feng T, Shao F, Wu Q, Zhang X, Xu D, et al. (2016) miR-124 downregulation leads to breast cancer progression via LncRNA-MALAT1 regulation and CDK4/E2F1 signal activation. Oncotarget 7: 16205-16216.
- Lv XB, Jiao Y, Qing Y, Hu H, Cui X, et al. (2011) miR-124 suppresses multiple steps of breast cancer metastasis by targeting a cohort of pro-metastatic genes in vitro. Chin J Cancer 30: 821-830.
- Cai QQ, Dong YW, Wang R, Qi B, Guo JX, et al. (2017) MiR-124 inhibits the migration and invasion of human hepatocellular carcinoma cells by suppressing integrin αV Sci Rep 7: 40733.
- Zhang J, Lu Y, Yue X, Li H, Luo X, et al. (2013) MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One 8: 70300.
- Wu Z, Huang W, Chen B, Bai PD, Wang XG, et al. (2017) Up-regulation of miR-124 inhibits invasion and proliferation of prostate cancer cells through mediating JAK-STAT3 signaling pathway. Eur Rev Med Pharmacol Sci 21: 2338-2345.
- Zhou L, Xu Z, Ren X, Chen K, Xin S (2016) MicroRNA-124 (MiR-124) Inhibits Cell Proliferation, Metastasis and Invasion in Colorectal Cancer by Downregulating Rho-Associated Protein Kinase 1(ROCK1). Cell Physiol Biochem 38: 1785-1795.
- Chen L, Xiao H, Wang Z-H, Huang Y, Liu Z-P, et al. (2014) miR-29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF-A. BMB Rep 47: 39-44.
- Tang W, Zhu Y, Gao J, Fu J, Liu C, et al. (2014) MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br J Cancer 110: 450-458.
- Pei YF, Lei Y, Liu XQ (2016) MiR-29a promotes cell proliferation and EMT in breast cancer by targeting ten eleven translocation 1. Biochim Biophys Acta 1862: 2177-2185.
- Wu Z, Huang X, Huang X, Zou Q, Guo Y (2013) The inhibitory role of Mir-29 in growth of breast cancer cells. J Exp Clin Cancer Res 32: 98.
- Li ZH, Xiong QY, Xu L, Duan P, Yang QO, et al. (2017) miR-29a regulated ER-positive breast cancer cell growth and invasion and is involved in the insulin signaling pathway. Oncotarget 8: 32566-32575.
- Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13: 472-482.
- Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330: 1340-1344.
- Draoui N, Feron O (2011) Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech 4: 727-732.
- Choi SY, Collins CC, Gout PW, Wang Y (2013) Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol 230: 350-355.
- Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ (2016) Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front Immunol 7: 52.
- Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, et al. (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118: 3930-3942.
- Miao P, Sheng S, Sun X, Liu J, Huang G (2013) Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life 65: 904-910.
- Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123: 3685-3692.
- Chen B, Li H, Zeng X, Yang P, Liu X, et al. (2012) Roles of microRNA on cancer cell metabolism. J Transl Med 10: 228.
- Chan B, Manley J, Lee J, Singh SR (2015) The emerging roles of microRNAs in cancer metabolism. Cancer Lett 356: 301-308.
- Potter CPS, Harris AL (2003) Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer 89: 2-7.
- Potter C, Harris AL (2004) Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle 3: 164-167.
- Masashi T, Regina S, Michael F (2010) Regulation and Isoform Function of the V-ATPases. Biochemistry 49: 4715-4723.
- Fais S, De Milito A, You H, Qin W (2007) Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res 67: 10627-10630.
- Stransky L, Cotter K, Forgac M (2016) The Function of V-ATPases in Cancer. Physiol Rev 96: 1071-1091.
- Cotter K, Capecci J, Sennoune S, Huss M, Maier M, et al. (2015) Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J Biol Chem 290: 3680-3692.
- Sennoune SR, Luo D, Martínez-Zaguilán R (2004) Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys 40: 185-206.
- Lee BS (2012) Regulation of V-ATPase expression in mammalian cells. Curr Protein Pept Sci 13: 107-116.
- Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM (2005) Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3’UTR evolution. Cell 123: 1133-1146.
- Cordat E, Casey JR (2009) Bicarbonate transport in cell physiology and disease. Biochem J 417: 423-439.
- Song LJ, Liu RJ, Zeng Z, Alper SL, Cui HJ, et al. (2012) Gastrin inhibits a novel, pathological colon cancer signaling pathway involving EGR1, AE2, and P-ERK. J Mol Med (Berl) 90: 707-718.
- Wang T, Zhao L, Yang Y, Tian H, Suo WH, et al. (2013) EGR1 is critical for gastrin-dependent upregulation of anion exchanger 2 in gastric cancer cells. FEBS J 280: 174-183.
- Wu TT, Hsieh YH, Wu CC, Tsai JH, Hsieh YS, et al. (2006) Overexpression of anion exchanger 2 in human hepatocellular carcinoma. Chin J Physiol 49: 192-198.
- Hwang JM, Kao SH, Hsieh YH, Li KL, Wang PH, et al. (2009) Reduction of anion exchanger 2 expression induces apoptosis of human hepatocellular carcinoma cells. Mol Cell Biochem 327: 135-144.
- Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF (2014) Regulation and roles of bicarbonate transporters in cancer. Front Physiol 5: 130.
- Yang Y, Wu PP, Wu J, Shen WW, Wu YL, et al. (2008) Expression of anion exchanger 2 in human gastric cancer. Exp Oncol 30: 81-87.
- Mahnensmith RL, Aronson PS (1995) The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res 56: 773-788.
- Slepkov ER, Rainey JK, Sykes BD, Fliegel L (2007) Structural and functional analysis of the Na+/H+ Biochem J 401: 623-633.
- Loo SY, Chang MK, Chua CS, Kumar AP, Pervaiz S, et al. (2012) NHE-1: A promising target for novel anti-cancer therapeutics. Curr Pharm Des 18: 1372-1382.
- Reshkin SJ, Cardone RA, Harguindey S (2013) Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov 8: 85-99.
Citation: Gupta VK, Jaiswara P, Kumar A (2018) The Emerging Role of miRNAs in Tumor Acidosis. J Cell Biol Cell Metab 5: 015.
Copyright: © 2018 Vishal Kumar Gupta, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

