
Enhancement of CC10 Production from Human Nasal Epithelial Cells by Histamine H1 Receptor Antagonists, Desloratadine and Levocetirizine in Vitro and in Vivo
*Corresponding Author(s):
Kazuhito AsanoDivision Of Physiology, School Of NRS, Showa University, Tokyo, Japan
Tel:81459856538,
Fax:81459857583
Email:asanok@med.showa-u.ac.jp
Abstract
Keywords
INTRODUCTION
Allergic rhinitis is well known to be allergic inflammatory responses in the nasal mucosa. It occurs when an allergen (e.g. pollen, dust and animal dander, etc.) is inhaled by an individual with a sensitized immune system. In such individuals, allergen triggers the production of IgE antibodies for specific allergen(s), which binds to receptors on the surface of mast cells and basophils [1,2]. On re-exposure to the relevant allergen(s), cross-linking of adjacent IgE molecules occurs, and mast cell degranulation takes place, releasing a variety of chemical mediators, such as histamine, leukotriene and prostaglandins, among others. These chemical mediators are responsible for the development of symptoms of allergic rhinitis including sneezing, swelling and inflammation of the nasal passages, and hyper-secretion of mucus [2]. From these established concept, histamine H1 receptor antagonists, so-called antihistamines are recommended as the first choice drugs for the treatment and prevention of allergic rhinitis.
It is well known that the human body is an exquisite machine and fends off many challenges from environments to its maintenance of balance. This ability of the body is called homeostasis and is nothing but its capability to regulate and control the inner environment physiologically, so that the body functions under a constant stability when exposed to certain fluctuating conditions in the external environment. The endocrine system and endogenous peptides secreted after several stimuli are well accepted to major part in maintaining homeostasis, as well as the sympathetic nervous system. From the point of view, we examined the influence of endogenous peptides on the development of allergic rhinitis and reported that nasal secretions from patients with allergic rhinitis contained much lower levels of Thioredoxin (TRX), which show the action of both anti-inflammatory and immunomodulation, as compared with that from normal subjects [3]. We also observed that oral administration of epinastine hydrochloride and fexofenadine hydrochloride, the second generation histamine H1 receptor antagonists, into allergic rhinitis patients for two weeks increases the contents of TRX in nasal secretions along with attenuation of clinical symptoms [3]. These reports may suggest that the activity of histamine H1 receptor antagonists on the production (or secretion) of endogenous peptides may contribute to the clinical efficacy of the agents on allergic rhinitis.
Clara cell 10-kDa protein (CC10) is a member of secretoglobin family and the major constituent of secretory granules of Clara cells in the bronchi and nasal epithelial cells. CC10 constitutively expressed by the epithelial lining of airways and isolated from mammals, including rats, rabbit and human [4]. It is reported that pro-inflammatory cytokines such as IFN-? and TNF-a modulate CC10 mRNA expression in Clara cells and results in increase in protein secretion from cells [5]. CC10 is also reported to exert anti-inflammatory effects through the suppression of the activity of both phospholipase A2 and transglutaminase, which are responsible for the development of allergic inflammation [4,6]. Furthermore, it is recognized that CC10 can inhibit inflammatory cell chemotaxis, and down-regulate Th2 T cell differentiation, including cytokine production [7-10,6]. In human cases, low levels of CC10 were detected in nasal inflammatory diseases, such as chronic rhinosinusitis and allergic rhinitis [11-13], suggesting that CC10 may play essential roles in the development of nasal inflammatory diseases.
Recently, third generation histamine H1 receptor antagonists, Desloratadine (DLT) and Levocetirizine (LCT), are developed and used for the treatment of allergic diseases such as allergic rhinitis and atopic dermatitis with remarkable success [14-16]. Although the attenuating effects of these agents on clinical condition of allergic patients has been recognized to depend, in part, on antagonistic action on the histamine H1 receptor [14,16], these drugs are also well known to exert inhibitory action on the synthesis and release of chemical mediators from mast cells and eosinophils in response to immunological and non-immunological stimulation [14]. It is reported that DLT can inhibit the production of cytokines and chemokines from mast cells and epithelial cells as well as the expression of adhesion molecules, which are involved in the development of allergic inflammation [14]. However, the influence of these agents on CC10 production is not well understood. The present study, therefore, was undertaken to examine the influence of the third generation histamine H1 receptor antagonists, DLT and LCT, on CC10 production from nasal epithelial cells in response to inflammatory stimulation in vitro. We also examined the influence of third generation histamine H1 receptor antagonists on the appearance of CC10 in nasal secretions through the choice of pollinosis patients against Japanese cedar pollen who were treated with LCT.
MATERIALS AND METHODS
Reagents
Subjects and treatment
| Controls | Patients | |
| Age, years (range) | 44-61 | 36-39 |
| Number of subjects | 4 | 10 |
| Sex | Male | Male |
| Disease | Nonallergic | Mild |
| Medication | None | None |
| Serum IgE (U/ml) | 41.6 ± 8.9 | 145.6 ± 12.7 |
| IgE RAST score | ||
| Cj | 0 | 50.8 ± 11.2 |
| Aa | 0 | 0 |
| Ap | 0 | 0 |
| Dg | 0 | 0 |
| Df | 0 | 0 |
| Af | 0 | 0 |
| Cd | 0 | 0 |
| Dd | 0 | 0 |
| Blood Eosinophil Count, % | 3.4 ± 0.5 | 15.5 ± 0.8 |
| Skin prick test | - | +++a |
| Nasal provocation test | ||
| Symptoms | - | +++b |
| Smear cytology | <1% eosinophils | >30% eosinophils |
b Positive for sneezing/itch, watery rhinorrhea and nasal blockage against C. japonica alone.
Recovery of nasal secretions
Nasal symptom scores
Nasal epithelial cell preparation and culture
Assay for CC10 mRNA expression
Preparation of TRX specific mRNA
Cell-free protein synthesis
Assay for CC10
Statistical analysis
RESULTS
Increased CC10 production induced by LCT and DLT in nasal epithelial cells in vitro
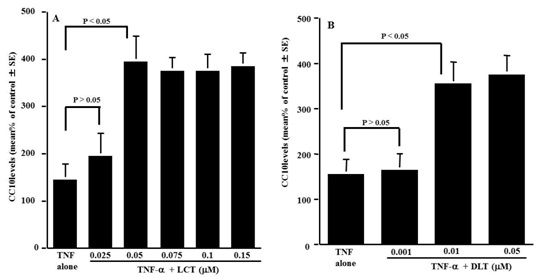
Figure 1: Influence of levocetirizine (LCT) and desloratadine (DLT) on CC10 production from nasal epithelial cells after TNF-a stimulation in vitro. Nasal epithelial cells at approximately 5 x 106 cells were stimulated with 20 ng/ml TNF-a in the presence of various concentrations of the agents. After 24h, the culture supernatants were obtained and assayed for CC10 levels by ELISA.
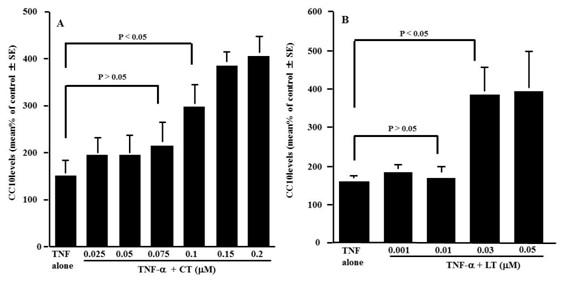
Figure 2: Influence of Cetrizine (CT) and Loratadine (LT) on CC10 production from nasal epithelial cells after TNF-a stimulation in vitro. Nasal epithelial cells were stimulated with 20 ng/ml TNF-a in the presence of various concentrations of the agents. After 24 h the culture supernatants were collected and assayed for CC10 levels by ELISA. The data are expressed as % of control (non-stimulated) ±SE of five different subjects.
Influence of LCT and DLT on CC10 mRNA expression in nasal epithelial cells in vitro
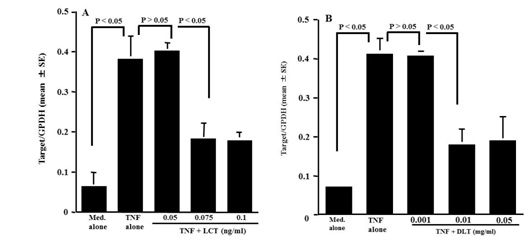
Figure 3: Influence of Levocetrizine and Desloratadine (DLT) on CC10 mRNA expression in nasal epithelial cells after TNF-a stimulation in vitro. Nasal epithelial cells were stimulated with 20ng/ml TNF-a in the presence of various concentrations of the agents. After 12h, Poly A+ mRNA was obtained from the cultured cells and CC10 mRNA expression was examined by real-time RT-PCR. The data are expressed as a mean ratio calculated as Target gene/GPDH.
Influence of LCT and DLT on cell-free protein synthesis
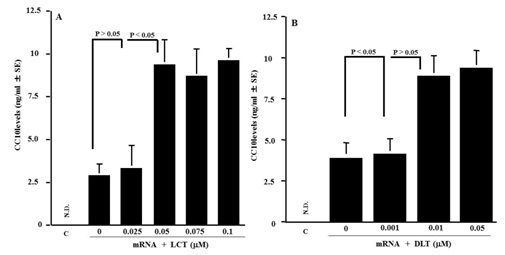
Figure 4: Influence of Levocetirizine (LCT) and Desloratadine (DLT) on CC10 production in cell-free protein synthesis system. CC10 levels in samples were examined by ELISA. The data expressed as the mean SE of five. ND: Not Detected (lower than 46 pg/ml) C: control.
Influence of LCT treatment on CC10 appearance in nasal secretions from patients with pollinosis
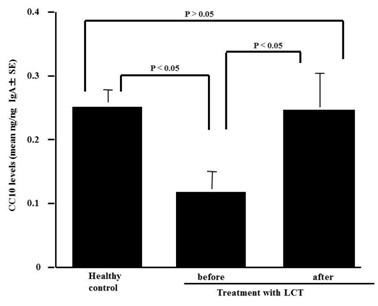
Figure 5: CC10 levels in nasal secretions from pollinosis patients before and after treatment with Levocetilizine (LCT) for 4 weeks.
Influence of LCT treatment on clinical symptoms in patients with pollinosis
| Treatment | ||
| Symptoms | Before | After* |
| Sneezing | 2.4±0.5 | 0.5±0.4 |
| Nasal discharge | 2.5±1.2 | 1.6±1.0 |
| Congestion | 2.2±1.1 | 0.8±0.4 |
DISCUSSION
The present results clearly show that the third generation histamine H1 receptor antagonists, LCT and DLT, enhance the ability of nasal epithelial cells to produce CC10 induced by TNF-a stimulation as well as their mother drugs, LT and CT in vitro. The minimum concentrations that cause significant increase in CC10 levels are 0.05 µM for LCT and 0.01 µM for DLT, which are lower levels that observed in their mother drugs and therapeutic blood levels [14, 16].
CC10 is a secretory protein composed of two identical subunits of 70 amino acids joined by two disulfide bonds [22,19]. Although CC10 was initially discovered in the rabbit uterus and believed to be a marker of the action of progesterone in a mammalian species [23], it is now revealed that CC10 possesses anti-inflammatory and immunomodulatory effects [7-10,6]. The role of CC10 on the development of allergic inflammation was extensively studied using experimental murine model. CC10 knock-out mice represent exaggerated allergic inflammation with intense infiltration of eosinophils in both lung [7,8] and nasal cavity [13]. In human cases, recent studies indicate that CC10 expression in nasal mucosa is down-regulated in patients with allergic rhinitis and chronic rhinosinusitis with nasal polyps [11,12]. Furthermore, it is also reported that CC10 levels in nasal secretions and nasal lavage fluids obtained from patients with allergic rhinitis was much lower than those from healthy control [11,24,25], indicating that CC10 may contribute to the induction and development of airway inflammatory diseases, especially allergic rhinitis. Together with these reports, the present results may suggest that enhancement of the ability nasal epithelial cells to produce CC10 by histamine H1 receptor antagonists underlie the therapeutic mode of action of the agent on allergic rhinitis. To further confirm this speculation, we then examine the influence of the agents on the production of CC10 in vivo using pollinosis patients treated with LCT. The present results clearly show that nasal secretions obtained from patients before treatment contained much lower levels of CC10 as compared with those from healthy control subjects, and that oral administration of LCT into pollnosis patients caused an increase in CC10 levels in nasal secretions along with favorable modification of clinical symptoms. Our previous observation [17] showing that oral administration of fexofenadine hydrochloride, a second generation histamine H1 receptor antagonist, into pollinosis patients for 2 weeks caused an increase in CC10 levels in nasal secretions and results in attenuation of clinical conditions in patients also supports our speculation.
Our previous works clearly showed that histamine H1 receptor antagonists inhibit the production of inflammatory mediators, such as cytokines and chemokines from both epithelial cells and fibroblasts after inflammatory stimulations through the suppression of mRNA expression [1,26,27]. On the other hand, the present results clearly show that treatment of nasal epithelial cells with DLT and LCT caused an increase in CC10 protein production in spite of the suppression of its mRNA expression induced by TNF-a stimulation. The reasons for this discrepancy are not clear at present. The process of protein synthesis in cells requires two different steps: in the first step, transcription, mRNA is synthesized from DNA in the nucleus. mRNA formed then comes out through nuclear membrane into cytoplasma where it binds to mRNA-binding site on ribosome and starts protein synthesis, which is call translocation. The final experiments, therefore, were undertaken to examine whether LCT and DLT could increase the translocation activity of CC10 mRNA. Cell free protein assay revealed that the addition of LCT and DLT at concentrations showing suppressive effects on CC10 mRNA expression could increase CC10 protein synthesis. These results strongly suggest that there is the possibility that LCT and DLT increase the translation of CC10 mRNA, resulting in production and secretion of large amount of CC10 from TNF-a stimulated epithelial cells. Although glucocorticoids, dexamethasone and cortisol, are well known to exert their immunosuppressive effects through the suppression of inflammatory protein mRNA expression, they are reported to enhance the ability of airway cells to produce CC10 after inflammatory stimulation, and this is due, in part, to an increase in translatable activity of CC10 mRNA by glucocorticoids [28-30]. These reports may also support our speculation that the translation of CC10 mRNA in nasal epithelial cells is enhanced by LCT and DLT and results in increase in the ability of cells to produce CC10 after TNF-a stimulation.
In conclusion, the present results may suggest that some of therapeutic mode of action of histamine H1 receptor antagonists, especially LCT and DLT, in allergic diseases such as allergic rhinitis and atopic allergy depend on their ability to increase the production of CC10 from epithelial cells in response to inflammatory stimulation through the enhancement of the translatable activity of CC10 mRNA.
CONFLICT OF INTEREST
The authors report no conflict of interest in this work.
REFERENCES
- Asano K, Kanai KI, Suzaki H (2004) Suppressive activity of fexofenadine hydrochloride on metalloproteinase production from nasal fibroblasts in vitro. Clin Exp Allergy 34: 1890-1898.
- Howarth PH (1998) Pathogenic mechanisms: a rational basis for treatment. British Med J. 316:757-761.
- Furuta A, AsanoK, Kanai K, Hirano K, Sanbe T, et al. (2010) Enhancement of thioredoxin production by epinastine hydrochloride in vitro and in vivo. Jpn Pharmacol Ther. 38: 965-70.
- Johansson S, Keen C, Ståhl A, Wennergren G, Benson M (2005) Low levels of CC16 in nasal fluid of children with birch pollen-induced rhinitis. Allergy 60: 638-642.
- Elia J, Aoki A, Maldonado CA (2003) Regulation of uteroglobin/Clara cell protein expression after acute lung exposure to an organophosphoreted insecticide. Histochem Cell Biol 120: 33-39.
- Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY (2003) Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest 111: 121-128.
- Chen LC, Zhang Z, Myers AC, Huang SK (2001) Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol 167: 3025-3028.
- Hung CH, Chen LC, Zhang Z, Chowdhury B, Lee WL, et al. (2004) Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J Allergy Clin Immunol 114: 664-670.
- Johansson S, Wennergren G, Aberg N, Rudin A (2007) Clara cell 16-kd protein downregulates T(H)2 differentiation of human naive neonatal T cells. J Allergy Clin Immunol 120: 308-314.
- Shiyu S, Zhiyu L, Mao Y, Lin B, Lijia W et al. (2011) Polydatin up-regulates clara cell secretory protein to suppress phospholipase A2 of lung induced by LPS in vivo and in vitro. BMC Cell Biol. 12: 31-44.
- Benson M, Fransson M, Martinsson T, Naluai AT, Uddman R et al. (2007) Inverse relation between nasal fluid Clara Cell Protein 16 levels and symptoms and signs of rhinitis in allergen-challenged patients with intermittent allergic rhinitis. Allergy 62: 178-183.
- Liu Z, Lu X, Zhang XH, Bochner BS, Long XB et al. (2009) Clara cell 10-kDa protein expression in chronic rhinosinusitis and its cytokine-driven regulation in sinonasal mucosa. Allergy 64: 149-157.
- Wang H, Long XB, Cao PP, Wang N, Liu Y, et al. (2010) Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med 181: 908-916.
- Henz BM (2001) The pharmacologic profile of desloratadine: a review. Allergy 65: 7-13.
- Sadowska-Woda I, Bieszczad-Bedrejczuk E, Rachel M (2010) Influence of desloratadine on selected oxidative stress markers in patients between 3 and 10 years of age with allergic perennial rhinitis. Eur J Pharmacol 640: 197-201.
- Schoepke N, Church MK, Maurer M (2013) The inhibition by levocetirizine and fexofenadine of the histamine-induced wheal and flare response in healthy Caucasian and Japanese volunteers. Acta Derm Venereol 93: 286-293.
- Nogaki T, Asano K, Furuta A, Kanai K, Suzaki I, et al. (2012) Enhancement of clara cell 10-kD protein (CC10) production from nasal epithelial cells by fexofenadine hydrochloride. Asian Pac J Allergy Immunol 30: 139-145.
- Cowan MJ, Huang X, Yao XL, Shelhamer JH (2000) Tumor necrosis factor alpha stimulation of human Clara cell secretory protein production by human airway epithelial cells. Ann N Y Acad Sci 923: 193-201.
- Yao XL, Levine SJ, Cowan MJ, Logun C, Shelhamer JH (1998) Tumor necrosis factor-alpha stimulates human Clara cell secretory protein production by human airway epithelial cells. Am J Respir Cell Mol Biol 19: 629-635.
- Yamamoto O, Takahashi H, Hirasawa M, Chiba H, Shiratori M, et al. (2005) Surfactant protein gene expressions for detection of lung carcinoma cells in peripheral blood. Respir Med 99: 1164-1174.
- Suzaki I, Asano K, Kanei A, Suzaki H (2013) Enhancement of thioredoxin production from nasal epithelial cells by the macrolide antibiotic, clarithromycin in vitro. In Vivo 27: 351-356.
- Bally R, Delettré J (1989) Structure and refinement of the oxidized P21 form of uteroglobin at 1.64 A resolution. J Mol Biol 206: 153-170.
- Lombardero M, Nieto A (1981) Glucocorticoid and developmental regulation of uteroglobin synthesis in rabbit lung. Biochem J 200: 487-494.
- Broeckaert F, Bernard A (2000) Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy 30: 469-475.
- Yang KD, Ou CY, Chang JC, Chen RF, Liu CA et al. (2007) Infant frequent wheezing correlated to Clara cell protein 10 (CC10) polymorphism and concentration, but not allergy sensitization, in a perinatal cohort study. J Allergy Clin Immunol. 120: 842-848.
- Kanai K, Asano K, Watanabe S, Kyo Y, Suzaki H (2006) Epinastine hydrochloride antagonism against interleukin-4-mediated T cell cytokine imbalance in vitro. Int Arch Allergy Immunol 140: 43-52.
- Shoji N, Asano K, Furuta A, Hirano K, Suzaki H (2011) Effect of histamine H1 receptor antagonists on TARC/CCL17 and MDC/CCL22 production from CD14+ cells induced by antigenic stimulation in vitro. Int Arch Allergy Immunol 155: 38-51.
- Cui YH, Wang YY, Liu Z (2011) Transdifferentiation of Clara cell 10-kDa protein secreting cells in experimental allergic rhinitis. Am J Rhinol Allergy 25: 145-151.
- Mukherjee AB, Zhang Z, Chilton BS (2007) Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev 28: 707-725.
- Roth FD, Quintar AA, Uribe Echevarría EM, Torres AI, Aoki A, et al. (2007) Budesonide effects on Clara cell under normal and allergic inflammatory condition. Histochem Cell Biol 127: 55-68.
Citation: Suzuki T, Asano K, Furuta A, Kanai KI, Hinohira Y, et al. (2014) Enhancement of CC10 Production from Human Nasal Epithelial Cells by Histamine H1 Receptor Antagonists, Desloratadine and Levocetirizine in Vitro and in Vivo. J Clin Immunol Immunother, 1: 002.
Copyright: © 2014 Takahiro Suzuki, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

