
Primary Male Breast Adenoid Cystic Carcinoma - A Case Report and Review of the Literature
*Corresponding Author(s):
Huaitao YangDepartment Of Pathology And Laboratory Medicine, University Of Cincinnati College Of Medicine, Cincinnati, United States
Tel:+1 5135843847,
Email:yanght@ucmail.uc.edu
Abstract
Adenoid Cystic Carcinoma (AdCC) of the breast is a rare subtype of breast cancer, accounting for less than 0.1% of all breast carcinomas. In contrast to the aggressive nature of AdCC of salivary glands, AdCC of the breast has a favorable prognosis, low rate of lymph node involvement and uncommon distant metastases. Many cases of adenoid cystic carcinoma are successfully treated with breast conserving surgery and sentinel lymph node biopsy. Chemotherapy, radiation and hormonal treatment are infrequently used. We report a rare case of primary adenoid cystic carcinoma of breast in a 75 year-old male with a subareolar, well-defined, 2.2 cm mass. The core-biopsy was diagnosed as AdCC with a basal-like phenotype (negative for ER-, PR-, and HER-2/neu) and was positive for c-Kit, p63, SM myosin, and e-cadherin. The Ki-67 labeling index was 28%. The patient underwent total mastectomy, with subsequent AJCC pathological staging pT2 pN0 pM0. He was treated with post-op methotrexate and 5-fluorouracil chemotherapy. We report this rare primary breast AdCC with in this 75-year-old male patient.
Keywords
INTRODUCTION
Adenoid Cystic Carcinoma (AdCC) is most commonly seen as a tumor of salivary glands, but is also a rare subtype of breast cancer, with an overall incidence of 0.1% (of all breast malignancies) [1-4]. AdCC is characterized by the presence of a dual cell population of luminal and basaloid cells. Breast AdCC generally affects older female patients (range 40-96 years) [1,5]. Primary AdCC of male breast is extremely rare and its’ clinical pathological features have not been completely characterized. We report this primary male breast AdCC due to its’ rarity.
CASE REPORT
General clinical appearance
Radiological images’ studies
Right breast core biopsy
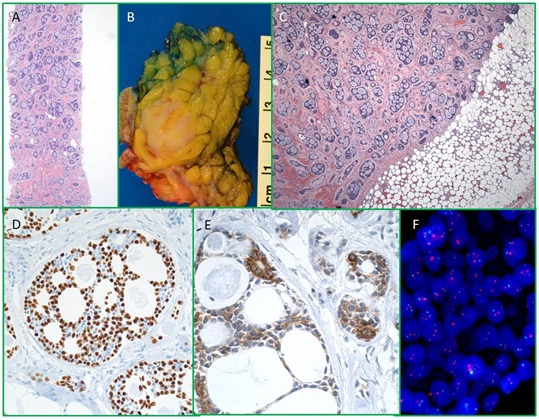
Figure 1: Right breast core biopsy reveals AdCC, H & E, (A) There is a well-defined mass in mastectomy specimen grossly, (B) and microscopically, (C) Carcinoma cells are positive for p63, (D) and c-Kit, (E) by immunohistochemical studies, and (F) HER2/neu FISH studies are negative for amplification.
Total mastectomy and sentinel lymph node dissection of right breast
Post-op management
DISCUSSION
We report a rare case of primary AdCC of the breast in a 75 year old male, with basal-like phenotype (negative for ER, PR, and Her-2) and pathological staging pT2 pN0pMX.
Most reported breast AdCC cases are in females. Breast AdCC has an excellent prognosis with a low incidence of lymph node metastasis and distant metastasesis uncommon [4-7]. Histologically, AdCC is composed of two cell populations, epithelial and myoepithelial cells, which are similar to that of their salivary gland counter parts. Similar to AdCCs of the salivary gland, breast AdCCs are characterized by the t (6;9) (q22-23; p23-24) chromosomal translocation, which generates fusion tran-scripts MYB-NFIB [8]. It is important to distinguish this from other types of breast cancer as breast AdCC has an excellent prognosis.
Male breast carcinoma is an uncommon malignancy, accounting for 1% of all breast carcinomas. Male AdCC is extremely rare. Few cases of male AdCC have been reported, thus its causes and clinical pathological features have not been completely characterized [9-11]. The clinical features of published male AdCC cases are summarized in table 1 [12-18]. Among these, AdCC of the male breast occurs in wide age distribution, which ranges from 13 to 82 years. AdCC usually presents as a firm mass, ranging from 1.7 cm to 6 cm, occurring equally on the left and right sides. It usually locates in the subareolar region. Nipple discharge is uncommon. AdCC of the male breast shows histopathological findings similar to AdCC of the salivary gland. Perineural invasion and axillary node metastasis are rare. The resection margin is virtually never involved by carcinoma. Immunohistochemically, the majority of male breast ACCs are immune phenotypically triple-negative (i.e., ER-, PR- and HER2-negative). However, in contrast to triple-negative breast carcinomas, AdCC of the breast usually has an excellent prognosis. Cytogenetically, breast AdCC is similar to AdCCs of the salivary gland, which characterized by the t (6;9) (q22-23; p23-24) chromosomal translocation, generating a fusion transcripts involving the oncogene MYB and the transcription factor gene NFIB. It has been reported that this chromosomal translocation is present in majority of breast AdCC cases. In these reported male breast cases as summarized in table 1, most patients are treated with mastectomy (simple, radical or total mastectomy) with excellent. However, one patient, reported by Kshirsaga, had a large 6 cm ulcerative AdCC. A modified radical mastectomy was done and found had three of the five axillary lymph nodes excised were positive for carcinoma. The patient refused postoperative radiotherapy. After 2 years, the patient had local recurrence at anterior chest wall, which then underwent wide excision and postoperative radiationtherapy. There was no evidence of recurrence for at least 9 months duration after wide excision [15]. The case reported by Yoo et al., had a small 1.7 cm AdCC with bone and lung metastasis at the time of diagnosis [17]. Although guidelines for diagnosis and treatment of male breast AdCC are not clear due to the extremely low numbers of reported cases; surgical management, such as mastectomy, is the main stay of treatment. Our reported case of primary male AdCC was treated with total mastectomy, which had negative resection margins and negative axillary lymph nodes. There was no evidence of recurrence or distant metastasis of AdCC at a follow up interval of 21 months.
| Cases | Age | Clinical finding | Side | Size | PNI | Margin | Axillary node | Treatment | Outcomes |
| Tang et al., | 19 Yr | Painless mass | Right | 2 cm | Absent | Negative | Negative | Radical mastectomy | No recurrence |
| Ferlito et al., | 60 Yr | Body nipple discharge | Left | “pea-like” | N/A | N/A | N/A | Radical mastectomy | N/A |
| Hjorth et al., | 21 Yr | Tender lump under nipple | Left | 2 cm | Present | N/A | Negative | Simple mastectomy | No recurrence |
| Miliauska et al., | 13 Yr | Tender and enlarged mass | Right | 3.8 cm | Absent | Negative | Negative | Subcutaneous mastectomy | No recurrence |
| kshirsaga, et al., | 82 Yr | Ulcer | Left | 6 cm | Absent | Negative | Positive | Radical mastectomy | Local recurrence after 2 years |
| Liu et al., | 20 Yr | Tender mass | Right | 2 cm | N/A | N/A | Negative | Simple mastectomy | N/A |
| Yoo et al., | 41 Yr | Cervical back pain (bone metastasis) | Left | 1.7 cm | N/A | N/A | Positive | N/A | Bone and lung metastasis |
| Present study | 75 Yr | Painless mass | Right | 2.2 cm | Absent | Negative | Negative | Total mastectomy | No recurrence |
Table 1: Summary of reported male breast AdCC cases.
In addition, this patient had two other neoplasms: one is a self-reported previous melanoma and the other a subsequently diagnosed urothelial carcinoma (six weeks later after mastectomy). Prior melanoma is very likely an independent event in relation to the AdCC. The later diagnosed urothelial carcinoma is also less likely associated with AdCC at the pathogenesis point. Multiple exogenous risk factors have been linked to bladder cancer, such as tobacco smoking, occupational risk, and lifestyle exposure to carcinogens, which are believed to take years for development of bladder cancer. However, this is the 1stcase report of male breast AdCC with co-existing bladder urothelial carcinoma. Further investigation will potentially elucidate the pathogenesis of male adenoid cystic breast carcinoma.
In conclusion, although rare, adenoid cystic carcinoma of male breast is a well-known entity. It is important to recognize this lesion as it has relatively excellent prognosis.
REFERENCES
- Arpino G, Clark GM, Mohsin S, Bardou VJ, Elledge RM (2002) Adenoid cystic carcinoma of the breast: molecular markers, treatment, and clinical outcome. Cancer 94: 2119-2127.
- Azzopardi JG, Ahmed A, Millis RR (1979) Problems in breast pathology. Major Probl Pathol 11: 1-466.
- Speirs V, Shaaban AM (2009) The rising incidence of male breast cancer. Breast Cancer Res Treat 115: 429-430.
- Miyai K, Schwartz MR, Divatia MK, Anton RC, Park YW, et al. (2014) Adenoid cystic carcinoma of breast: Recent advances. World J Clin Cases 2: 732-741.
- Leeming R, Jenkins M, Mendelsohn G (1992) Adenoid cystic carcinoma of the breast. Arch Surg 127: 233-235.
- Hopkins GB, Tullis RH (1972) Adenoid cystic carcinoma of the breast. Calif Med 117: 9-11.
- Peters GN, Wolff M (1983) Adenoid cystic carcinoma of the breast. Report of 11 new cases: review of the literature and discussion of biological behavior. Cancer 52: 680-686.
- Wetterskog D, Lopez-Garcia MA, Lambros MB, A'Hern R, Geyer FC, et al. (2012) Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol 226: 84-96.
- Fentiman IS, Fourquet A, Hortobagyi GN (2006) Male breast cancer. Lancet 367: 595-604.
- Contractor KB, Kaur K, Rodrigues GS, Kulkarni DM, Singhal H (2008) Male breast cancer: is the scenario changing. World J Surg Oncol 6: 58.
- White J, Kearins O, Dodwell D, Horgan K, Hanby AM, et al. (2011) Male breast carcinoma: increased awareness needed. Breast Cancer Res 13: 219.
- Ferlito A, Di Bonito L (1974) Adenoid cystic carcinoma of the male breast: report of a case. Am Surg 40: 72-76.
- Hjorth S, Magnusson PH, Blomquist P (1977) Adenoid cystic carcinoma of the breast. Report of a case in a male and review of the literature. Acta Chir Scand 143: 155-158.
- Miliauskas JR, Leong AS (1991) Adenoid cystic carcinoma in a juvenile male breast. Pathology 23: 298-301.
- Kshirsagar AY, Wader JV, Langade YB, Jadhav KP, Zaware SU, et al. (2006) Adenoid cystic carcinoma of the male breast. Int Surg 91: 234-236.
- Liu J, Jia W, Zeng Y, Deng H, Rao N, et al. (2012) Adolescent male adenoid cystic breast carcinoma. The Am surg 78: 288-289.
- Yoo SJ, Lee DS, Oh HS, Kim HJ, Kim MH, et al. (2013) Male breast adenoid cystic carcinoma. Case Rep Oncol 6: 514-519.
- Tang P, Yang S, Zhong X, Yao J, Zhang Y, et al. (2015) Breast adenoid cystic carcinoma in a 19-year-old man: a case report and review of the literature. World J Surg Oncol 13: 19.
Citation: Sharma D, Lowder L, Zhao C, Khan S, Zhou M, et al. (2015) Primary Male Breast Adenoid Cystic Carcinoma - A Case Report and Review of the Literature. J Clin Stud Med Case Rep 2: 023.
Copyright: © 2015 Divya Sharma, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

