
Rapid Inferior Olivary Hypertrophy Secondary to Reversible Metronidazole Neurotoxicity
*Corresponding Author(s):
Granell-Moreno EDepartment Of Radiology, Neuroradiology Unit, Hospital De La Santa Creu I Sant Pau, Barcelona, Spain
Email:egranell@santpau.cat
Abstract
We report the case of a 55 years old man, who developed progressive ataxia one month after metronidazole treatment. Initial brain MRI showed typical findings suggesting metronidazole toxicity. Discontinuation of metronidazole induced a quick improvement of symptoms and 15 days later, the patient was almost asymptomatic. A second brain MRI obtained two months later demonstrated resolution of all lesions, but appearance of bilateral inferior olivary hypertrophy. Clinical follow-up during 4 years showed cognitive difficulties in verbal memory and in retaining new information.
Keywords
INTRODUCTION
Several neurological complications associated to metronidazole therapy have been reported, including sensory polyneuropathy, cerebellar dysfunction and encephalopathy. Proposed risk factors for toxicity include liver dysfunction [1], duration of treatment and total cumulative dose [2], but this is less clear for Central Nervous System (CNS) involvement, as has been recently suggested [3].
CASE REPORT
A 55 years old man was admitted to the Neurology department with a three days history of fluctuating walking instability, and dysarthria. There was no history of alcoholism or drug addiction. One week after admission, cerebellar dysfunction progressively worsened, becoming permanent, but fluctuating, depending on the day. The pattern of the fluctuation was neither predictive nor related with the moment of administration of metronidazole. Neurological examination revealed gaze-evoked nystagmus, slurred speech, mild limb dysmetria, truncal ataxia and unstable gait. The patient had been under treatment with metronidazole for vascular necrosis and infection of the first finger of the right toe. After 2 months of treatment, the symptoms began (total duration of treatment: 2 months and 3 weeks; dose: 500mg/8h). There was no liver or renal dysfunction, and vitamin levels were normal (B1: 4,5mcg/dl and B12: 188pmol/L). CSF was also normal. Brain MRI at admission showed bilateral and symmetric hyperintensities in the cerebellar White Matter (cWM) and Dentate Nuclei (DN), Superior Cerebellar Peduncles (SCP), medulla, medullary Pyramid (P), pons, midbrain, Splenium of the Corpus Callosum (SCC) and subcortical White Matter (WM). Diffusion-Weighted Imaging (DWI) demonstrated high signal intensity within the SCC and low apparent diffusion coefficients, suggesting cytotoxic edema, whereas lesions involving the brainstem showed faint hyperintensity on DW and increased ADCs, consistent with T2 shine-through effect and/or vasogenic edema (Figure 1).
MRI findings suggested metronidazole neurotoxicity, and recognition of this entity was crucial for the patient’s management. Interruption of metronidazole treatment quickly induced clinical improvement, and the patient was almost asymptomatic after fifteen days. Two months later, a second brain MRI demonstrated resolution of most lesions, but the appearance of bilateral inferior olivary hypertrophy (Figures 1g and h).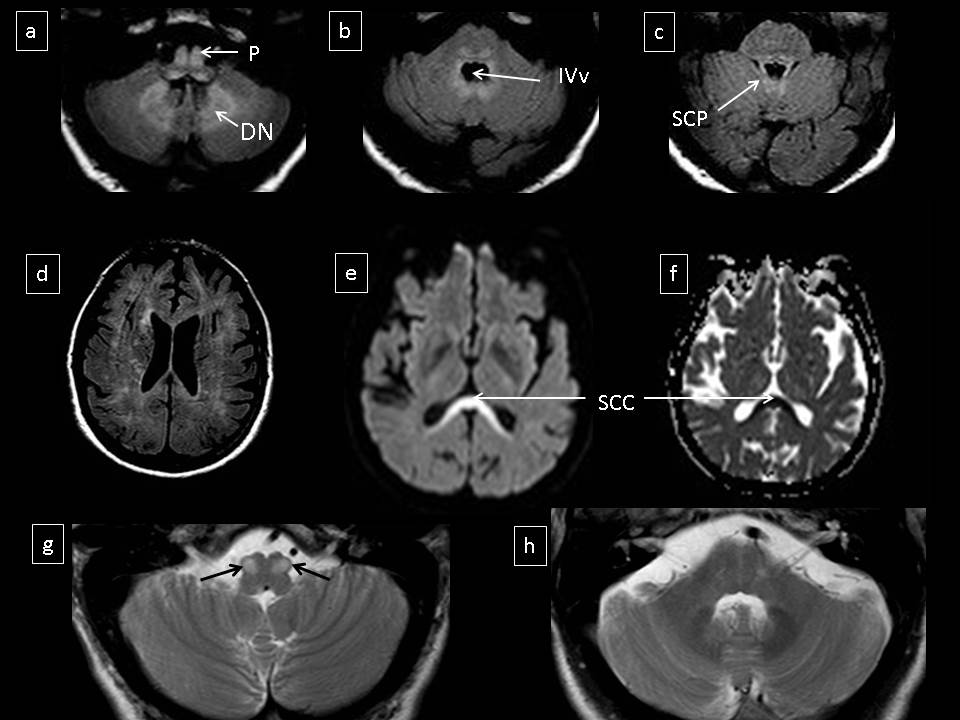
Figure 1: FLAIR images demonstrated hyperintense lesions in the posterior fossa (a-c) and supratentorial WM (d) DWI showed hyperintensity and lowed ADC values within the SCC (e and f). T2 weighted images at the level of the medulla (g) and pons (h), showed resolution of most lesions, but the appearance of bilateral inferior olivary hypertrophy.
DISCUSSION
Metronidazole is a five-nitro-imidazole derivative antibiotic commonly prescribed for the treatment of anaerobic and protozoal infections of the gastrointestinal and genitourinary tracts. Sensory-motor neuropathy is occasionally seen in patients treated with metronidazole, but other rare neurological complications including cerebellar dysfunction, seizures or encephalopathy have been also reported [4]. Neurological features usually become apparent when the drug is used with a dose exceeding 2g/day for prolonged periods. The cumulative dose implicated ranges from 13.2 to 228g, with neuropathy developing 2 weeks to 6 months following initiation [4]. Quick recognition of the clinical and radiological picture is crucial to avoid persistent disability in patients suspected of having metronidazole-related complications [5,6].
Here, we report a patient with highly suggestive MRI findings of neurotoxicity, who presented with cerebellar and brainstem dysfunction after metronidazole treatment. Previously reported cases of metronidazole neurotoxicity include similar findings to those of our patient, with bilateral, symmetric T2/FLAIR-hyperintensities in the cWM and DN, midbrain, dorsal pons, and SCC. On DWI, most of the lesions probably reflect underlying vasogenic edema, whereas involvement of the corpus callosum rather suggests underlying cytotoxic edema [7]. The lesion distribution in the brainstem is heterogeneous, as many structures can be affected. Lesions in the medulla may involve the vestibular and inferior olivary nuclei [5,6].
Restricted ADC values in the corpus callosum could indicate irreversible damage. Corpus callosum degeneration may produce memory impairment and slowdown retaining information [8]. Clinical follow-up of our patient after 4 years, showed a minor cognitive impairment, and neuropsychology demonstrated impairment of verbal memory, and difficulties in retaining new information. Therefore, we suggest clinical follow-up of cases having metronidazole neurotoxicity, as there may be long impairment, similarly to our case.
Inferior olivary hypertrophy in metronidazole toxicity has only been described previously in 2 cases [9,10]. In these, inferior olivary hypertrophy is suggested to be secondary to lesions involving the midbrain and cerebellum (interruption of the triangle of Guillain Mollaret), rather resulting from direct metronidazole neurotoxicity. In our case, both the central tegmental tract and the superior cerebellar peduncles were involved, and olivary hypertrophy occurred rapidly. Follow-up MRI two months after metronidazol withdrawal showed improvement of all lesions, except for the inferior olivary nucleus, which became hypertrophic. The findings in our case thus seem to support the view that olivary hypertrophy is secondary to disruption of the Guillain Mollaret triangle caused by metronidazole neurotoxicity, even if this is reversible [11].
The Guillain Mollaret triangle connects the red nucleus, via the central tegmental tract, to the inferior olivary nucleus, via the inferior cerebellar peduncle, to the contralateral DN, back to the red nucleus, via the superior cerebellar peduncle. When both the central tegmental tract and superior cerebellar peduncle are involved, bilateral hypertrophic olivary degeneration can occur. On pathology, gliosis appears 15-20 months after the primary lesion. Clinically, olivary hypertrophic degeneration may produce palatal myoclonus [10], which was nevertheless absent in our patient Hypertrophic olivary degeneration may last for almost three years after the initial presentation [12].
In the case described here, the combination of clinical and radiological signs reported were suggestive of metronidazole neurotoxicity, but the differential diagnosis should include other neurological conditions, especially Wernicke’s encephalopathy [13]. Classical MRI features in Wernicke’s encephalopathy consist on bilateral, symmetric T2 hyperintensities involving the mammillary bodies, and other structures, such as the medial thalamus, the floor of the third and fourth ventricles, the periaqueductal grey matter, midbrain tectum, and less commonly the pericentral cortex and superior cerebellar vermis [14]. The DN can also be affected in cases of malnourished Wernicke’s encephalopathy, thus suggesting that vitamin B1 antagonism may play a role in the neurotoxicity associated to metronidazole [13]. Moreover, bilateral DN involvement has also been reported in cases of methyl bromide intoxication [15], metabolic conditions such as maple syrup urine disease [16] and enteroviral and other viral encephalomyelitis [17-19].
The pathophysiology of the brain changes related to imidazole toxicity is unclear, but similar clinical and radiological findings as metronidazole have been described in others 5-nitroimidazole drugs, such as tinidazole, secnidazole and ornidazole [20,21].
CONCLUSION
Differential diagnosis of a subacute progressive ataxia in adults should include drug intoxications/toxicity, among others. Metronidazole neurotoxicity should be considered in patients undergoing metronidazole treatment presenting characteristic MRI findings, especially in the DN and SCC, similarly to our patient. Rapid olivary hypertrophy may appear secondary to metronidazole neurotoxicity. Even though this entity is reversible, minor cognitive impairment may occur, therefore follow-up of these patients is recommended.
REFERENCES
- Horlen CK, Seifert CF, Malouf CS (2000) Toxic metronidazole-induced MRI changes. Ann Pharmacother 34: 1273-1275.
- Patel K, Green-Hopkins I, Lu S, Tunkel AR (2008) Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 12: 111-114.
- Kuriyama A, Jackson JL, Doi A, Kamiya T (2011) Metronidazole-induced central nervous system toxicity: a systematic review. Clin Neuropharmacol 34: 241-247.
- Park KI, Chung JM, Kim JY (2011) Metronidazole neurotoxicity: sequential neuroaxis involvement. Neurol India 59: 104-107.
- Kalia V, Vibhuti, Saggar K (2010) Case report: MRI of the brain in metronidazole toxicity. Indian J Radiol Imaging 20: 195-197.
- Kim E, Na DG, Kim EY, Kim JH, Son KR, et al. (2007) MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol 28: 1652-1658.
- McKinney AM, Kieffer SA, Paylor RT, SantaCruz KS, Kendi A, et al. (2009) Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am J Roentgenol 193: 192-206.
- Erickson RL, Paul LK, Brown WS (2014) Verbal learning and memory in agenesis of the corpus callosum. Neuropsychologia 60: 121-130.
- Cazals X, Omoumi P, Agnard P, Bibi R, Ferquel C, et al. (2010) [Reversible metronidazole-induced encephalopathy and hypertrophic olivary degeneration]. J Radiol 91: 304-306.
- Seok JI, Yi H, Song YM, Lee WY (2003) Metronidazole induced encephalopathy and inferior olivary hypertrophy: Lesion analysis with diffusion weighted imaging and apparent diffusion coefficient maps. Arch Neurol 60: 1796-800.
- Choh NA, Choh SA, Jehangir M (2009) Hypertrophic olivary degeneration: the forgotten triangle of Guillain and Mollaret. Neurol India 57: 507-509.
- Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, et al. (2000) Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol 21: 1073-1077.
- Zuccoli G, Pipitone N, Santa Cruz D (2008) Metronidazole-induced and Wernicke encephalopathy: two different entities sharing the same metabolic pathway? AJNR Am J Neuroradiol 29: 84.
- Bae SJ, Lee HK, Lee JH, Choi CG, Suh DC (2001) Wernicke’s encephalopathy: atypical manifestation at MR imaging. AJNR Am J Neuroradiol 22: 1480-1482.
- Suwanlaong K, Phanthumchinda K (2008) Neurological manifestation of methyl bromide intoxication. J Med Assoc Thai 91: 421-426.
- Steinlin M, Blaser S, Boltshauser E (1998) Cerebellar involvement in metabolic disorders: a pattern-recognition approach. Neuroradiology 40: 347-354.
- Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T (2006) Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. AJNR Am J Neuroradiol 27: 1983-1986.
- Shen WC, Chiu HH, Chow KC, Tsai CH (1999) MR imaging findings of enteroviral encephaloymelitis: an outbreak in Taiwan. AJNR Am J Neuroradiol 20: 1889-1895.
- Hagemann G, Mentzel HJ, Weisser H, Kunze A, Terborg C (2006) Multiple reversible MR signal changes caused by Epstein-Barr virus encephalitis. AJNR Am J Neuroradiol 27: 1447-1449.
- Chacko J, Pramod K, Sinha S, Saini J, Mahadevan A, et al. (2011) Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: Insights into possible pathogenesis. Neurol India 59: 743-747.
- Taskapilioglu O, Seferoglu M, Kaygili E, Hakyemez B, Zarifoglu M (2010) Reversible cerebellar toxicity during treatment with ornidazole: the first case report. J Neurol Neurosurg Psychiatry 81: 349-350.
Citation: Granell-Moreno E, Díaz-Manera J, Rojas-García R, Gómez-Ansón B (2015) Rapid Inferior Olivary Hypertrophy Secondary to Reversible Metronidazole Neurotoxicity. J Clin Stud Med Case Rep 2: 019.
Copyright: © 2015 Granell-Moreno E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

