
Polishing of Water Contaminated with Diclofenac or Antimony using the Clinoptilolite Tuff
*Corresponding Author(s):
Eva ChmielewskáFaculty Of Natural Sciences, Comenius University, Bratislava, Slovakia
Tel:+421 260296410,
Email:chmielewska@fns.uniba.sk
Abstract
Environmental requirements are becoming of great importance in today´s society, since there is an increased interest in the industrial use of renewable resources. The main objective of this contribution was to provide some literature review of state-of-the-art and the future prospects of green synthesis, with special emphasis on membrane based processing as one part and on another with pharmaceuticals as emerging contaminants in environment. Both parts are completed briefly with laboratory results dealing with antimony removal onto various Fe-oxihydroxides and FeO(OH) covered clinoptilolite tuff and diclofenac uptake onto carbon-rich adsorption materials incl. zeolites. Clinoptilolite tuff occurs in Slovakia in huge deposits and as natural resource is considered for economic accessible adsorption material, potentially useful also for water polishing. Based on the preliminary results, diclofenac adsorption proceeds with the highest efficiency especially onto various carbon-rich materials, incl. onto zeolite (commercial product KlinoCarb), however antimony removal onto FeO(OH)-zeolite could be considered for competitive especially in treatment of large volumes of highly acidic mine waters.
Keywords
MEMBRANE BASED TECHNOLOGIES UNDER PROGRESSIVE DEVELOPMENT
Also, surface or immobilized membranes of industrial adsorbents are considered to be relevant for fulfilling all above functions by pollutants removal. Here, a membrane could be defined as a thin sheet of natural or synthetic material that is permeable to substances in solution or any thin, flexible layer or material designed to separate, filter, purify, treat, etc., Thin-film composite membranes are semipermeable membranes manufactured principally for use in water purification or water desalination systems. This membrane can be considered as a molecular sieve constructed in the form of a film from two or more layered materials. Other materials, usually zeolites, are also used in the manufacture of such thin-film membranes. Zeolites are basically crystalline solids and their structure is made from silicon, aluminium and oxygen atoms, that form a characteristic framework with cavities and channels inside, where cations, water and/or small molecules may reside. According to a new definition, zeolites are clathrates or inclusion compounds, able to host various guest substances in their versatile structure. The most industrially used natural zeolite is clinoptilolite (here indicated as clinoptilolite tuff) [3,7].
Metal oxides such as iron oxide, titanium dioxide and alumina are effective, low cost materials for heavy metals and radionuclides removal as well as pathogen detection. Their cleanup process of pollutants removal is mainly controlled by complexation. When their particle size is reduced to below 20 nm, the specific surface area of normalized adsorption capacity increased 10-100 times, suggesting a "nanoscale effect". They may be combined with other carriers, like in presented paper with zeolite (clinoptilolite tuff), to avoid technological disadvantages of their nano-scale form. Current immobilization techniques usually result in significant loss of treatment efficiency. Therefore, research is needed to develop simple, low-cost methods to immobilize nanomaterial without significantly impacting its performance. Nevertheless, to overcome a potential human risk from environmental spreading, nanomaterials need to be embedded in a solid matrix, respectively, to have minimum release until they are disposed of [8-11].
Electrospinning is another simple, efficient and inexpensive way to make ultra fine fibers using various materials like polymers, ceramics or even metals, that may be incorporated as above nano-oxides onto porous support or membrane. However, mainly nano-zeolites have been the most frequently used dopants in 250 nm-thin film nanocomposite membranes since several years and have shown potential in enhancing membrane permeability. Moreover, nano Ag usually incorporated into zeolite matrix is currently the most widely used antimicrobial nanomaterial damaging proteins and suppressing DNA replication, which possess low human toxicity and a broad antimicrobial spectrum [1-3]. Certain emerging membrane based technologies, such as nanocomposite membranes, show substantial promise for energy reduction and have been recently commercialized. When zeolite nanoparticles were embedded within the polyamide active layer, the water permeability accross such as membrane was enhanced. Also, mimic aquaporin membranes constructed onto porous support were considered to be 100 times more permeable than commercial reverse osmosis membrane [2-5].
Membranes incorporating carbon nanotubes have been found to be promising candidates for water desalination, as the size and uniformity of the tubes can achieve the desired salt rejection. They have shown higher efficiency than activated carbon on removal of various organic chemicals due to diversed contaminant interactions, larger surface area and porous system. While nanomaterials embedded in a solid matrix may expect minimum release into the environment, research is also needed to develop simple, low cost methods to ensure their integrity with the existing infrastructure either as slurry reactor or in form of pellets/beads fixed adsorber [1-4].
Also, at last but not least, has to be mentioned the development of wastewater treatment systems using oyster shells as the biological growth media that prove enhanced affinity to microorganisms or for even trace concentrations of emerging contaminants in water, development of biomimetic materials imitating lipids from white whales, arctic wolves, South African fur seals, marketable fish and mussels, based on high accumulation affinity of organism´s lipids especially towards polyaromatic substances [10-13].
One of the most fascinating examples of microbial synthesis of nanostructures is the biomineralization of magnetosomes (magnetic nanoparticles) by magnetotactic bacteria. Magnetosomes consist of a magnetic mineral crystal magnetite Fe3O4 or greigite Fe3S4 and were identified in fossilized sediments or rocks. To date about 60 biomineral deposits were identified on the Earth, developed by such a biomineralization process (e.g., silica, calcium carbonates, sulfates, calcium phosphates, etc.). When iron is stored as a nanoparticle of iron oxide (ferrihydrite) inside the protein cage ferritin, it is completely sequestered and rendered inert. Thus the encapsulation and sequestration of the iron oxide nanoparticle in biological systems highlights its tremendous potential for use as a synthesis platform for material design. From understanding direct biomineralization in ferritin, scientists developed a model for surface-induced metal oxide formation and have used this as a guiding principle for the synthesis of metal oxide nano-particles in other natural or engineered architectures [5,7,14-25].
Materials designed using components derived from biological sources such as collagen, chitosan, three-dimensional polymeric hydrogels like surfactants, alginate, plant proteins and polysaccharides have also been investigated thoroughly for use in environmental remediation. These biomaterials possess some advantages over their synthetic counterparts, such as their capability to be environmentally viable and thus recognized by the living microenvironment. Since the beginning of 21st century, several types of hydrogels with excellent mechano-chemical properties have been developed through applying different synthesis routes, while especially biomimetic sol-gel strategy presents the most frequently one [14-16]. Using mostly biomimetic sol-gel method, we also prepared the octadecylammonium (surfactant) coated zeolite, chitosan and alginate composed zeolites as well as lately iron oxihydroxide immobilized zeolite, which showed improved adsorption properties to broaden range of pollutants [20].
Another progress in the new synthesis development presents simultaneously cavitational and microwave processing. Cavitational reactors based on the use of sound energy or the energy associated with the fluid flow, offer immense potential for process intensification. The driving mechanism in the case of cavitational reactors is the generation of cavities based on alterations of pressure followed by growth and collapse of the cavities, while releasing a significant amount of the accumulated energy during the growth phase [26]. Due to significant energy release over a very small active area, it is expected that conditions of very high pressures and temperatures (a few thousand atmospheres and a few thousand degrees) are generated locally by producing free hydroxyl radicals with a strong oxidizing power, while simultaneously avoiding agglomeration. Also, microwave processing for various chemical transformations is more efficient than conventional heating. Solvents like water, methanol or acetone with high dielectric constants are easily heated with microwaves by providing the necessary driving force for mass transfer. Above described systems employ lower capital and operating costs and higher throughput as well [22,23,26].
Antimony removal onto ferrihydrite coated zeolite (experimental results)
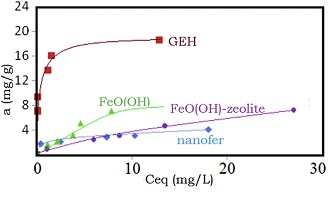 Figure 1: Adsorption isotherms for aqueous Sb species uptake on the GEH, FeO(OH), FeO(OH)-zeolite and nanofer (a means adsorption capacity and Ceq equilibrium concentration).
Figure 1: Adsorption isotherms for aqueous Sb species uptake on the GEH, FeO(OH), FeO(OH)-zeolite and nanofer (a means adsorption capacity and Ceq equilibrium concentration).Sb concentration in all water solutions was analysed on AAS instrument ZEEnit 700 in cooperation with Zeocem Company, Bystré (Slovakia). In highly acidified solutions (pH<2.7) antimony was predominantly dissolved in Sb(III) valency as Sb(OH)3, SbO+, Sb(OH)2+ and H3SbO3. A broad variety of physically and chemisorbed Fe species in zeolite rock may serve then in enhanced Sb(OH)3, SbO+, Sb(OH)2+, H3SbO4 and H3SbO3 uptake via intercalation, electrostatic interaction and other sorption processes. It was supposed that considerably part of Fe neutral species as well ionic clusters were probably deposited outside zeolite framework because clinoptilolite structure contains channels with the size of 0.33×0.46 nm; 0.3×0.76 nm and 0.26×0.47 nm not sufficient large for their entrance. The plotting of adsorption isotherms in the system studied clearly confirmed the increasing uptake capacity of the adsorbents with the increased S(BET) data. As aforementioned, the highest surface area possess GEH, then ferrihydrite, followed by FeO(OH) zeolite, whereas the lowest surface area belongs to nanofer [14-16,20].
PHARMACEUTICALS-EMERGING CONTAMINANTS IN ENVIRONMENT
Pharmaceuticals, as today called emerging contaminants, can enter the environment by a number of pathways and can be further distributed to various environmental media. One prominent pathway could be the use of wastewater sludge or waste water for field fertilization and irrigation. In water environments, a large variety of these compounds and their metabolites have been detected and also soil could be an important source of water contamination [12,13,27,28].
The presence and distribution of pharmaceuticals in the soil via land application are far from known because of a lack of appropriate methodologies. Liquid Chromatography combined with Mass Spectrometry (LC-MS) or with tandem Mass Spectrometry (LC-MS/MS) is popular techniques currently being used in pharmaceutical analyses. The latter allows detection of extremely low concentrations (ng/L or ng/g) of these compounds in various complex liquid or solid matrices. The presence of emerging contaminants in the environment is mainly attributed to the discharge of treated wastewater from water treatment facilities. Conventional secondary processes (activated sludge and trickling filters) represent the most extensively used and studied processes. An increase of drugs in surface waters may be anticipated during music festivals, public holidays, major sporting events and by students during exam periods [12,13,27,28]. Unfortunatelly, above mentioned bioprocesses are not designed to remove emerging contaminants thoroughly resulting in their discharge to receiving surface waters including rivers, lakes and coastal discharge. Moreover, during the anaerobic digestion, biosolids (or treated sludge) is generated that is often applied to agricultural land as a fertiliser in many countries. Despite lengthy digestion (in avarage 4 weeks) and outdoor storage for up to six months following treatment, some emerging contaminants have shown to persist. The presence of these chemicals in the environment is more serious considering that they do not appear individually, but as a complex mixture, which could lead to unwanted synergistic effects. Therefore a tertiary adsorption process mostly onto active coke used to be proposed for water polishing [12,13,27,28].
Parent chemicals are often excreted from the human body also with a number of associated metabolites. As an example, the ibuprofen is excreted as the unchanged drug. Approximately 70 pharmaceuticals, belonging to a variety of therapeutic classes, have been reported only in UK waters [12]. The analgestic tramadol has been observed in river water at the highest concentration up to a maximum of 7731 ng/L [12,29]. The hallucinogen 3,4-Methylenedioxy-N-Methyl-Amphetamine (MDMA) and the stimulant cocaine have been observed in river water at concentrations of 25 and 17 ng/L, respectively [12,30-32]. To date more than 200 different pharmaceuticals alone have been reported in river waters globally, with concentrations up to a maximum of 6.5 mg/L for the antibiotic ciprofloxacin [12,29,33].
Methamphetamine (Figure 2, locally called pervitin) is an extremely addictive stimulant drug that is chemically similar to amphetamine [34]. It takes the form of a white, odorless, bitter-tasting crystalline powder. Methamphetamine is taken orally, smoked, snorted, or dissolved in water or alcohol and injected. Smoking or injecting the drug delivers it very quickly to the brain, where it produces an immediate, intense euphoria. According to monitoring provided by the national water authorities and researchers, consumption of this chemical in Czech and Slovak Republics belongs to the highest in Europe [34-36].
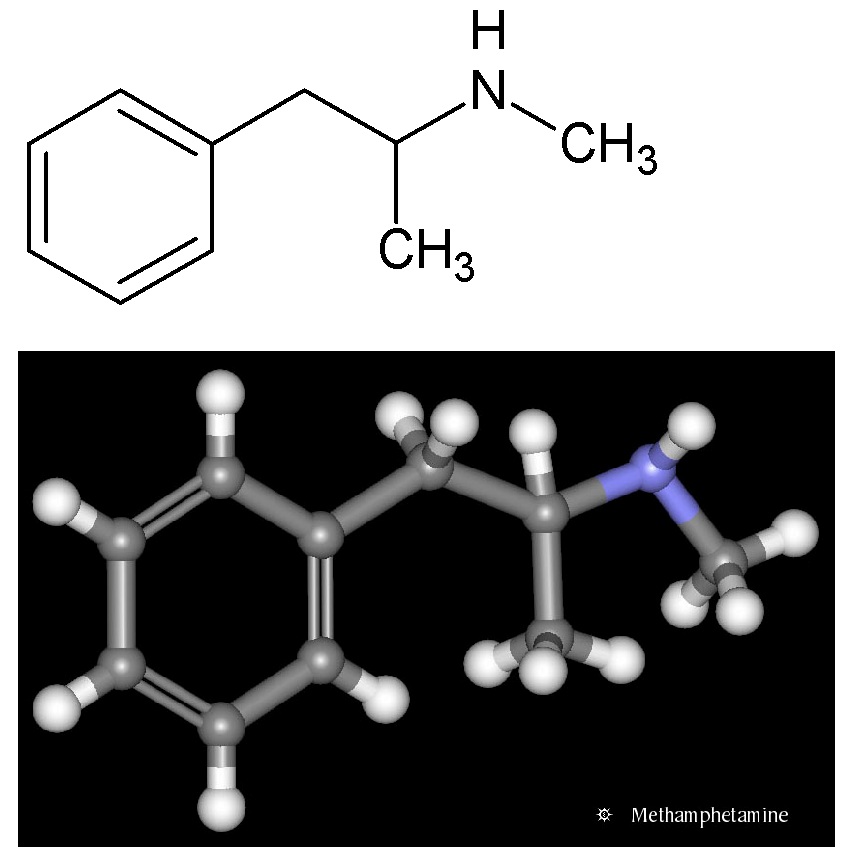 Figure 2: Structural formula of methamphetamine.
Figure 2: Structural formula of methamphetamine.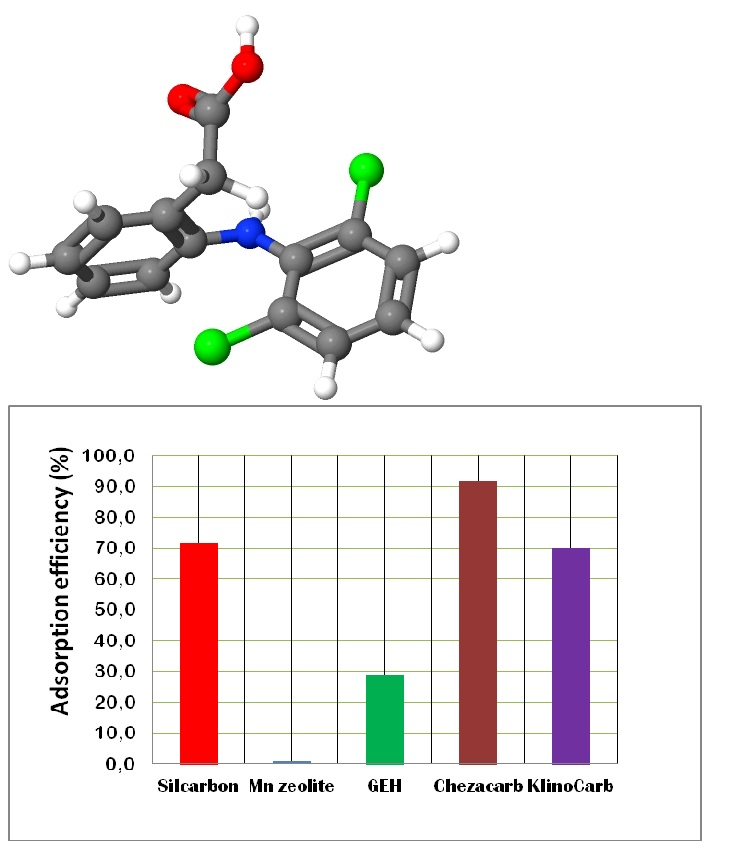
Measurements of diclofenac in aqueous solutions were performed on an Agilent Technologies 1200 Series Liquid Chromatography, in conjunction with an automatic dispenser and a DAD detector. The ZorbaxSB-C18 Chromatographic Column 3.5 μm, 150x2.1 mm was used for the analysis. Diclofenac was detected at 278 nm and quantified by the calibration curve method at a concentration range of 0.45-450 mg/L.
As the figure 3b displays, the highest adsorption efficiency towards diclofenac exhibits Chezacarb, Silcarbon and KlinoCarb, ordered according to their adsorption capacity data. So called magnetic adsorbents like GEH and Mn-zeolite show considerably lower performance.
We observed approximately 10% difference between Silcarbon and KlinoCarb capacities in diclofenac uptake and found out that 80% of natural powdered zeolite (clinoptilolite tuff) was added into activated carbon (20%) and mixed to get KlinoCarb product currently selling by Zeocem Company.
Since the natural zeolite possess any affinity to hydrophobic and anionic species due to its net negative charge and presence of exchangable cations (Ca2+, Mg2+, Na+ and K+) at the hydrophilic surface, it was confirmed that neither natural zeolite nor MnOx coated zeolite (Mn-zeolite) were effective in diclofenac adsorption (data for natural zeolite are not shown). Nevertheless, pure zeolite (clinoptilolite tuff) was the most effective in diclofenac adsorption among the examined modifications like ODA-, carbonized, FeO(OH)- or Mn-zeolite (also not recorded). We may conclude following: Based on our preliminary results, diclofenac adsorption proceeds with the highest efficiency especially onto various carbon-rich materials and commercial activated carbon (Silcarbon). In the literature, there are published many procedures how to produce low cost carbon-rich adsorbents, using even plenty waste products from agriculture, chemical industry or communal branche [37-40]. In our case, industrial ashes Chezacarb as waste product from Chemopetrol Litvínov (Czech Republic) was considered for the most effective in diclofenac removal. Above discussed research and diclofenac removal onto various adsorption materials is currently under further investigation.
CONCLUSION
Metal oxides such as iron oxide are natural, low cost adsorbents for aqueous pollutants removal, however their nanoscale counterparts with higher specific surface area must be usually compressed into porous pellets or to be impregnated onto some carriers (like zeolite) to achieve better filtration performance. Therefore, we prepared and at laboratory examined antimony removal onto iron oxide nano-particles embedded on the Slovakian clinoptilolite tuff which based on its nano-porous structure worked as a nanoreactor.
Main advantages of such a synthesized FeO(OH)-zeolite might be the relatively low capital cost and therefore applicability to a large volume of waters, especially to highly acidic mine waters. Domestic zeolite is available in the local market for the price which is approximatelly 100 times lower that the price of commercial iron oxihydroxide (GEH product).
Based on our preliminary results, diclofenac adsorption proceeds with the highest efficiency especially onto various carbon-rich materials and commercial activated carbon (Silcarbon). Natural zeolite or some zeolite modified samples did not prove more or less any afinity towards diclofenac except commercial product of Zeocem Company KlinoCarb.
ACKNOWLEDGEMENT
This project is supported by the APVV agency of Slovakia under the code: SK-SRB-2015-0001-Zeolite-based adsorbents for environmental remediation as well as VEGA grant under the code: 1/0039/18-Magnetic nanocomposites and biomimetics as new generation of multifunctional nanoproducts. The authors thank also for financial support of the projects encoded as ITMS 26240220007 and ITMS 26240220086.
REFERENCES
- Qu X, Alvarez PJJ, Li Q (2013) Application of nanotechnology in water and wastewater treatment. Water Research 47: 3931-3946.
- Chmielewská E, Xu F (2015) Functional gradient adsorbents processed with biogenic components for ecologically benign water purification. Current Green Chemistry 2: 362-370.
- Mann S (1996) Biomimetic Materials Chemistry. John Wiley & Sons, Inc, UK.
- Behrens P, Bauerlein E (2007) Handbook of biomineralization; Biomimetic and bioinspire chemistry.Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany.
- Altshuller GS (1999) The innovation algorithm: TRIZ, Systematic innovation and technical creativity. Technical Innovation Center Inc., Worcester, Massachusetts, USA.
- Lehn JM (1995) Supramolecular chemistry: Concepts and perspectives. WCH Service Bureau, New York.
- Favret EA, Fuentes NO (2009) Functional properties of bio-inspired surfaces: Characterization and technological applications. World Scientific Publishing Co Pte Ltd, 5 Toh Tuck Link, Singapore.
- Yang X, Song J, Xu W, Liu X, Lu Y, et al. (2013) Anisotropic sliding of multiple-level biomimetic rice-leaf surfaces on aluminium substrates. Micro & Nano Letters 8: 801-804.
- Jabbari E (2014) Handbook of biomimetics and bioinspiration. World Scientific, Singapore.
- Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Research 72: 3-27.
- Sabourin L, Duenk P, Bonte-Gelok S, Payne M, Lapen DR, et al. (2012) Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci Total Environ 431: 233-236.
- Doula MK (2009) Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Research 43: 3659-3672.
- Jaiswal A, Banerjee S, Mani R, Chattopadhyaya MC (2013) Synthesis, Characterization and application of goethite mineral as an adsorbent. J Environ Chem Eng 1: 281-289.
- Jing-Hua R, Lena QM, Hong-Jie S, Fei C, Jun L (2014) Antimony uptake, translocation and speciation in rice plants exposed to antimo-nite and antimonate. Sci Total Environ 475: 83-89.
- Subramani A, Jacangelo JG (2015) Emerging desalination technologies for water treatment: A critical review. Water 75: 164-187.
- Šiljeg M, Stefanivi? ŠC, Mazaj M, Tušar NN, Ar?on I, et al. (2009) Structure investigation of As(III)- and As(V)-species bound to Fe-modified clinoptilolite tuffs. Microporous Mesoporous Mat 118: 408-415.
- Aredes S, Klein B, Pawlik M (2012) The removal of arsenic from water using natural iron oxide minerals. Journal of Cleaner Production 29-30: 208-213.
- Chmielewská E, Tylus W, Drábik M, Majzlan J, Krav?ak J, et al. (2017) Structure investigation of nano-FeO(OH) modified clinoptilolite tuff for antimony removal. Microporou Mesoporous Materials 248: 222-233.
- Kim ES, Hwang G, El-Din MG, Liu Y (2012) Development of nanosilver and multiwalled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J Membrane Sci 394-395:: 37 -48.
- Savage N, Diallo MS (2005) Nanomaterials and water purification: Opportunities and challenges. J Nanopart Res 7: 331-342.
- Quang DV, Pradi B, Sarawade SJ (2013) Effective water disinfection using silver nanoparticle containing silica beads. Appl Surf Sci 287: 84-90.
- Watkins R, Weiss D, Dubbin W, Peel K, Coles B, et al. (2006) Investigations into the kinetics and thermodynamics of Sb(III) adsorption on goethite (alpha-FeOOH). J Colloid Interface Sci 303: 639-646.
- Yang X, Song J, Xu W, Liu X, Lu Y, et al. (2013) Anisotropic sliding of multiple-level biomimetic rice-leaf surfaces on aluminium substrates. IET Micro& Nano Letters 8: 801-804.
- Stefanidis G, Stankiewicz A (2013) Alternative energy sources for green chemistry. The Royal Society of Chemistry, New York, USA.
- Ort C, Lawrence MG, Reungoat J, Mueller JF (2010) Sampling for PPCPs in wastewater systems: Comparison of different sampling modes and optimization strategies. Environ Sci Technol 44: 6289-6296.
- Miao XS, Yang JJ, Metcalfe CD (2005) Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ Sci Technol 39: 7469-7475.
- Heal DJ, Smith SL, Gosden J, Nutt DJ (2013) Amphetamine, past and present-a pharmacological and clinical perspective. J Psychophar-macol 27: 479-496.
- Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43: 9216-9222.
- European Chemicals Bureau (2003) Technical guidance document on risk assessment. European Commission, Dublin, UK.
- European Protection Agency (2012) Nanomaterial case study: Nanoscale silver in disinfectant spray. European Protection Agency, Washington, DC., USA.
- Gehrke I, Geiser A, Somborn-Schulz A (2015) Innovations in nanotechnology for water treatment. Nanotechnol Sci Appl 8: 1-17.
- Macku?ak T, Jaroslav Škubák, Roman Grabic, Jozef Ryba, Lucia Birošová, et al. (2014) National study of illicit drug use in Slovakia based on wastewater analysis. Sci Total Environment 494: 158-165.
- Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, et al. (2002) Removal of pharmaceuticals during drinking water treatment. Envron Sci Tlechnol 36: 3855-3863.
- Tome?ková V, Reháková M, Mojžišová G, Wadsten T, Zele?áková K, et al. (2016) Spectral study of modified natural clinoptilolite with pharmacologically active escin and horse chestnut extract. Spectroscopy Letters 49: 63-72.
- Danmaliki GI, Saleh TA (2016) Influence of conversion parameters of waste tires to activated carbon on adsorption of dibenzothiophene from model fuels. Journal of Cleaner Production 117: 50-55.
- Saleh TA, Gupta KV (2016) Nanomaterial and polyme membranes: Synthesis, characterization and applications, Elsevier, Amsterdam, Netherlands.
- Solmin J, Nam SH, E JK, S YO, Hyun UL, et al. (2016) Feasibility test of waste oyster shell powder for water treatment. Process Safety and Environmental Protection 102: 129-139.
- Martucci A, Pasti L, Marcetti N, Cavazzini A, Dondi F, et al. (2012) Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microp Mesoporous Mat 148: 174-183.
Citation: Chmielewská E, Górová R, Hawash HBI (2018) Polishing of Water Contaminated with Diclofenac or Antimony using the Clinoptilolite Tuff. J Environ Sci Curr Res1: 001.
Copyright: © 2018 Eva Chmielewská, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

