
Journal of Environmental Science Current Research Category: Environmental science
Type: Review Article
Surface Modified Zeolite and other Nanogeocomposites: An Effective and Sustainable Natural Resources for the Removal of Pollutants
*Corresponding Author(s):
Eva ChmielewskáFaculty Of Natural Sciences, Comenius University, Bratislava, Slovakia
Tel:+421 260296410,
Email:chmielewska@fns.uniba.sk
Received Date: Jun 06, 2019
Accepted Date: Jul 15, 2019
Published Date: Jul 24, 2019
Abstract
Considering the proactive approach to green agenda and sustainable development goals the use of natural resources instead of non-environmentally friendly processed adsorbents is one of the way how to save environment as well as not to widespread industrial pollution. EU Directive 2013/39/EU recommends to monitor in waters already 45 priority compounds incl. pharmaceuticals. Among the traditional geomaterials used also in biomedicine as pharmaceutical excipients or nanotransporters (nanobots) occurs lately also clinoptilolite-rich tuff with hydrophobized external surface, as potential representant of tectosilicates, able effectively remove much broader range of environmental pollutants than some other natural or commercial adsorbents. This review deals with some aspects of water pollutants (cefazoline, acid red, cresol and decane) removal using the clinoptilolite-rich tuff and some other perspective natural resources regarding to conventional adsorbents.
Keywords
Adsorption based technologies; Biomimetics; Emerging contaminants; Nanogeocomposites; Zeolite
NATURAL RESOURCES OR PRODUCTS OF NATURE HELP TO SAVE ENVIRONMENT
Inspiration from nature has been widely used in the development of new materials and in the improvement of their properties since long time. Environmental requirements are becoming increasingly important in today's society, since there is an increased interest in the industrial use of renewable resources. Simultaneously, it is believed that nature's pattern may indicate that in the near future the synthesis development of traditional adsorption materials might change. E.g., using the hydrophilic, expandable and permeable hydrogels with low interfacial tension for the novel hybridized adsorbent synthesis, substances which resemble to soft living tissues, is an excellent example for advanced surface treatment strategy to broaden the native zeolite properties for a wide variety of applications [1-2].
Zeolites occurring in the nature are highly porous aluminosilicates of volcanic origin. Zeolite rock is basically composed of active component, i.e., industrially most frequently used clinoptilolite and some other geological minerals, like volcanic glass, feldspar, quartz and clays, which may reach up to 30%. Their structures consist of a three-dimensional framework, having a negatively charged lattice. The negative charge is balanced by cations which are exchangeable with certain cations in solutions. Zeolites consist of a wide variety of species, i.e. more than 80 natural species have been identified till today. However, the most abundant and frequently studied zeolite is clinoptilolite, a mineral of the heulandite group. Its characteristic tabular morphology shows an open reticular structure of easy access, formed by open channels of 8-10 member rings (Figures1,2). High ion-exchange capacity and relatively high specific surface area, and more importantly their relatively low prices, make zeolites attractive adsorbents. Their price is about US$ 0,03-0,12/kg, depending on the quality of the mineral [1-4]. Another advantage of zeolites over synthetic resins is their ion selectivity’s generated by their rigid porous structures. Zeolites are becoming widely used as alternative materials in areas where adsorptive applications and thermal and radiation resistance are required. They have been intensively studied for several decades, because of their applicability in removing trace quantities of pollutants such as heavy metal ions, ammonium, phosphates, azodyes, some pesticides or pharmaceuticals and other emerging pollutants [4].
Zeolites occurring in the nature are highly porous aluminosilicates of volcanic origin. Zeolite rock is basically composed of active component, i.e., industrially most frequently used clinoptilolite and some other geological minerals, like volcanic glass, feldspar, quartz and clays, which may reach up to 30%. Their structures consist of a three-dimensional framework, having a negatively charged lattice. The negative charge is balanced by cations which are exchangeable with certain cations in solutions. Zeolites consist of a wide variety of species, i.e. more than 80 natural species have been identified till today. However, the most abundant and frequently studied zeolite is clinoptilolite, a mineral of the heulandite group. Its characteristic tabular morphology shows an open reticular structure of easy access, formed by open channels of 8-10 member rings (Figures1,2). High ion-exchange capacity and relatively high specific surface area, and more importantly their relatively low prices, make zeolites attractive adsorbents. Their price is about US$ 0,03-0,12/kg, depending on the quality of the mineral [1-4]. Another advantage of zeolites over synthetic resins is their ion selectivity’s generated by their rigid porous structures. Zeolites are becoming widely used as alternative materials in areas where adsorptive applications and thermal and radiation resistance are required. They have been intensively studied for several decades, because of their applicability in removing trace quantities of pollutants such as heavy metal ions, ammonium, phosphates, azodyes, some pesticides or pharmaceuticals and other emerging pollutants [4].
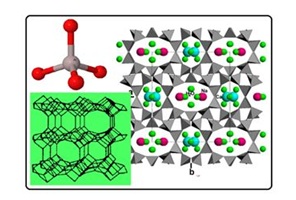
Figure 1: Structural characteristics of zeolite (clinoptilolite type) with its basic tetrahedral unit of SiO44-or AlO45-in upper left.

Figure 2: SEM micrograph of raw clinoptilolite-rich tuff from Nižný Hrabovec (East Slovakia), magnification 3700 X, SEM of clinoptilolite-rich tuff hydrophobized with octadecyl ammonium surfactant (ODA), AFM of ODA-clinoptilolite-rich tuff, STM of ODA- clinoptilolite-rich tuff (from the left to the right).
Several types of hydrogels with excellent mechano-chemical properties have been developed through applying different synthesis routes (biomimetic sol-gel strategy) using the layer by layer (LbL) self assembling of hydrocarbons chains to build surfactant hydrophobic mono - or bilayers on the surfaces of various solids. Various aspects regarding inland octadecylammonium ODA - zeolite adsorbents have been examined several years ago, too, however due to the hydrocarbons degradation on the sunlight caused by photolysis, they were recommended preferentially for underground barriers construction. Especially, Bowman school in USA iniciated synthesis and investigation of hydrophobized grain-sized zeolites so far in pilot measure, due to its superior hydrodynamic properties [1-4].
Combined zeolite filters, on one site in natural form with a high selectivity toward ammonium and phosphate and on another in hydrophobized form, may control also pesticides, polyaromatic hydrocarbons and other emerging pollutants of waters and thus become in water treatment more effective [5-10]. EU Directive 2013/39/EU recommends to monitor in waters already 45 priority compounds incl. pharmaceuticals. Wastewater treatment with activated sludge process, depending on its age and other parameters, may partially decrease the pharmaceuticals concentration in water, but not thoroughly. Membrane processes, based on high solubility of pharmaceuticals in waters, are not appropriate for their removal and therefore some German plants prefer ozonization with activated carbon adsorption. Also, despite of some water contamination with microplastics, this task is in Europe not classified as acute till today, however, it is necessary to deal with this subject and propose the removal and analytical methods for microplastics already. Based upon the problem with plastics and food chain intoxication known for several years, especially in sea fauna, attention was focused also on contamination of gastro-intestinal tract of humans. In 10 g of faecal discharge several tens of microplastics were found [11-13].
SURFACTANT COATED ZEOLITE WITH ENHANCED ADSORPTION POTENTIAL
Generally, the fabrication of surfactant modified zeolite requires the contacting of solid material with the template (surfactant) solution, mechanical shaking for several hours, followed by removal of the supernatant, centrifugation or filtration and finally air drying. This sol-gel process is suitable wet route to the synthesis of hydrophobized, i.e., surfactant couted zeolite (mostly clinoptilolite-rich tuff used to be selected for this treatment). The process is based on the condensation of molecular precursors in solution, originating a „sol“, while further condensation leads to the final wet „gel“. Because these reactions are performed at room temperature, further heat treatment is needed to acquire the final hydrophobization product.
The main parameters that influence the kinetics, growth reactions, hydrolysis, condensation reactions and consequently, the structure and properties of the gel are solvent, temperature, nature, concentration of the salt precursors employed, pH and agitation. In principle, the negative charged clinoptilolite framework use to be easy modified via the addition of positive charged mostly quaternary ammonium surfactant in the above hydrophobization process.
Long-chain quaternary ammonium salts on clay minerals have been applied already to remove petrochemical spills and to protect ground-water contaminant migration by construction of subsurface impermeable barriers since several decades. In contrast to the clays, the surface of which has been so far coated by surfactant modifiers, natural zeolitic minerals modified with hydrophobic long-chain amines present a fairly new potential in water protection, especially due to their superior hydraulic characteristics, that are by clays missing. The presence of surfactants, usually quaternary ammonium salts like hexadecyltrimethyl-ammonium chloride (HDTMA), cetylpyridinium chloride (CPC) and the others, at the solid zeolitic surface can enhance adsorption of different nonpolar organic molecules and/or anions. Since the modifications of the zeolites with surfactants take place only at the outer surface of the crystallites, therefore, the external cation exchange capacity of the zeolite is an important parameter in the synthesis of organozeolites. Cationic surfactants exchange only native inorganic cations at the external surfaces of the zeolites, forming relatively stable organic coating on their surface. Depending on the amount of surfactant at the zeolitic surface, this modification drastically alters the surface chemistry of zeolites, enhancing adsorption of anions and cations as well as removal of organic nonpolar solutes through partitioning into the hydrophobic surfactant tail core [1,2,4].
In our research, octadecyl ammonium (ODA) surfactant was selected for this treatment as inland economically accessible product, applying with a long tradition for domestic bentonite hydrophobization. This surfactant consists of the long straight of hydrocarbon chains (18C) with the polar ammonium head at the one end of their tails [1-4]. To establish the most efficient surfactant counterion uptake by ODA-clinoptilolite, a batch sorption procedure using various initial ODA concentrations (all greater than the critical micelle concentration - CMC) and the same weight/volume ratios, i.e. 20 g of raw clinoptilolite rich-tuff and 100 mL of different ODA aqueous solutions (0.001, 0.01, 0.1, 0.2 mol/L) was selected. The suspensions were stirred using the MLW ER 10 lab assembly for 24 h at 60°C in glass containers. This stirring period was considered to be sufficient to achieve the equilibrium. The pH value of the solutions were kept at around 3.0 by the addition of 98% acetic acid. Equilibrated mixtures were then paper-filtered on the warmed Buchner funnel to yield a clear supernatant solution and ODA-modified clinoptilolite-rich tuff. Zeolitic samples were thereafter rinsed with deionized (D.I.) water and allowed to dry in Premed KBC laboratory dryer at 60°C. Finally, the samples were ground by mortar and pestle. The adsorption experiments with that ODA-modified clinoptilolite-rich tuff was performed, which was prepared using the procedure of 0.2 mol/L initial ODA concentration, because this concentration proved the most efficient counterion uptake [4,9,17].
Photomicrographs of the raw clinoptilolite-rich tuff in Figure 2 showed well-defined, tabular-shaped crystals with excellent crystal edges. As surface coverage with the polymeric ODA-surfactant occurred, smaller, more agglomerated crystals and more poorly defined crystal edges were observed in about 1-?m scale SEM image. The apparent sharpness of the image decreased with increasing surfactant coverage. Among Scanning Electron Micrograph (SEM), Atomic Force Microscopy image (AFM) as well as Scanning Tunneling Microscopy image (STM) in Figure 2 the clearest imaging of ODA-surfactant tails are visible by AFM technique. Figures 3 and 4 illustrate simultaneously some comparable measurements on cefazoline and acid red removal using the various adsorption products incl. ODA-zeolite. Detailed description of the applied adsorption materials is discussed in next section.
The main parameters that influence the kinetics, growth reactions, hydrolysis, condensation reactions and consequently, the structure and properties of the gel are solvent, temperature, nature, concentration of the salt precursors employed, pH and agitation. In principle, the negative charged clinoptilolite framework use to be easy modified via the addition of positive charged mostly quaternary ammonium surfactant in the above hydrophobization process.
Long-chain quaternary ammonium salts on clay minerals have been applied already to remove petrochemical spills and to protect ground-water contaminant migration by construction of subsurface impermeable barriers since several decades. In contrast to the clays, the surface of which has been so far coated by surfactant modifiers, natural zeolitic minerals modified with hydrophobic long-chain amines present a fairly new potential in water protection, especially due to their superior hydraulic characteristics, that are by clays missing. The presence of surfactants, usually quaternary ammonium salts like hexadecyltrimethyl-ammonium chloride (HDTMA), cetylpyridinium chloride (CPC) and the others, at the solid zeolitic surface can enhance adsorption of different nonpolar organic molecules and/or anions. Since the modifications of the zeolites with surfactants take place only at the outer surface of the crystallites, therefore, the external cation exchange capacity of the zeolite is an important parameter in the synthesis of organozeolites. Cationic surfactants exchange only native inorganic cations at the external surfaces of the zeolites, forming relatively stable organic coating on their surface. Depending on the amount of surfactant at the zeolitic surface, this modification drastically alters the surface chemistry of zeolites, enhancing adsorption of anions and cations as well as removal of organic nonpolar solutes through partitioning into the hydrophobic surfactant tail core [1,2,4].
In our research, octadecyl ammonium (ODA) surfactant was selected for this treatment as inland economically accessible product, applying with a long tradition for domestic bentonite hydrophobization. This surfactant consists of the long straight of hydrocarbon chains (18C) with the polar ammonium head at the one end of their tails [1-4]. To establish the most efficient surfactant counterion uptake by ODA-clinoptilolite, a batch sorption procedure using various initial ODA concentrations (all greater than the critical micelle concentration - CMC) and the same weight/volume ratios, i.e. 20 g of raw clinoptilolite rich-tuff and 100 mL of different ODA aqueous solutions (0.001, 0.01, 0.1, 0.2 mol/L) was selected. The suspensions were stirred using the MLW ER 10 lab assembly for 24 h at 60°C in glass containers. This stirring period was considered to be sufficient to achieve the equilibrium. The pH value of the solutions were kept at around 3.0 by the addition of 98% acetic acid. Equilibrated mixtures were then paper-filtered on the warmed Buchner funnel to yield a clear supernatant solution and ODA-modified clinoptilolite-rich tuff. Zeolitic samples were thereafter rinsed with deionized (D.I.) water and allowed to dry in Premed KBC laboratory dryer at 60°C. Finally, the samples were ground by mortar and pestle. The adsorption experiments with that ODA-modified clinoptilolite-rich tuff was performed, which was prepared using the procedure of 0.2 mol/L initial ODA concentration, because this concentration proved the most efficient counterion uptake [4,9,17].
Photomicrographs of the raw clinoptilolite-rich tuff in Figure 2 showed well-defined, tabular-shaped crystals with excellent crystal edges. As surface coverage with the polymeric ODA-surfactant occurred, smaller, more agglomerated crystals and more poorly defined crystal edges were observed in about 1-?m scale SEM image. The apparent sharpness of the image decreased with increasing surfactant coverage. Among Scanning Electron Micrograph (SEM), Atomic Force Microscopy image (AFM) as well as Scanning Tunneling Microscopy image (STM) in Figure 2 the clearest imaging of ODA-surfactant tails are visible by AFM technique. Figures 3 and 4 illustrate simultaneously some comparable measurements on cefazoline and acid red removal using the various adsorption products incl. ODA-zeolite. Detailed description of the applied adsorption materials is discussed in next section.
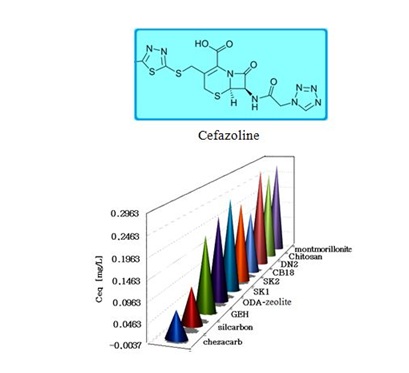 Figure 3: Antibiotic cefazoline removal onto selected materials.
Figure 3: Antibiotic cefazoline removal onto selected materials.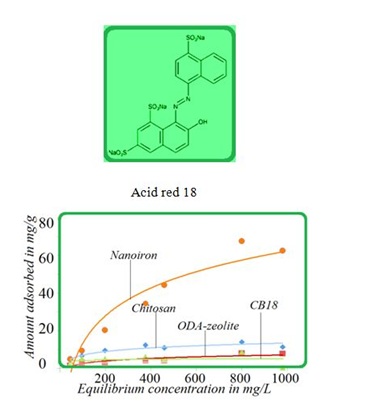 Figure 4: Adsorption isotherms for acid red onto selected adsorption materials at ambient temperature.
Figure 4: Adsorption isotherms for acid red onto selected adsorption materials at ambient temperature.NATURAL OR OTHER NANOGEOCOMPOSITES IN COMPETITION WITH ZEOLITES
Based on the economic feasibility and local availability, natural materials such as chitosan, zeolites, clays, certain industrial or agricultural waste products, i.e. fly ash, low rank coal, lignite, natural metal oxides, peat, sawdust, waste slurry, lignine, shungite, coconut shell charcoal, waste biomass, cork (Quercus suber L.) seem to be perspective and sustainable adsorption materials, especially for water post treatment in the countries characterized as developing economies.
An organo-inorganic composite, termed as a hybrid material too, may be defined hereto as a combination of a polymerous substance immobilized onto surface of the inorganic, e.g. zeolite carrier to avail advantages of both zeolitic and polymerous constituents as well. Accordingly, hybridization can be used to modify organic or inorganic materials and hybrids should therefore be considered as the new generation of composites that may encompass a wide variety of applications [14-19]. The conversion of inorganic ion exchange materials into hybrid fibrous or nanoscale ion exchangers is considered to be the latest development of the discipline. These nanomaterials are drawing a great attention as they exhibit a high efficiency and rate of sorption with short diffusion path toward environmental pollutants [6,7]. Advances in nanoscale science and engineering are providing unprecedent opportunities to develop more cost effective and environmentally acceptable water purification processes, respectively [19-23]. For the water purification, besides the metal-containing nanoparticles, carbonaceous materials and dendrimers, also the zeolites are being evaluated as the most progressive functional and nanosized materials of the millennium.
Shungite is elementary carbon with amorphous structure, although XRD studies confirmed the existence of fragments with graphitic structures of an interlayer distance of 3.4 Å. Recently it has been stated that the mineral has important amount of fullerenes, mainly C60. Shungite rocks have a peculiar structure. The rock contains highly dispersed silicate mineral grains distributed in the shungite carbon matrix. Shungite can replace coke, graphite, carbon black or glassy carbon, and usually serves as a complex raw material in thermal processes. Our sample of shungite originated from the deposit nearby St. Petersburg in Russia [24].
In the last decade, chitin and its deacetylated derivative chitosan, respectively, are appearing as potential biosorbents. Chitosan possesses apropriate mechanical strength, rigidity and settling characteristics, which are a perfect alternative to other adsorbent - biomass, despite fragile structure and poor mechanical strength. Chitin is found in fungi, arthropods and marine invertebrates. It is one of the most abundant, easily obtainable and renewable natural biopolymers (polysaccharides).
The alginite fossil was of domestic origin. Alginite is an ecological raw material, which originates from algae stock deposition (Botryococcus braunii Ktz.) situated at the bottom of a maar lake together with the clay weathering, products washedout from the maar ring and composed of basalt tuffs. This fossil possesses a high humus content, macro- and micronutritive and trace elements with considerable dehydration ability, which supports its utilization as a soil fertilizer, pharmaceutical additive or environmental adsorbent [4].
Metal oxides such as iron oxide, titanium dioxide and alumina are effective, low cost materials for heavy metals and radionuclides removal and pathogen detection as well. Their cleanup process of pollutants removal is mainly controlled by complexation. When their particle size is reduced to below 20 nm, the specific surface area of normalized adsorption capacity increased 10 - 100 times, suggesting a „nanoscale effect“. To overcome a potential human risk from environmental spreading, nanomaterials need to be embedded in a solid matrix, respectively, to have minimum release until they are disposed of [6,7,10,11]. Current immobilization techniques usually result in significant loss of treatment efficiency. Therefore, research is needed to develop simple, low-cost methods to immobilize nanomaterial without significantly impacting its performance. Based upon these facts, we prepared and thoroughly investigated iron and manganese oxides supported clinoptilolite-rich tuff, which due to its nanoporous structure worked as nanoreactor and in such a treated form provided enhanced functionalities. A detailed description of biomimetic FeO(OH) - zeolite sol-gel synthesis was published in paper [17]. It should be pointed, that usually novel and state-of-the art biosynthesis protocols allow simplifying experimental design and applying renewable and ubiquitous biological materials, what minimalizes energy requirements and waste generation. MnOx-zeolite was prepared by simple and inexpensive large-scale redox-synthesis at ambient temperature using the 5% KMnO4 solution. The price of its commercial product (Mn-Klinopur) on the market is 1500 Euro per ton.
As Figures 3,4 illustrate, the selected pollutants, i.e., the azodye (acid red 18) and prophylaxis antibiotic cefazoline by means of ODA-clinoptilolite-rich tuff was examined. Its performance was compared with other on the market accessible adsorption products, e.g. active coke of German provenience silcarbon, industrial ashes chezacarb (amorphous carbon) from Chemopetrol Litvínov (Czech Republic), German Granulated Ferric Hydroxide (GEH) with the main components of akaganeite (ß-FeOOH) and goethite [α-FeO(OH)], montmorillonite from the deposit in Slovak Republic, obtained from the rock after sedimentation and purification procedures and commercial Absodan (Great Britain), mostly natural resources derived and mixed products denoted as SK1, SK2, DN2 and CB18.
The SK1 and SK2 contain the mixture of cellulose, calcium carbonate and clays, while DN2 is manufactured mainly of natural silicate resources, based on the former commercial absodan and CB18 means a high quality peat (Figures 3-6). Peat contains lignin, cellulose, fulvic and humic acids as major constituents that bear polar functional groups, such as alcohols, aldehydes, ketones, phenolic hydroxides and ethers, using them in chemical bonding. To characterize the voluminous pigment molecule from the point of its molecular structure, the well dissociated and polar naphtalene disulfonic acid, i.e. acid red, consists of 3 benzene rings interlinked with characteristic azo - N = N - group. Actually, the azodye in water represents dissolved sodium salts of polyaromatic disulfonic acid. According to Figure 4, the acid red was better adsorbed onto ODA-clinoptilolite-rich tuff than onto peat. Somewhat higher adsorption performance toward acid red proved also biopolymeric chitosan, however the best or even superior adsorption efficiency toward the pigment (azodye) was observed by the Czech commercial product Nanofer 25S or nanoiron. When providing the column arrangement, the GEH product showed a higher efficiency toward acid red than the ODA-clinoptilolite-rich tuff did. Despite to a little higher adsorption capacity of above examined adsorption materials (Figsures 5,6), the adsorption performance of cost effective surfactant modified clinoptilolite-rich tuff was comparable and thus assessed as competitive. Finally, Figures 3,4 summarize clearly competitive position of ODA-clinoptilolite-rich tuff (here denoted as ODA-zeolite) among the other described mostly commercial and costly products and their removal efficiency for acid red and cefazoline. On the other hand, as can be seen from Figures 3,5 and 6, carbon based adsorbents (chezacarb and silcarbon) proved the best removal performance toward m-cresol, n-decane and cefazoline [3-11,19-22].
An organo-inorganic composite, termed as a hybrid material too, may be defined hereto as a combination of a polymerous substance immobilized onto surface of the inorganic, e.g. zeolite carrier to avail advantages of both zeolitic and polymerous constituents as well. Accordingly, hybridization can be used to modify organic or inorganic materials and hybrids should therefore be considered as the new generation of composites that may encompass a wide variety of applications [14-19]. The conversion of inorganic ion exchange materials into hybrid fibrous or nanoscale ion exchangers is considered to be the latest development of the discipline. These nanomaterials are drawing a great attention as they exhibit a high efficiency and rate of sorption with short diffusion path toward environmental pollutants [6,7]. Advances in nanoscale science and engineering are providing unprecedent opportunities to develop more cost effective and environmentally acceptable water purification processes, respectively [19-23]. For the water purification, besides the metal-containing nanoparticles, carbonaceous materials and dendrimers, also the zeolites are being evaluated as the most progressive functional and nanosized materials of the millennium.
Shungite is elementary carbon with amorphous structure, although XRD studies confirmed the existence of fragments with graphitic structures of an interlayer distance of 3.4 Å. Recently it has been stated that the mineral has important amount of fullerenes, mainly C60. Shungite rocks have a peculiar structure. The rock contains highly dispersed silicate mineral grains distributed in the shungite carbon matrix. Shungite can replace coke, graphite, carbon black or glassy carbon, and usually serves as a complex raw material in thermal processes. Our sample of shungite originated from the deposit nearby St. Petersburg in Russia [24].
In the last decade, chitin and its deacetylated derivative chitosan, respectively, are appearing as potential biosorbents. Chitosan possesses apropriate mechanical strength, rigidity and settling characteristics, which are a perfect alternative to other adsorbent - biomass, despite fragile structure and poor mechanical strength. Chitin is found in fungi, arthropods and marine invertebrates. It is one of the most abundant, easily obtainable and renewable natural biopolymers (polysaccharides).
The alginite fossil was of domestic origin. Alginite is an ecological raw material, which originates from algae stock deposition (Botryococcus braunii Ktz.) situated at the bottom of a maar lake together with the clay weathering, products washedout from the maar ring and composed of basalt tuffs. This fossil possesses a high humus content, macro- and micronutritive and trace elements with considerable dehydration ability, which supports its utilization as a soil fertilizer, pharmaceutical additive or environmental adsorbent [4].
Metal oxides such as iron oxide, titanium dioxide and alumina are effective, low cost materials for heavy metals and radionuclides removal and pathogen detection as well. Their cleanup process of pollutants removal is mainly controlled by complexation. When their particle size is reduced to below 20 nm, the specific surface area of normalized adsorption capacity increased 10 - 100 times, suggesting a „nanoscale effect“. To overcome a potential human risk from environmental spreading, nanomaterials need to be embedded in a solid matrix, respectively, to have minimum release until they are disposed of [6,7,10,11]. Current immobilization techniques usually result in significant loss of treatment efficiency. Therefore, research is needed to develop simple, low-cost methods to immobilize nanomaterial without significantly impacting its performance. Based upon these facts, we prepared and thoroughly investigated iron and manganese oxides supported clinoptilolite-rich tuff, which due to its nanoporous structure worked as nanoreactor and in such a treated form provided enhanced functionalities. A detailed description of biomimetic FeO(OH) - zeolite sol-gel synthesis was published in paper [17]. It should be pointed, that usually novel and state-of-the art biosynthesis protocols allow simplifying experimental design and applying renewable and ubiquitous biological materials, what minimalizes energy requirements and waste generation. MnOx-zeolite was prepared by simple and inexpensive large-scale redox-synthesis at ambient temperature using the 5% KMnO4 solution. The price of its commercial product (Mn-Klinopur) on the market is 1500 Euro per ton.
As Figures 3,4 illustrate, the selected pollutants, i.e., the azodye (acid red 18) and prophylaxis antibiotic cefazoline by means of ODA-clinoptilolite-rich tuff was examined. Its performance was compared with other on the market accessible adsorption products, e.g. active coke of German provenience silcarbon, industrial ashes chezacarb (amorphous carbon) from Chemopetrol Litvínov (Czech Republic), German Granulated Ferric Hydroxide (GEH) with the main components of akaganeite (ß-FeOOH) and goethite [α-FeO(OH)], montmorillonite from the deposit in Slovak Republic, obtained from the rock after sedimentation and purification procedures and commercial Absodan (Great Britain), mostly natural resources derived and mixed products denoted as SK1, SK2, DN2 and CB18.
The SK1 and SK2 contain the mixture of cellulose, calcium carbonate and clays, while DN2 is manufactured mainly of natural silicate resources, based on the former commercial absodan and CB18 means a high quality peat (Figures 3-6). Peat contains lignin, cellulose, fulvic and humic acids as major constituents that bear polar functional groups, such as alcohols, aldehydes, ketones, phenolic hydroxides and ethers, using them in chemical bonding. To characterize the voluminous pigment molecule from the point of its molecular structure, the well dissociated and polar naphtalene disulfonic acid, i.e. acid red, consists of 3 benzene rings interlinked with characteristic azo - N = N - group. Actually, the azodye in water represents dissolved sodium salts of polyaromatic disulfonic acid. According to Figure 4, the acid red was better adsorbed onto ODA-clinoptilolite-rich tuff than onto peat. Somewhat higher adsorption performance toward acid red proved also biopolymeric chitosan, however the best or even superior adsorption efficiency toward the pigment (azodye) was observed by the Czech commercial product Nanofer 25S or nanoiron. When providing the column arrangement, the GEH product showed a higher efficiency toward acid red than the ODA-clinoptilolite-rich tuff did. Despite to a little higher adsorption capacity of above examined adsorption materials (Figsures 5,6), the adsorption performance of cost effective surfactant modified clinoptilolite-rich tuff was comparable and thus assessed as competitive. Finally, Figures 3,4 summarize clearly competitive position of ODA-clinoptilolite-rich tuff (here denoted as ODA-zeolite) among the other described mostly commercial and costly products and their removal efficiency for acid red and cefazoline. On the other hand, as can be seen from Figures 3,5 and 6, carbon based adsorbents (chezacarb and silcarbon) proved the best removal performance toward m-cresol, n-decane and cefazoline [3-11,19-22].
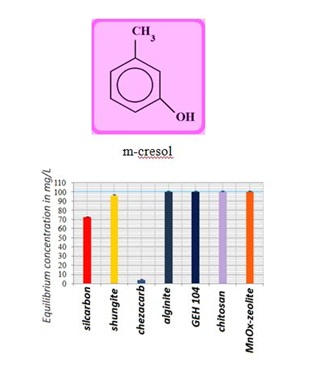 Figure 5: Comparable measurements for m-cresol removal using the commercial, natural and waste products.
Figure 5: Comparable measurements for m-cresol removal using the commercial, natural and waste products.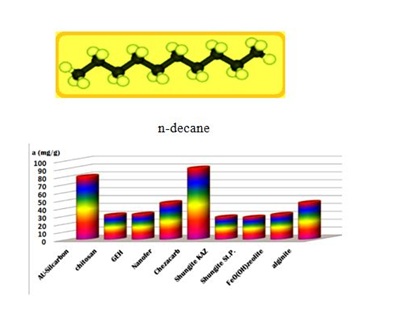 Figure 6: Illustration of adsorption capacities for n-decane uptake using the various natural and commercial adsorption materials.
Figure 6: Illustration of adsorption capacities for n-decane uptake using the various natural and commercial adsorption materials.CONCLUSION
The problem of water pollution presents today an increasing trend and it is expected to become more urgent in near future, due to rapid economic development of some highly populated regions of the world. Considering the proactive approach to green agenda and sustainable development goals the use of natural resources instead of non-environmentally friendly processed adsorbents is one of the way how to save environment as well as not to widespread industrial pollution. All these facts caused that the clay minerals and zeolites supplemented with progressive surface modification or various combination (compressing) with another natural or even waste products, have become increasingly popular, effective and economic feasible materials in adsorption based water purification technologies during the recent several decades. As discussed before, individual adsorbents, either natural or originated from some kind of industrial waste, possess specific affinity and sorption activity toward environmental pollutants, oppositelly to natural zeolite (clinoptilolite-rich tuff), which either in native form or after surface modification became nearly universal, able to remove much broader range of pollutants.
REFERENCES
- Li Z, Bowman RS (1996) Environ Sci Technol 29: 2400.
- Sullivan EJ, Hunter DB, Bowman RS (1997) Clays and Clay Miner 45: 42.
- Chmielewská E, Xu F (2015) Functional gradient adsorbents processed with biogenic components for ecologically benign water purification, Current Green Chemistry 2: 362-370.
- Chmielewská E (2014) Environmental zeolites and aqueous media: Examples of practical solutions. Bentham Science Publishers , Sharjah, United Arab Emirates.
- Ort C, Lawrence MG, Reungoat J, Mueller JF (2010) Sampling for PPCPs in wastewater systems: comparison of different sampling modes and optimization strategies. Environ Sci Technol 44: 6289-6296.
- Gehrke I, Geiser A, Somborn-Schulz A (2015) Innovations in nanotechnology for water treatment. Nanotechnol Sci Appl 8: 1-17.
- Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43: 9216-9222.
- USEPA (2014) Nanomaterial case study: Nanoscale silver in disinfectant spray. United States Environmental Protection Agency, Washington, D.C., USA.
- Tome?ková V, Reháková M, Mojžišová G, Wadsten T, Zele?áková K, et al. (2016) Spectral study of modified natural clinoptilolite with pharmacologically active escin and horse chestnut extract. Spectroscopy Letters 49: 63-72.
- Doula MK (2009) Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Research 43: 3659-3672.
- Dyachenko A, Mitchell J, Arsem N (2017) Extraction and identification of microplastic particles from secondary Wastewater Treatment Plant (WWTP) effluent. Anal Meth 9: 1412-1418.
- Lares M, Ncibi MC, Sillanpää M, Sillanpää M (2018) Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res 133: 236-246.
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, et al. (2011) Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ Sci Technol 45: 9175-9179.
- Jaiswal A, Banerjee S, Mani R, Chattopadhyaya MC (2013) Synthesis, Characterization and application of goethite mineral as an adsorbent. J Environ Chem Eng 1: 281-289.
- Subramani A, Jacangelo JG (2015) Emerging desalination technologies for water treatment: A critical review. Water Research 75: 164-183.
- Šiljeg M, Stefanivi? ŠC, Mazaj M, Tušar NN, Ar?on I, et al. (2009) Structure investigation of As(III)- and As(V)-species bound to Fe-modified clinoptilolite tuffs. Microporous Mesoporous Mat 118: 408- 415.
- Chmielewská E, Tylus W, Drábik M, Majzlan J, Krav?ak J, et al. (2017) Structure investigation of nano-FeO(OH) modified clinoptilolite tuff for antimony removal, Microporou Mesoporous Materials 248: 222-233.
- Kim ES, Hwang G, El-Din MG, Liu Y (2012) Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J Membrane Sci 394: 37-48.
- Nora S, Mamadou SD (2005) Nanomaterials and water purification:opportunities and challenges. J Nanopart Res 7: 331-342.
- Quang DV, Pradi B, Sarawade SJ (2013) Effective water disinfection using silver nanoparticle containing silica beads. Appl Surf Sci 266: 280-287.
- Watkins R, Weiss D, Dubbin W, Peel K, Coles B, et al. (2006) Investigations into the kinetics and thermodynamics of Sb(III) adsorption on goethite (α-FeOOH). J Colloid Interface Sci 303: 639-646.
- Yang X, Song J, Xu W, Liu X, Lu Y, et al. (2013) Anisotropic sliding of multiple-level biomimetic rice-leaf surfaces on aluminium substrates. Micro & Nano Letters 8: 801-804.
- Stefanidis G, Stankiewicz A (2016) Alternative energy sources for green chemistry. The Royal Society of Chemistry, New York.
- Cascarini de TLE, Fertitta AE, Flores ES, Llanos JL, Bottani EJ (2004) Characterization of shungite by physical adsorption of Gases. J Argent Chem Soc 92: 51-58.
Citation: Chmielewská E (2019) Surface Modified Zeolite and other Nanogeocomposites: An Effective and Sustainable Natural Resources for the Removal of Pollutants. J Environ Sci Curr Res 3: 009.
Copyright: © 2019 Eva Chmielewská, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2024, Copyrights Herald Scholarly Open Access. All Rights Reserved!

