Introduction
An impressive number of modern drugs have been isolated from natural sources, especially, plants that have been used as a source of medicinal agents [1]. However, active ingredients of many medicinal plants have not been previously characterized. A Liliaceae is a large family of around 230 genera and 3500 species, characterized by showy flowers with six perianth segments, six stamens, and a superior ovary and usually producing bulbs or rhizomes [2]. Many Liliaceae plants are of high medical values, such as antifungal activity, anti tumour-promoter, insecticidal activity and anti-inflammatory [3-6].
Disporopsis pernyi (Hua) diels, which belongs to the genus Disporopsis of the family Liliaceae, is widespread in South Asia; however, it has not yet been successfully cultivated. This plant has been widely used for curing chronic cough and cancer in China [7]. The volatile compositions of Disporopsis pernyi have been previously characterized, but its effective components have seldom been reported [8].
Disporopsis pernyi is often confused with rhizoma polygonati odorati and Panax japonicus C.A. Mey. rhizoma polygonati is the dried rhizome of Polygonatum odoratum. The underground portion of Polygonatum odoratum has an appearance similar to that of Disporopsis pernyi. Hence, the two herbs are often confused with each other. Panax japonicus is commonly known as ‘Zhujieshen’ in China, although ‘Zhujieshen’ is also an alias of Disporopsis pernyi. The phenomenon of homonyms also creates confusion. Therefore, to exploit these species resources commercially, comprehensive research on their chemical constituents is necessary.
This study aimed to investigate the cytotoxic properties of the extracts and chemical compositions of the three herbs.
Materials and Methods
Plant material
Fresh Disporopsis pernyi were collected from an alpine site in August 2015. The site was located in the Guizhou province (longitude: 106°95’-106°98’E, latitude: 26°44’-26°47’N; altitude: 1200-1800m). Polygonatum odoratum (90226) and Panax japonicus (90149) were purchased from Pengxiang Pharmaceuticals Co., Ltd. The origins of them were showed in table 1. The air-dried plant material was pulverized, screened through 40-mesh sieves, and stored in the refrigerator.
Chemicals and reagents
Methanol, petroleum ether, ethanol, ethyl acetate, anhydrous sodium sulphate and ferrous sulphate (analytical grade) were purchased from Tianjin Damao Chemical Reagent Co., Ltd. (Tianjin, China). Purified water was purchased from Hangzhou Wa ha ha Co. Ltd. (Hangzhou, China) n-Alkanes (9-35) were purchased from Aladdin Chemical Reagent Co. Ltd. (Shanghai, China).
Extraction and isolation of Disporopsis pernyi
Air-dried and finely powdered roots and rhizomes (12.3kg) of Disporopsis pernyi were sequentially and exhaustively extracted with 70% ethanol, four times at 90ºC for 2h, and the ethanol solutions were combined and concentrated in vacuo. The residue (1.8kg) was suspended in H2O and sequentially extracted three times each with petroleum ether, chloroform, ethyl acetate and n-butanol to afford a petroleum ether fraction (PEF, 92g), a chloroform fraction (CF, 180g), an ethyl acetate fraction (EAF, 86g) and an n-butanol fraction (BF, 54g).
The PEF was subjected to silica-gel column chromatography (200-300 mesh, 1.2kg) by eluting with petroleum ether (6.0L) and then with a gradient of petroleum ether-acetone by gradually increasing the polarity of the elution solvent system. The eluents were collected and monitored by TLC to yield 9 fractions (P1 to P9). P5 was isolated by silica gel and Sephadex LH-20 column chromatography to obtain compounds 1 (yield 0.2382g, 0.0019%)and 2 (yield 1.8514g, 0.0150%).
The CF was subjected to silica-gel column chromatography (200-300 mesh, 1.2kg) by eluting with chloroform (6.0L) and then with a gradient of chloroform-acetone by gradually increasing the polarity of the elution solvent system. The eluents were collected and monitored by TLC to yield 10 fractions (C1 to C10). C10 was isolated by silica gel and Sephadex LH-20 column chromatography to obtain compound 3 (yield 0.4681g, 0.0038%).
The EAF was subjected to silica-gel column chromatography (200-300 mesh, 1.2kg) by eluting with chloroform (6.0L) and then with a gradient of chloroform-methanol by gradually increasing the polarity of the elution solvent system. The eluents were collected and monitored by TLC to yield 8 fractions (E1 to E8). E3 was isolated by silica gel and Sephadex LH-20 column chromatography to obtain compound 4 (yield 0.1923g, 0.0016%). E6 was isolated by silica gel and Sephadex LH-20 column chromatography to obtain compounds 5 (yield 0.5251g, 0.0043%) and 6 (yield 0.3072g, 0.0025%).
The BF was subjected to macroporous resin D-101 (1.0kg) and then to a gradient of water-ethanol by gradually decreasing the polarity of the elution solvent system. The eluents were collected and monitored by TLC to yield 28 fractions (B1 to B28). B13 was isolated by silica gel and Sephadex LH-20 column chromatography to obtain a green substance, and the pH was adjusted to 4 by adding HCl. Compound 7 (yield 0.3175g, 0.0026%) was then obtained by re crystallization. B20 was isolated by silica gel and Sephadex LH-20 column chromatography to obtain compound 8 (yield 0.1248g, 0.0010%).
Identification of purity
The purified compounds were identified by 1H NMR spectrometry, 13C NMR spectrometry and ESI-MS (micoOTOFQ, Bruker). 1H NMR spectra and 13C NMR spectra were recorded on a Bruker Avance DMX-600 spectrometer (Bruker Biospin GmbH, Germany) operating at 300 MHz for 1H and 13C. Chemical shifts were expressed in δ (ppm) downfield from TMS as an internal standard, and coupling constants were reported in hertz.
Cytotoxicity assay
Cell culture: MCF-7 human breast cancer cells were cultured in an RPMI 1640 nutritive medium supplemented with 10% Foetal Bovine Serum (FBS) at 37ºC in a 5% CO2 incubator. Fluorouracil was included as a reference substance.
Cell proliferation: tumor cells in the logarithmic growth phase were adjusted to 5×103 per well in a 96 well plate, and different concentrations of the tested samples were added after culturing for 24h. Then, 15µL MTT (5mg/mL) was added to the 96 well plate and was cultured in a CO2 incubator for 48h. Stock solutions were made in 100% DMSO-d6 and were diluted in the medium to a final concentration of 0.2% (v/v) DMSO-d6 before treatment. The cells were fixed in DMSO-d6 and stained with Acridine Orange (AO) and Ethidium Bromide (EB), and the cell morphology was observed using a fluorescence microscope.
The Absorbance (A) value was measured by a micro plate reader at a wavelength of 490nm. The cell inhibition rate was calculated according to the following formula: Inhibition ratio (%) = [A490(control) - A490(test)]/[A490(control) - A490(blank)] × 100.
Preparation of the extracts
According to the methods described in the literature, the air-dried powdered roots and rhizomes (20g) of Polygonatum odoratum and Panax japonicus were extracted using a steam distillation apparatus [8]. The Essential Oils (EO) of Polygonatum odoratum (yield 0.158 ± 0.008g, 0.79%) and Panax japonicus (yield 0.241 ± 0.014g, 1.21%) were stored at 4ºC.
According to the methods described in the literature, the air-dried powdered roots and rhizomes (50g) of Polygonatum odoratum and Panax japonicus were successively extracted with 70% ethanol and ethyl acetate successively [8]. Ethyl Acetate Extracts (EAE) of the two herbs were evaporated to dryness under reduced pressure using a rotary evaporator. The EAE of Polygonatum odoratum (yield 3.591 ± 0.141g, 7.18%) and Panax japonicus (yield 4.627 ± 0.248 g, 9.25%) were stored at 4ºC.
Gas Chromatography-Mass Spectrometry (GC-MS)
The EO of Polygonatum odoratum and Panax japonicus were injected into a SHIMADZU QP5050A series instrument for GC-MS analysis. An HP-5 MS capillary column (30m × 0.25mm; 0.25μm) was used for the separation. Helium was used as a carrier gas at a flow rate of 1.0ml/min. As described previously, essential oil analysis was performed by gas chromatography under the same conditions [8].
Results and Discussion
Identification of purity
Compound 1 was obtained as a white powder. ESI-MS (m/z): 283[M-H]-; The 1H-NMR spectrum (CDCl3, 300MHz):11.023 (1H, s), 2.346(2H, t, 7.5Hz), 1.633(2H, t, J = 7.5Hz), 1.301(28H, s), 0.880 (3H, t, 6.5Hz). Thus, the structure of compound 1 was determined to be Stearic acid.
Compound 2 was obtained as a white crystalline substance. ESI-MS (m/z): 255[M-H]-; The 1H-NMR spectrum (CDCl3, 300MHz): 2.346(2H, t, J = 7.5Hz), 1.633(2H, m), 1.257 (24H, brs), 0.880(3H, t, J = 6.6Hz); IR spectrum showed absorptions at: 2954.5, 2916.7, 2848.5, 1702.9, 1470.9cm-1. Thus, the structure of compound 2 was determined to be Palmitic acid.
Compound 3 was obtained as an odorless white powder: mp256-257ºC; The 1H-NMR spectrum (DMSO, 300MHz): 0.812(3H, s), 0.822 (3H, s), 1.004(6H, s), 1.059(3H, s), 1.067(3H, s), 1.788(3H, s), 3.530(1H, dd, 11.4, 4.2Hz), 4.765(1H, s), 4.942(1H, s); 13C-NMR spectrum: (DMSO, 300MHz), 150.258 (C-20), 109.947 (C-29), 78.156 (C-3), 56.659 (C-17), 51.002 (C-9), 49.819 (C-19), 47.838 (C-18), 42.880 (C-14), 41.154 (C-8), 39.543 (C-4), 38.689 (C-13), 37.557 (C-10), 34.870 (C-7), 32.929 (C-16), 28.675 (C-23), 28.334 (C-2), 21.232 (C-11), 19.505 (C-30), 16.362 (C-26); IR spectrum showed absorptions at 3381, 2954, 2871, 1462, and 1373cm-1. Thus, the structure of compound 4 was determined to be Betulin.
Compound 4 was also obtained as an odorless white powder: 1H-NMR (CDCl3, 300MHz) δ:4.64(1H, s), 4.51(1H, s), 3.06(1H, m), 1.68(3H, s), 1.21(3H, s), 0.98(3H, s), 0.91(3H, s), 0.80(3H, s), 0.70(3H, s); 13C-NMR (DMSO, 300MHz) δ: 14.4 (C-27), 15.1 (C-25), 15.9 (C-26), 16.0 (C-24), 18.9 (C-6), 19.9 (C-30), 20.7 (C-11), 25.2 (C-12), 28.2 (C-2), 28.8 (C-23), 29.9 (C-15), 29.9 (C-21), 32.7 (C-16), 33.8 (C-7), 36.5 (C-22), 37.3 (C-10), 38.0 (C-13), 39.3 (C-1), 40.4 (C-4), 40.9 (C-8), 42.9 (C-14), 48.4 (C-19), 48.6 (C-18), 49.2 (C-9), 55.1 (C-5), 55.0 (C-17), 79.0 (C-3), 109.5 (C-29), 150.9 (C-20), 179.0 (C-28); IR spectrum showed absorptions at 3452, 3076, 2943 2870 1688cm-1. Thus, the structure of compound 4 was determined to be Betulinic acid.
Compound 5 was obtained as a white powder: 1H-NMR (DMSO, 300MHz) δ: 5.31(1H, m, H-2), 4.87(1H, m, H-1’), 4.20-4.41(6H, m, H-2’, 3’, 4’, 5’, 6’), 0.64-1.00(18H, m, 6CH3), 2.88(1H, m, H-9), 2.49, 1.93(2H, m, H-10), 13C-NMR (DMSO, 300MHz), δ140.502 (C-1), 121.242 (C-2), 100.921 (C-1’), 78.576 (C-9), 76.831 (C-5’), 70.156 (C-4’), 73.516 (C-2’), 61.160 (C-6’), 56.227 (C-14), 55.483 (C-17), 50.638 (C-5), 49.667 (C-21), 45.199 (C-13), 41.905 (C-10), 40.423 (C-12), 38.754 (C-6), 38.365 (C-7), 36.877 (C-18), 36.261 (C-19), 35.526 (C-3), 31.467 (C-4), 29.294 (C-22), 29.056 (C-8), 28.775 (C-20), 27.848 (C-15). TLC: Silica gel thin-layer chromatography was used and developed in three kinds of solvent systems; β-Daucosterol was used as a positive control and 10% sulphuric acid-ethanol (v/v) as a colour developing reagent; the TLC results showed that the compound and β-Daucosterol were similar. Thus, the structure of compound 5 was determined to be β-Daucosterol.
Compound 6 was obtained as a yellow powder: 1H-NMR (DMSO, 300MHz) δ: 12.986(1H, s), 10.793(brs), 7.437(1H, s), 7. 417(1H, m), 6. 915(1H, d, J = 9.0Hz), 6. 673(1H, s), 6. 463(1H, d, J = 2.4Hz), 6. 210(1H, d, J = 2. 4 Hz); 13C-NMR (DMSO, 300MHz), δ: 163.979 (C-2), 102.966 (C-3), 181.736 (C-4), 157.374 (C-5), 98.924 (C-6), 164.218 (C-7), 93.928 (C-8), 161.568 (C-9), 103.796 (C-10), 121.622 (C-1’), 113.476 (C-2’), 145.823 (C-3’), 149.773 (C-4’), 116.119 (C-5’), 119.066 (C-6’). Thus, the structure of compound 6 was determined to be Luteolin.
Compound 7 was obtained as a green powder: EI-MS m/z: 609.0[M-H]-, 633.1[M+Na]+, 611.1[M+H]+; 1H-NMR (MeOD, 300MHz) δ: 6.203(1H, S), 6.389(1H, S), 6. 874(1H, d, J = 8.4Hz), 7.641(2H, dd, J = 6.8Hz); 13C-NMR(MeOD, 300MHz) δ: 158.467 (C-2), 135.627 (C-3), 179.381 (C-4), 162.925 (C-9), 99.939 (C-6), 165.973 (C-7), 94.866 (C-8), 159.316 (C-5), 105.612 (C-10), 123.109 (C-1’), 116.048 (C-2’), 149.774 (C-4’), 117.700 (C-5’), 123.109 (C-6’), 102.399 (C-1”), 75.715 (C-5”), 77.186 (C-3”), 71.377 (C-4”), 68.546 (C-6”), 104.730 (C-1”’), 72.079 (C-2”’), 72.232 (C-3”’), 73.929 (C-4”’), 69.691 (C-5”’), 17.870 (C-6”’); IR spectrum showed absorptions at 3421.5, 2923.6, 2853.3, 1656.2, 1602.0, 1503.5cm-1. Thus, the structure of compound 7 was determined to be Rutin.
Compound 8 was obtained as a white crystalline substance. 1H-NMR (CDCl3, 300MHz) δ: 7. 936(1H, dd, J = 7.8, 0.9Hz, 3-H), 7. 528(1H, ddd, J = 7.2, 7.2, 0. 9Hz, 5-H), 7. 016(1H, dd, J = 8. 4, 0.9Hz), 6. 942(1H, ddd, J = 7. 2, 7.2, 1.2Hz); IR spectrum showed absorptions at 3237.6, 3008.5, 2857.6, 2594.4, 1657.5, 1611.8, 1030.4, 760.1, 698.6cm-1. Thus, the structure of compound 8 was determined to be Salicylic acid (Table 2).
Evaluation of cytotoxicity
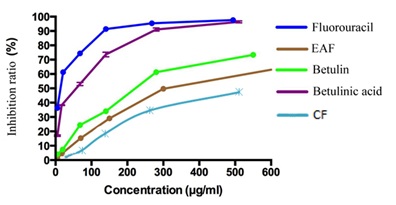
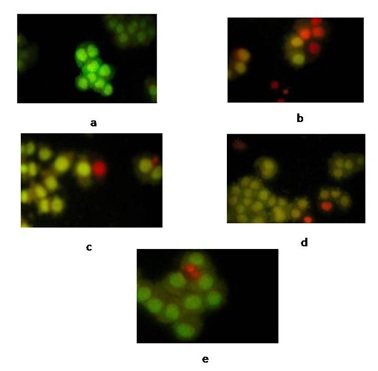
We initially tested the effect of the 4 fractions (PEF, CF, EAF and BF) of the three herbs on the growth of MCF-7 human breast cancer cells for 24h by adding fresh compounds. All fractions of Polygonatum odoratum and Panax japonicus did not show Cytotoxicity. The EAF of Disporopsis pernyi significantly reduced the cell number compared to the vehicle alone (2% DMSO-d6). The effective concentration of the samples is shown in figure 1, and there was a 48% reduction in cell number at 315.8µg/ml. As showed in figure 2, the observed change in the color of the cell from green to yellow or red fluorescence appeared to be due to increased membrane permeability and apoptosis. While its counterfeits and substitutes, Polygonatum odoratum and Panax japonicus, do not show cytotoxicity against MCF-7 cells in this test.
Betulinic acid and Betulin have been reported to have activity against various pathogenic microbes, including anti-HIV activity and inducing apoptosis [9-11]. The Cytotoxicity of EAF against MCF-7 cells might be related to Betulinic acid and Betulin [12,13]. The other fractions did not show significant activity against MCF-7 cells, so did the fractions of Polygonatum odoratum and Panax japonicus.
Chemical compositions of the EO
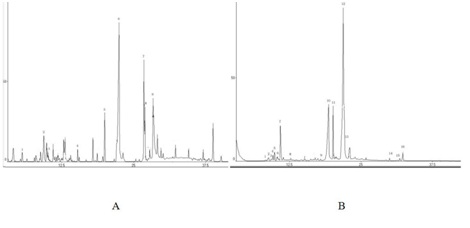
The chemical compositions of the EO from Disporopsis pernyi have been previously reported by our research group [8]. In the present study, the chemical compositions of the EO from Polygonatum odoratum and Panax japonicus were detected using the same conditions and methods. Figure 3 shows the Total Ion Chromatograms (TIC) of the EO. The GC-MS analysis of these two herbs resulted in the identification of 9 and 14 constituents, representing 61.25% and 92.70% of the volatile components, respectively (Table 3 and 4). Twelve volatile compounds from Disporopsis pernyi were identified according to the literature [8]. Pentadecenoic acid methyl ester, hexadecanoic acid methyl ester, n-hexadecanoic acid, and 9, 12-octadecadienoic acid were the major volatile components identified in Disporopsis pernyi. The comparison of the main volatile components of the three herbs showed remarkable differences.
Conclusion
Eight pure compounds, which were identified as Stearic acid, Palmitic acid, β-Daucosterol, Luteolin, Rutin, Salicylic acid, Betulinic acid and Betulin, were obtained and characterized from Disporopsis pernyi. Some of the compounds, including Stearic acid, Luteolin, Rutin Salicylic acid, Betulinic acid and Betulin, which we found in Disporopsis pernyi, have not been reported in Polygonatum odoratum and Panax japonicus.
The EAF from Disporopsis pernyi conspicuously restrained the growth of MCF-7 human breast cancer cells. Betulin and Betulinic acid have been used against a variety of cell lines, such as malignant brain tumor, primitive neuro ectodermal tumor [12], human chronic myelogenous leukemia, and against most of the prevalent human cancer types, such as cervical, prostate, breast, lung or colorectal cancer [13]. Betulinic acid and Betulin are plant products found in the genera Betulla, Diospiros, Paeonia, Syzigium or Ziziphus [14]. Betulinic acid and Betulin belong to triterpenoids, which represent a large group of plant products with a broad variety of biological activity [15-17]. The results suggested that the two triterpenes, Betulin and Betulinic acid, contributed significantly to the cytotoxicity of Disporopsis pernyi against MCF-7cells.
Although Disporopsis pernyi and its confused varieties share some similar compositions of EO, such as palmitic acid 9, 12-Octadecadienoic acid and Linolenic acid, their volatile constituents are different from each other.
Acknowledgment
This project was supported by National Natural Science Foundation of China (NSFC No. 21708033) and Jiangsu Natural Science Foundation (BK20140225). The authors declare that there is no conflict of interest regarding the publication of this paper.