
Food Proteins and Bioactive Peptides, Functional Diets
*Corresponding Author(s):
Valdemiro Carlos SgarbieriDepartment Of Food And Nutrition, School Of Food Engineering, University Of Campinas, São Paulo, Brazil
Tel:+55 1932877400,
Email:vsgarbieri@gmail.com
Abstract
This review article is an attempt to update on research and recent published data on food proteins as a source of bioactive peptides contained in their primary structure sequences in inactive forms, which can be released and activated by enzymatic action in vitro or digestive system. Once absorbed in the active form they can perform various biochemical and biological functions to improve human metabolism and health. The main bioactivities and functions already described in the literature have been summarized. Methodology for identification of these peptides as food ingredients or functional food has already been applied in some countries. These potential applications have reached different degrees of advancement according to technology and specific needs in various countries. Considerations are also made on the development in this area of food science and technology in Brazil and other countries in South America.
INTRODUCTION
Throughout the second half of the last century and early this century the concept of the biological function of food proteins has changed significantly. While in the past the food proteins were regarded only as macronutrients capable of providing the amino acids to form the protein component of organs and tissues, also participating in the generation of metabolic energy for the normal functioning of living organisms, today it is known that certain proteins present in food, as well as fragments or peptides derived thereof, may participate in the general metabolism in a much more interactive manner, through bioactivities that go far beyond the nutritional functions that had been established by the end of the first half of the prior century.
By making an analogy with the aeronautical activity we can say that in the past the food proteins functioned as black boxes holding numerous important pieces of information, which research and technology have only revealed, in part, in recent decades. In this period it was found that certain proteins present in foods are naturally bioactive, and can be absorbed from the Gastrointestinal System (GIS) in their intact or slightly modified form and exercise specific bioactivities in the systemic metabolism [1], or, in addition, resist the action of digestive enzymes exercising different bioactivities in the gastrointestinal system [2]. Even more interesting were the findings that food proteins, of both plant and animal origin, contain, in their primary structures, specific amino acid sequences in their polypeptide chains, which when cleaved in the form of peptides by proteolytic enzymes or by specific chemical reagents may exercise diverse bioactivities that were latent in the original structure of the protein from which they originated.
Bioactive peptides have been obtained from precursor proteins through the use of methodologies that have reached a high degree of sophistication and complexity, such as: (a) enzymatic hydrolysis by digestive enzymes; (b) proteolysis of the protein source by enzymes derived from microorganisms or plants; (c) fermentation process using culture with high proteolytic power and specificity. The bioactive potential of the peptides originating from the proteolysis of food proteins can be explored in the integral hydrolysates, produced in special conditions. However, in the current literature there are numerous research works and literature reviews emphasizing the importance of the isolation, purification, characterization, study of mechanisms and impact of these peptides on human health [3-8].
PRODUCTION AND PROCESSING OF BIOACTIVE PEPTIDES DERIVED FROM FOOD PROTEINS
A large number of plants and animals have been object of study as source of Bioactive Peptides (BAPs). Several studies were conducted, particularly with milk proteins [9,10], eggs [11,12], and muscle proteins from pigs [13], cattle [14], poultry [15], and fish [16]. BAPs have been obtained from vegetable proteins such as soybeans [17,18], lentils, chickpeas, peas, beans, and oilseeds such as canola and flaxseed [8]. As to fish, the fishing industry has shown much interest in obtaining bioactive peptides and hydrolysates from fishery by-products, including carcasses and viscera of aquatic animals, of very low commercial value. Based on the current literature [6], food proteins are selected as potential source of bioactive peptides based on two main criteria: (a) with the objective of adding value and increasing the use of abundant sources of under-utilized proteins; (b) utilization of proteins containing specific peptide sequences or amino acid residues of special interest for the pharmaceutical or food industry. Recently a strategy was developed and it enables to shorten the time and investment in seeking proteins with high potential of bioactive peptides. This strategy is the Quantitative Structure-Activity Relationship (QSAR) analysis, based on prior knowledge of the amino acid sequence in the polypeptide chain and of the specificity of a certain number of proteolytic enzymes tested. Based on these two parameters, computer programs (software) are established that are capable of predicting the number of peptides released by specific proteases with potential to exercise different bioactivities, a methodology now known as proteolysis or digestibility in silico [19]. However, a detailed experimental work should be conducted with the peptide identified and isolated to confirm possible bioactivities and repeatability as well as the feasibility of the process in silico. A general scheme of methodologies available for production and processing of BAPs is presented in Undenigwe and Aluko [6].
The several bioactivities described for peptides isolated from food proteins include the following: antihypertensive; antimicrobial; antioxidant; antitumor; immunomodulatory activity; binders and mineral ion carriers; hypocholesterolemic; anti-inflammatory; multifunction activity. Among the main food sources of bioactive peptides should be highlighted the milk proteins (caseins and whey proteins), egg proteins, fish proteins, meat proteins (cattle, pigs, poultry), some proteins of cereal grains and legumes [2].
ANTIHYPERTENSIVE PEPTIDES
A significant number of proteins release into the Gastrointestinal System (GIS) peptides containing 3 to 10 AA residues capable of inhibiting the enzyme Angiostensin-Converting Enzyme (ACE), enzyme that converts angiotensin I into angiotensin II [20]. Angiotensin I, an inactive decapeptide, is produced in the liver by action of the proteolytic enzyme renin on the angiotensinogen polypeptide. Angiotensin I is then converted by ACE into Angiotensin II, an octapeptide that is a potent vasoconstrictor. The ACE also acts on the hormone bradykinin, a vasodilator, converting it into inactive peptides. Moreover, angiotensin II stimulates the release of aldosterone, a hormone produced by the suprarenal glands that promotes the retention of Na+ and water, hence contributing even more to elevate blood pressure. Thus, ACE inhibitors contribute to maintain or lower blood pressure through three mechanisms: a) by inhibiting the formation of angiotensin II (a potent vasoconstrictor); b) by inhibiting the degradation of bradykinin (a vasodilator); and c) by helping maintain high levels of aldosterone that acts stimulating the retention of Na+ and water [21]. The ACE enzyme is found in various tissues and its inhibition can influence different systems involved in the regulation of blood pressure, in addition to the immune and nervous systems [22].
ACE inhibitors have been isolated from the primary sequences of &alph;S1-casein (fs: 23-34; 23-27; 294-299), of bovine β-casein (f: 177-183), human (f: 43-52) and of kappa-casein (f: 63-65). These peptides are generally called casokinins [23]. Antihypertensive activity has been described in numerous hydrolysates and peptides isolated from a large number of food proteins. Ariyoshi [2] in a literature review presents ACE inhibitory activity in a number of peptides isolated from animal and vegetable proteins in terms of micromolar concentration capable of inhibiting 50% of the ACE activity represented by IC50(μM); the lower the IC50 the greater the inhibitory activity of the peptide. Examination of the composition and structure of the ACE inhibitory peptides has shown a trend of greater inhibitory activity as there is a decrease in the Molecular Mass (MM) of the peptide and a very accentuated importance of the presence of proline and/or hydroxyproline at the C-terminal extremity and of leucine and/or isoleucine at the – NH2 extremity.
From the 2000s the studies on ACE inhibitors were intensified with the use of several methodologies of obtainment and characterization, from different protein sources. Wu and Ding [24] obtained ACE inhibitors from the alkaline hydrolysis of soy protein after ultra filtration and chromatography in cation-exchange resin. The final yield was of 14.42% (protein base) and IC50 of 0.065 mg protein/mL. The peptides maintained their activity under various conditions of temperature and pH and showed resistance to digestion in vitro by gastrointestinal proteases. The two most active peptides were sequenced after successive chromatographic isolations. Their structures Asp-Leu-Pro and Asp-Gly with IC50 4.8 and 12.3μM, respectively. In later research, Wu et al., [25,26] based on database with 168 dipeptides and 140 tripeptides applied the QSAR analysis – Quantitative Structure-Activity Relationship analysis – designed through computational-statistical modeling that enables to estimate, based on activities already described for peptides and primary structures of different protein chains published in databases, possible ACE inhibitory peptides. They managed to isolate 3 dipeptides and 6 tripeptides of very high ACE inhibitory power, as shown in (Table 1).
| Peptide | Position in the Chain | Protein of Origin | Log IC50 | |
| Predicted | Observed | |||
| FW | f: 150-151f: 149-150 | Legumin A2 (garden pea)Legumin A (garden pea) | 0.60 | 0.77 |
| WW | f: 150-151f: 147-148 | Glycine G1 (soy)Glycine G2 (soy) | 0.52 | 1.91 |
| YW | f: 122-123f: 113-114f: 219-220f: 153-154f: 13-14f: 27-28f: 147-148f: 156-157 | a-lactalbumin (bovine milk)Albumin 2 (PA2); (garden pea)Legumin J (garden pea)Legumin K (garden pea)b-conglycinin (soy)Glycinin G3 (soy)Glycinin G4 (soy)Glycinin (soy) | 0.92 | 1.64 |
| VRF | f: 4-6 | b-conglycinin (soy) | 0.14 | 1.38 |
| IKP | f: 265-267f: 279-281f: 6-8 | Glycinin G1 (soy)Legumin J (garden pea)Vicilin 47kDa (garden pea) | 0.37 | 0.44 |
| LRW | f: 377-379f: 374-376 | Legumin A2 (garden pea)Legumin A (garden pea) | -0.11 | -0.64 |
| LRF | f: 36-38 | aS1-casein (bovine milk) | 0.22 | 1.33 |
| VPP | f: 99-101 | b-casein (bovine milk) | 1.28 | 1.42 |
| IPP | f: 89-91f: 129-131 | b-casein (bovine milk)k-casein (bovine milk) | 1.18 | 1.75 |
Table 1: Prediction, experimental validation, and location of potent ACE inhibitory peptides.
Adapted from Wu et al., [25] ExPASy - Expert Protein Analysis System - Primary Sequences. F, Phenylalanine; W, Tryptophane; Y, Tyrosine; V, Valine; R, Arginine; I, Isoleucine; K, Lysine; P, Proline; L, Leucine.
In a recent work, Gu et al., [19] systematically researched the potential of the main food proteins as precursors of ACE inhibitory peptides, with the aid of the QSAR in silico technique in order to establish the rationality of choice of more appropriate proteins as substrate in the preparation of these peptides. The in silico digestion of proteins present in 15 foods that are common in the human diet, of vegetable origin (soybeans, peas, barley, oats, canola), milk proteins (&alph;s1 and &alph;s2 caseins, K-casein, β-lactoferrin, &alph;-lactalbumin), meat (pigs, cattle, poultry), and fish (tuna, salmon, hake) generated, based on the specificity of the proteolytic enzyme thermolysine, 5,709 peptides in the range of 2 to 6 amino acid residues. A significant number of these peptides was resistant to the action of pepsin and trypsin; therefore, potentially absorbable and antihypertensive. These peptides, in addition to inhibiting the activity of ACE in vitro (IC50<10μM) after hydrolysis by thermolysine, also resisted the action of pepsin and trypsin, remaining intact and active, which highly increases the probability they are absorbed and exercise in the general metabolism the antihypertensive functions.
The results obtained by Gu et al., [19] indicated that the proteins of meat (pigs, cattle, and poultry) released the highest number of active peptides (IC50<10μM), followed by the proteins of milk, egg, soybean, and canola, while the proteins of fish (except for salmon) and cereals (except for oats and barley) contain the lowest number of peptides of high activity. A high number of these peptides remained active after being subjected to the action of pepsin and trypsin. It is important to emphasize that the ACE inhibitory activity alone does not prove the antihypertensive power of the peptides. They need to be tested in vivo with SHR rats (naturally spontaneous hypertensive rats), and the ultimate test will be conducted in clinical trials with humans. A large number of recent researches, including literature reviews, has focused on the study of the antihypertensive peptides, with important contribution for the development of food and/or food supplements that contribute to the control of hypertension in humans such as egg proteins [27] dipeptides derived from pig muscle proteins [28], milk proteins, proteins in seeds of cereals, oilseeds, fruits and vegetables [29], milk proteins and fish [30], sarcoplasmatic proteins in bovine brisket [14], gastrointestinal contents of swine meat [13].
ANTIMICROBIAL ACTIVITY
Peptides obtained from cleavage of Lactoferrin (LF) by swine pepsin or acid protease of Penicillium duponti showed strong activity against Escherichia coli. Low MM peptides generated by cleavage of LF by porcine pepsin presented a broad spectrum of antibacterial activity, inhibiting the growth of both gram-negative and gram-positive species, including lineages resistant to native LF. A precise way to study the antimicrobial activity of a hydrolysate and/or peptide is to determine the minimum concentration to inhibit proliferation or “Minimum Inhibitory Concentration” (MIC). The MIC value defines the minimum concentration of an antimicrobial agent to inhibit 100% the growth of a given microorganism. The antibacterial power of the hydrolysate was at least 8 times higher than that of the intact LF. The selective cleavage of LF by pepsin generates the Lactoferricin (LFcin) with a very high bactericidal potential [31].
The proteolytic action of the enzymes pepsin, trypsin, and chymotrypsin on β-lactoglobulin (β-LG) originated 3 peptides with bactericidal properties. Two fragments were obtained by the action of trypsin, one pentapeptide with the sequence Glu-GLn-leu-Thr-Lys (f: 1-5) and another one Gly-Tyr-Gly-Gly-Val-Ser-Leu-Pro-Glu-Trp-Val-Cys-Thr-Thr-Phe-Ala-Cys-Ser-Glu-Lys (fs: 17-31-S-S-109-114), composed of 2 peptides linked by a disulfide bond. The fragmentation of the &alph;-LA by chymotrypsin produced the peptide Cys-Lys-Asp-Gln-Asn-Pro-His-Ile-Ser-Cys-Asp-Lys-Phe (fs: 61-68-S-S-75-80), also composed of 2 polypeptides linked by a disulfide bond. The 3 polypeptides were synthesized and shown to have antimicrobial action, having been more active against Gram (+) bacteria. Digestion of the &alph;-Lactalbumin (&alph;-LA) by pepsin produced several polypeptides; however, they showed no antimicrobial activity. The intact &alph;-LA does not present antimicrobial activity [32]. In later research, Pellegrini et al., [33] isolated, after action of trypsin on (β-LG), 4 polypeptides with bactericidal activity. The sequences of the 4 peptides were determined, as follows: Val-Ala-Gly-Thr-Trp-Tyr (f: 15-20); Ala-Ala-Ser-Asp-Ile-Ser-Leu-Leu-Asp-Gln-Ser-Ala-Pro-Leu-Arg (f: 25-40); Ile-Pro-Ala-Val-Phe-Lys (f: 78-83); Val-Leu-Val-Leu-Asp-Thr-Asp-Tyr-Lys (f: 92-100). The 4 peptides were synthesized and exercised bactericidal activity only against Gram (+) bacteria. These results suggest a possible antimicrobial function of LG after partial digestion by pancreatic endopeptidases.
Caseinomacropeptide (CMP) is the heterogeneous C-terminal fragment (f: 106-169) of bovine K-casein composed of glycosylated and phosphorylated forms of different genetic variants. Malkoski et al., [34] showed that CMP presented inhibitory activity against the opportunistic pathogens of the oral cavity, Streptococcus mutans and Porphyromonas gingivalis and against Escherichia coli. It was shown that only the phosphorylated form inhibited the bacteria, while the glycosylated form showed no activity. The active form (phosphorylated) was called kappacin. The kappacin was submitted to hydrolysis by endoproteinase Glu-C and the peptides generated were separated by RP-HPLC and HPLC-gel filtration, then tested for inhibition of the pathogen S. mutans. Only the peptide isolated from the K-casein A (f: 138-158) containing serine-P in position 149 presented inhibitory activity against the aforementioned pathogen.
Peptides with antimicrobial activity have been isolated from different types of fish and by-products of the fishery industry [3]. Fish hydrolysates can be obtained by different techniques, namely: a) liquid extraction [35]; b) chemical treatment with acid or alkali [36]; c) microbiological fermentation of protein [37]; d) enzymatic hydrolysis by various enzymes [38]. According to Urakova et al., [39] peptides obtained from fish hydrolysates are generally multifunctional and can exercise activities such as antimicrobial, antihypertensive, antiviral, antioxidant, immuno stimulating, antitumor, in addition to other effects. Antimicrobial peptides act through the disintegration of the cell membrane of the microorganism. Most antimicrobial peptides have &alph;-helical structure and are cationic, others are &alph;-helical and hydrophobic. The cationic property of peptides enables their binding with the lipid-rich anionic cell membrane and the beginning of cell membrane lysis.
Some examples of peptides with antimicrobial activity isolated from fish are presented below. From the liver tissue of Atlantic salmon an antimicrobial polypeptide with MM 20,734Da was isolated, which after purified was characterized as a Histone and named H1. Histones are polypeptides found in chromatin of the cell nucleus and involved in the organization of the DNA structure. Three basic polypeptides with antimicrobial activity were isolated from an extract with acetic acid of skin of catfish (Ictalurus punctatus). The molecular mass of these peptides were reported as 15.5kDa (HLP-1), 30kDa (HLP-2), and 15kDa (HLP-3), respectively. HLP-1 showed to be the dominant peptide and to be closely related to the histone H2B, with inhibitory effect against pathogenic bacteria of fish such as Escherichia coli D31. It has been shown that the activity of the HLP-1 was stable in acidic conditions and in heat [40]. Other catfish (Parasilurus asotus) also contained a peptide with strong antimicrobial activity named Parasin I [40]. This peptide consists of 19 amino acid residues including 3 arginine residues and 5 lysine residues with MM of 2,000Da. It was shown the existence of homology between parasin I and the N-terminal region of histone H2A, suggesting that parasin I may have derived from H2A through cleavage by specific protease. It was also shown [40] that parasin I has immunomodulatory activity. A peptide called pleurocidin was found in epithelial mucosal cells of winter flounder, with an aliphatic conformation in &alph;-helix similar to other antimicrobial peptides. This structure enables the binding to the cellular membrane rich in anionic phospholipids acting similarly to detergents in lysis of the membrane and destruction of the microbial cell. From the yellow head catfish (Pelteobagrus fulvidrago) a peptide with 19 amino acid residues was isolated, Pelteobagrin (Gly-Lys-Leu-Asn-Leu-Phe-Leu-Ser-Arg-Leu-Glu-Ile-leu-Lys-Phe-Val-Gly-Ala-Leu), with activity against Gram (+) and Gram (-) bacteria [41]. The best studied antimicrobial peptides were isolated from hydrolysates of the intestine of the fish known as Atlantic bonito. The C111 (Gly-Val-Tyr-Pro-His-Lys) and C112 (Ile-Arg-Pro-Val-Gln) peptides isolated from autolysates of liver and intestine of bonito also inhibited the activity of ACE in vitro with IC50 of 1.6 and 1.4, respectively. The antihypertensive effects of these peptides were also shown in vivo in SHR and Sprague-Dawley® rats [16,42]. A JF2 peptide containing 26 amino acid residues was isolated from Japanese flounder (Paralichthys olivaceus) which showed antimicrobial activity against Gram (-) Escherichia coli and Gram (+) Streptooccus aureus and Lacrococcus garvieae, but had no effect on the Gram (-) bacteria Edwardsiella tarda [43]. Expression of the JF2 peptide was observed in the liver, heart, kidneys, spleen, and stomach of the flounder.
Fogaca et al., [44] identified and sequenced the first antimicrobial peptides from bovine Hemoglobin (Hb). The sequence of amino acids was identified by mass spectrometry: Phe-Leu-Ser-Phe-Pro-Thr-Thr-Lys-Thr-Tyr-Phe-Pro-His-Phe-Asp-Leu-Ser-His-Gly-Ser-Ala-Gly-Val-Lys-Gly-Hys-Gly-Ala-Lys (MM 3,205.7Da) corresponding to the (f: 33-61) of &alph;-hemoglobin. A second peptide with antimicrobial activity was obtained from bovine &alph;-hemoglobin (f: 1-23) by hydrolytic action of pepsin [45] and purified by ion-exchange and Reversed-Phase Chromatography (RP-HPLC). The following amino acid composition was established through the use of mass spectrometry: Val-Leu-Ser-Ala-Ala-Asp-Lys-Gly-Asn-Val-Lys-Ala-Ala-Trp-Gly-Lys-Val-Gly-Gly-His-Ala-Ala-Glu (MM 2,236.9Da). Activity was tested against growth of M. luteus A270 bacteria in liquid culture medium.
A more recent research [46] submitted bovine hemoglobin to hydrolysis by pepsin (pH 4.5, 23°C), isolating and purifying from the hydrolysate the peptide: Val-Thr-Leu-Ala-Ser-His-Leu-Pro-Ser-Asp-Phe-Thr-Pro-Ala-Val-His-Ala-Ser-Leu-Asp-Lys-Phe-Leu-Ala-Asn-Val-Ser-Thr-Val-Leu (MM 3.150Da). Antimicrobial activity was tested against 9 lineages: 3 Gram (-) lineages, Escherichia coli, Shigiella sonnei, and Salmonella enteritidis; 6 Gram (+) lineages, M. luteus A270, Listeria innocua, Enterococcus faecalis, Bacillus cereus, Staphylococcus saprophyticus, and Staphylococcus similans. After purification by RP-HPLC the peptide showed antimicrobial activity against M. luteus A270, Listeria innocua, E. coli, and S. enteritidis. Jang et al., [47] isolated from hydrolysate of bovine sarcoplasmatic protein a peptide with antimicrobial action, whose composition and structure is the following: Gly-Phe-His-Ile-Phe-His-Gly-Asp-Phe-His-Ile-Asn-Gly-Gly-Leu-Ser-Asp-Gly-Glu-Trp-Gln (MM 2,438.6Da). This peptide presented inhibitory activity against all lineages tested in the 3 previous researches.
Ovotransferrin, a protein from egg yolk presents an antimicrobial domain as characteristic of the transferrin family. Ibrahim et al., [48] reported that practically all bactericidal activity of ovotransferrin can be attributed to the N-lobe. An antimicrobial peptide (OTAP-92) with 92 AA res (Leu 109 – Asp 200) contains 6 cysteines involved in the formation of 3 intra chain disulfide bonds. The peptide was obtained by partial acidic proteolysis. Maintenance of the tertiary structure of OTAP-92 is essential for antimicrobial activity, which is exercised through a mechanism of degradation of the microorganism’s membrane [49]; however, the OTAP-92 peptide does not exercise Fe binding [48,50].
ANTIOXIDANT ACTIVITY
Peptides with antioxidant action have been isolated and characterized from a significant number of food proteins, specially: fish, meat, eggs, milk proteins, cereals and legumes [5,8,14,39,51,52]. In addition to the Caseinophosphopeptides (CPPs) with antioxidant action, several peptides are contained in the primary sequences of the &alph;S1-caseins, β-caseins, K-caseins, and β-LG. The antioxidant activity of these peptides has been attributed to the high contents of histidine and some hydrophobic amino acids. A hexapeptide, Tyr-Phe-Tyr-Pro-Gln-Leu, with antioxidant activity was released from &alph;S1-casein (f: 144-149) by the proteolytic action of pepsin in vitro [53]. It has been shown by Frong et al., [15] that ovotransferrin acts by retarding oxidation in turkey meat. The antioxidant activity of ovotransferrin was enhanced by the rupture of its structure, allowing the approach of amino acid residues that scavenge free radicals and form chelates with pro-oxidant metals [54]. Recent research by Wu and Acero-Lopez [50] showed that hydrolysis of ovotransferrin increases the antioxidant activity. Fourteen peptides were isolated and characterized from ovotransferrin [55]. Two tetrapeptides – Trp-Asn-Ile-Pro and Gly-Trp-Asn-Ile – showed higher antioxidant activity, suggesting that (Trp-Asn-Ile) should represent the nucleus responsible for the high antioxidant activity of these peptides.
Di Bernardini et al., [14] conducted systematic research of sarcoplasmatic proteins extracted from bovine brisket. The extraction was carried out according to the procedure described by Jang and Lee [56] and the extract contained mostly myoglobin, troponin and enzymes of the metabolism of muscle tissue. The proteins were submitted to hydrolysis by papain (24 h at 37°C). The hydrolysate was submitted to UF with 10kDa and 3kDa cut-off membranes. Total hydrolysate and fractions were tested for antioxidant activity, by the 3 usual methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH), which measures free radical scavenging activity; antioxidant power to reduce ferric ion (FRAP); and Fe+2 chelation capacity. The peptidic content and the composition of the peptides were studied by spectrophotometric and chromatographic methods that identified and characterized 11 peptides in the total hydrolysate, 15 peptides in the 10-kDa fraction, and 9 peptides in the 3-kDa fraction, all showing antioxidant activity for the 3 methods.
A significant number of recent publications reports the presence of peptides with antioxidant action in hydrolysates from fish proteins and in by-products of the fishery industry [5,39,57,58]. Je et al., [57] obtained peptides from dark muscles of a variety of tuna (Thunnus obesus) through the enzymatic action of several proteases and evaluated their antioxidant potential. A peptic hydrolysate with potent antioxidant action was obtained, from which it was possible to isolate the peptide (Leu-Asn-Leu-Pro-Thr-Ala-Val-Tyr-Met-Val-Tyr), MM 1,222 Da, with strong free radical scavenging action and antioxidant effect on inhibition of Peroxidation of Unsaturated Fatty Acids (PUFAs). The scavenging power of this peptide was also tested in cellular systems, showing high degree of effectiveness. The authors conclude that the peptide obtained from dark muscles of this species of tuna may have great potential as antioxidant ingredient in the protection of functional foods and/or medicinal products. Urakova et al., [39] also describe antioxidant activity in peptides derived from hydrolysates of several species of fish. A peptidic fraction with MM of about 10kDa isolated from a hydrolysate of cod skeleton proteins showed high antioxidant activity, as opposed to the fractions with MMs of 5kDa and 30kDa. According to Klompong [59] the antioxidant properties of hydrolysates of trevally (Selaroides leptolepsis) with yellow stripes was dependent on the Degree of Hydrolysis (DH) and on the type of product. The peptidic fraction with MM of 1.77kDa, obtained from the hydrolysate by the enzyme flavourzyme presented the highest antioxidant effect. A hydrolysate obtained with the alcalase enzyme originated a fraction (2.44kDa) that also showed antioxidant activity. Ranathunga et al., [60] isolated from the marine species Conger (Conger myriaster) a peptide that presented high antioxidant action, with the following structure: Leu-Gly-Leu-Asn-Gly-Asp-Asp-Val-Asn (MM 1,060Da).
Najafian and Babji [5] reported on a number of peptides with antioxidant action, isolated from different species of fish, with their characteristic structures and molecular masses, using different proteolytic enzymes and hydrolysis techniques.
PEPTIDES WITH ANTITUMOR ACTIVITY
Although antitumor activity has been described for protein hydrolysates of various foods that are common in human diet, very few peptides with antitumor action have been isolated from food proteins with proven antitumor action. Freiburghaus et al., [61] describe the action of Lactoferricin (LFcin), polypeptide derived from the milk protein lactoferrin by pepsin hydrolysis, as being capable of partially inhibiting, in vitro, the proliferation of human colon cancer. On the other hand, Mader et al., [62] demonstrated that LFcin inhibits some vasoendotelial growth factors and angiogenesis, competing for a similar binding site to heparin, in endothelial cells. Angiogenesis is an important component in carcinogenesis, especially in the phenomena of metastasis.
Ohami et al., [63] demonstrated cytotoxic effect in the-β unit of Ovalbumin (OVA) in culture of tumor cells, using scanning electron microscopy. The β-subunit and glycoprotein fragments obtained from the digesta of glycoproteins presented in vitro antitumor potential similar to that described for intact glycoproteins. Antitumor effects of heavily glycosylated peptides (220 and 120kDa) separated from egg white ovomucin, after treatment with pronase, were tested on a dual tumor-implantation system. Mice of BALB/c lineage received simultaneous inoculation of fibrosarcoma cells Meth-A (1×106 cells) in the right flank and (2×105 cells) in the left flank, on day zero (beginning) of the experiment. There was injection of (100μg/animal/d) of the two peptides in the right flank in days 3, 4, and 5 and the mice were maintained for 21 days. The 2 peptides cured the tumors injected in the right side and slightly inhibited the growth of the tumors implanted in the left flank. With the removal of sialic acid of the peptide of 120kDa it was observed that the sialic acid residue was not necessarily indispensable to the direct action, in loco in the elimination of the tumor, but it seems to have been indispensable to the regression of the distant tumors (left flank). The absence of inhibitory activity when the animals received inoculation of tumor cells only in the left flank and the increase of acid immunosuppressive protein in blood serum suggests a slight activation of the immune system by the fragments with antitumor action [64]. Castro et al., [65] investigated, in vitro, the antitumor effect of a Whey Protein Isolate (WPI), a Bovine Collagen Hydrolysate (BCH), and fractions of this hydrolysate on B16F10 cells of human melanoma. In this study they considered the percentage of non-viable cells, cell cycle phases, and possible mechanisms involved in the anti proliferative action on cancer cells, in an ideal culture medium. The collagen derivatives for this study were obtained from a bovine skin collagen hydrolysate provided by “Gelita South America,” Cotia, SP, Brazil. The hydrolysate fractions were obtained through the techniques of molecular exclusion (membranes) and fractionation by Reversed-Phase Chromatography (RP-HPLC). The fractions obtained through molecular exclusion of the BCH fraction (mean MM 3kDa) were: permeate P1 (BCH-P1, mean MM 2.5kDa); permeate P2 (BCH-P2, mean MM 1.7kDa); permeate P3 (BCH-P3, mean MM 1.3kDa). From fraction BCH-P1, they obtained 5 retentates (R1, R2, R3, R4, and R5), by UF in membranes (UFC Millipore, SP, Brazil) whose final retentate R5 (MM<3kDa) was also tested in the experiments. From BCH-P1 they also obtained 4 fractions (F1-F4) through reversed-phase chromatography (preparative column Vydac C18). Permeate BCH-P1 and all its fractions were tested comparatively to a negative control (B16F10 cells+culture medium) and a positive control (B16F10+culture medium+WPI).
Product concentration required to inhibit 50% of proliferation of B16F10 cells (IC50) ranged from 0.19 to 156.9mg/mL for all products tested (Table 2). Hydrolysate fractions from BCH-P1 and F1–F4 with IC50 of less than 1mg/mL were the most active in inhibiting the proliferation of melanoma cells B16F10. Alterations in cell cycle phases were characterized by a strong decrease in the G2/M transition that characterizes an inhibition of cell reproduction, some increase in S phase for (BCH-P1 and F4) but a strong increase of cells in the G0/G1 transition by the hydrolysates BCH-P1 and F4. Caspase-3 expression increased significantly in all mediums containing the fractions F1-F4 and retentates R, increase also observed in the presence of the integral hydrolysate (BCH) or of the WPI. Apoptosis was extremely high in the presence of the fractions F1-F3, at low concentrations (400μg/mL). Considering these results, the authors suggest that the mechanism of tumorigenesis inhibition by these peptides should involve the cascade action of caspases and apoptosis.
| Sample | IC50 (mg/mL) | Concentration Range Studied |
| HCB | 70.87 | 6 - 100mg/mL |
| HCB-P1 | 100.74 | 6 - 100mg/mL |
| HCB-P1 | 0.31 | 0.002 - 1mg/mL |
| HCB-P2 | 177.11 | 6 - 100mg/mL |
| HCB-P3 | 72.42 | 6 - 100mg/mL |
| R1 | 6.11 | 0.004 - 8mg/mL |
| R2 | 2.10 | 0.004 - 8mg/mL |
| R3 | 1.79 | 0.004 - 8mg/mL |
| R4 | 3.65 | 0.004 - 8 mg/mL |
| R5 | 1.78 | 0.004 - 8mg/mL |
| P5 | 2.35 | 0.004 - 8mg/mL |
| F1 | 0.34 | 0.098 - 500mg/mL |
| F2 | 0.19 | 0.098 - 500mg/mL |
| F3 | 0.42 | 0.098 - 500mg/mL |
| F4 | 0.41 | 0.098 - 500mg/mL |
| WPI | 6.8 | 0.15 - 100mg/mL |
Some peptides with antitumor action are naturally expressed in some foods such as milk (Ubiquitin) and soy, rye, barley, wheat, oats, which contain lunasin. Lunasin, a peptide formed of 43 AA res (MM 4.8kDa) was described in soy [66], wheat [67], oats [68], barley [69], and rye [70].
Freiburghaus et al., [71] described the presence of a new peptide in bovine milk, Ubiquitin (Ub), with molecular mass of approximately 8.6kDa. The presence of Ub in bovine milk was confirmed by Western blot technique and its concentration in milk was estimated at 0.003μmol/L of milk. The authors investigated the effect of the treatment of CaCo-2 cells of human colon cancer with Ub and in higher concentration than in bovine milk. CaCo-2 cells were treated with Ub in the range of concentrations of 0.02 to 0.2μmol/L. The method of flow cytometry using DNA labeled with bromodesoxiuridine was used in the study of cell cycle kinetics of CaCo-2 cells treated with Ub. The data pointed to an extension of the G1 phase. The levels of various cell cycle regulatory proteins were affected. The research data suggest that Ubiquitin (Ub) is possibly one of the milk components capable of reducing the risk of colon cancer. The milk components and mechanisms that contribute to decreased risk of colon cancer are not yet fully known.
BIOACTIVE GLYCOPEPTIDES AND PHOSPHOPEPTIDES
As the names suggest, phosphopeptides are peptides derived from phosphoproteins. Phosphoproteins are quite common in nature; however, those best known for their bioactivity are the phosphocaseins in milk and the proteins found in egg, particularly in egg yolk. It is also commonly found in foods phospholipoproteins and phosphoglycoproteins or even phospholipoglycoproteins.
A large number of phosphopeptides have been obtained from caseins. However, a subunit of casein contains only 1 to 13 phosphoserine residues to stabilize the amorphous calcium phosphate, while an egg yolk protein molecule (phosvitin) contains approximately 120 phosphoserine residues, suggesting that several phosphopeptides with different sizes capable of binding and releasing calcium ions can form from phosvitin [72]. The granular fraction of egg yolk (after centrifugation, 10,000g, 30 min) is composed of phosvitin (60%) and lipovitelin. Phosvitin contains 90% of all phosphorus of egg yolk. Phosvitin is considered one of the most phosphorylated proteins found in nature with phosphorus content ranging from 3% to 10% of the molecule [73]. Phosvitin is highly hydrophilic, it presents a high number of negative charges and a very low percentage of hydrophobic side chains [74]. Although phosvitin can be considered a potential source of bioactive phosphopeptides, it is highly resistant to proteolytic digestion in vitro due to its extraordinarily complex primary structure, composed of long continuous segments of oligophosphoserine [75]. Goulas et al., [11] submitted phosvitin to hydrolysis by pepsin, trypsin, and &alph;-chymotrypsin. Pepsin produced 3 peptides: Gly4 – Glu 41 (38 res); Asn 44 – Leu 193 (150 res); and a C-terminal fragment, Leu 193 – Glu 214 (21 res). Action of trypsin resulted in 2 main peptides, Ala1 – Arg 35 (35 res) and Gln 49 – Arg 212 (164 res). Digestion by &alph;-chymotrypsin released 2 peptides, Gly4 – Gln 49 (46 res) and Ala 50 – Trp 210 (161 res). Electrophoretic analysis (SDS-PAGE) of the tryptic digestion of phosvitin indicated that the molecular mass of the largest fragment was 28kDa. Phosvitin dephosphorylation by treatment with alkali (NaOH at 37°C) increased the susceptibility of phosvitin to proteolysis, resulting in peptides with 10 to 20 AA res. (1 to 3kDa) after digestion with trypsin [76].
According to a recent review by Samaraweera et al., [77], although phosvitin is an attractive source of phosphopeptides, the high price of phosvitin limits its application for the production of bioactive phosphopeptides. Therefore, research aimed at lowering the cost of obtaining phosvitin needs to be developed in order to enable its viable application in the food industry. The authors propose the use of phosphopeptides derived from phosvitin in the pharmaceutical and cosmetics industries in the production of antioxidant and anti-aging derivatives in the form of mineral chelating peptides. To this end, specific studies should be conducted in this area.
CASEINOPHOSPHOPEPTIDES (CPPS) AND GLYCOMACROPEPTIDE (GMP)
The term CPP was introduced in the 1950s to describe phosphorylated peptides derived from caseins and capable of improving the calcification of children with rickets. These peptides presented high phosphoserine content and were capable of increasing the calcium balance by 39% to 70% in newborn children with rickets. Later studies showed that the CPPs could also bind, in addition to Ca, otherminerals like Mg, Fe, Ba, Cr, Ni, Co, and Se [78,79].
New studies have shown that the CPPs are a mixture of peptides with different molecular masses in the range from 1.4 to 9.6kDa and that about 50% of the CPPs identified presented the sequence (SerP-SerP-SerP-Glu-Glu), responsible for the property of binding minerals [80]. In addition to increasing the solubility, absorption, and fixation of minerals to bones and teeth, the CPPs along with the caseinoglycomacropeptide (GMP) also act by inhibiting the adherence of Streptococcus sobrinus and S. mutans, thus decreasing the incidence of tooth decay and dental erosion [81]. CPPs also act as antioxidant at intestinal level, protecting the intestine against oxidative stress and helping to maintain intestinal health [82]. The glycomacropeptide originates from Kappa casein in the milk-clotting process by action of the enzyme chymosin and represents the C-terminal portion of κ-casein (f: 106-169), which due to being water soluble remains in whey after coagulation and separation of caseins. In addition to protecting the teeth against caries by its adhesive action, GMP also presents prebiotic, immunomodulatory, and antithrombotic action (res 106-116) and stimulates the action of the intestinal hormone cholecystokinin, produced in the small intestine (duodenum) and regulator of appetite and food intake [4,83].
OPIOID AND ANTIOPIOID PEPTIDES
Opioids are released by all caseins and by some whey proteins. Three types of receivers have been recognized in the membranes of cells of the central and peripheral nervous systems called δμ, μ, and δ. These receptors may bind to the opioids that start to modulate the functioning of cells [84]. Natural opioids are peptides known as enkephalin, endorphin, and dynorphin and are derived respectively from proopiomelanocortin, proenkephalin, and prodynorphin. All these opioids present the N-terminal sequence (Tyr-Gly-Gly-Phe). Opioid peptides were obtained from β-casein (f: 60-70), which are the β-casomorphins, and from &alph;-S1-casein (f: 90-96), called &alph;-casomorphins. Unlike the &alph;- and β-caseins, the Kappa-caseins release antagonist opioid or antiopioid [85]. It has been shown that casomorphins can produce analgesia, increase the intestinal transit time of food bolus, produce antidiarrheal effect, stimulate the absorption of amino acids and electrolytes as well as the secretion of insulin and somatostatin [85-88] demonstrated that opioids can influence the choice and intake level for certain types of foods, especially foods that are rich in sugar and fat, stimulating the preference and intake by consumers.
IMMUNOMODULATORY PEPTIDES
Fewer investigations have been conducted with immunomodulatory peptides isolated from food proteins. Models in vitro enable the study of different parameters of cellular metabolism, such as: proliferation and activation of lymphocytes, antibody production, impacts on non-specific immune response, among others. There has been major interest in the study of B-cell proliferation for production of antibodies and T cells that control the specific immune response to antigens, including inflammatory reactions that can lead to degradation of tissue in diseases of the Gastrointestinal System (GIS). According to Gauthier et al., [10] there are many discrepancies between authors about the positive effect of peptides on lymphocyte proliferation, with far greater agreement about the effect of milk peptides on antibody production. The discrepancies observed should be attributed to differences in the methodologies applied, in the materials studied, as well as in the study models used.
Two synthetic peptides corresponding to &alph;-LA, Tyr-Gly (f:50-51) and Tyr-Gly-Gly (f: 18-20), stimulated protein synthesis and the proliferation of peripheral lymphocytes isolated from human blood, when stimulated by Concanavalin A (Con A), a mitogenic protein. Maximum stimulation was reached at a concentration of 10-4mol/L for Tyr-Gly and 10-8mol/L for Ty-Gly-Gly [89]. Peptides released during milk fermentation by lactic bacteria modulate the proliferation of human lymphocytes, by controlling the production of certain cytokines and stimulating the phagocytic activity of macrophages [90,91]. It was also suggested that immunomodulatory peptides from milk can alleviate atopic allergic reactions in humans and strengthen the immunity of the gastrointestinal tract mucosa [92].
The Glycomacropeptide (GMP) and its derivatives have shown to exercise various immunomodulatory functions, such as: immunosuppressive effects on the production of IgG antibodies [93,94], in addition to stimulatory effects on the proliferation of phagocytic activities in human cells similar to macrophages, such as U937 [95]. The oligopeptide lactoferricin, derived from lactoferrin, stimulated the production of human neutrophil-activating Interleukin-8 (IL-8) by human polymorphonuclear leukocytes [96]. Whey Protein Concentrate (WPC) enriched with the Glycomacropeptide (GMP) suppressed the secretion of Interleukin-4 (IL-4) and of Interferon-Gamma (INF-γ) in culture of mouse lymphocytes; an effect that was partly ceased after enzymatic digestion with pepsin and pancreatin. The GMP also induced the production of the family of Interleukins-1 (IL-1) in mouse macrophage cells [97,98]. A synthetic peptide (Gly-Leu-Leu), corresponding to the sequence (51-53) of &alph;-lactalbumin significantly increased phagocytosis of peripheral blood cells by mouse peritoneal macrophages, protecting the animals against infection by lethal Klebsiella pneumonia [99]. Bovine lactoferricin also promoted phagocytic activity in human neutrophils. Recently it has been shown that the strengthening of the immune system, partly attributed to the presence of bioactive peptides in food, has contributed decisively to reduce the risk of chronic diseases [100].
The bioactive peptides action mechanism is related to direct interaction with pathogens, as well as with the suppression or stimulation of immune responses [101]. Studies show that both immunosuppression and immunostimulation can be important in preventing and combating several pathological conditions of the organism, and the biopeptides may act against inflammation, autoimmune diseases, preventing rejection in transplants and improving general health [10]. There are numerous examples in the literature of such interactions, most with peptides derived from milk [102].
ANTIHYPERLIPIDEMIC AND ANTITHROMBOTIC PEPTIDES
A significant number of peptides with anticholesterolemic (antilipidemic) and antithrombotic properties has been described recently [6,12,30,53,103], among other studies. The vast majority of the studies have been conducted with milk proteins. Pihlanto [53] described 2 peptides with hypocholesterolemic action resulting from hydrolysis of β-lactoglobulin by trypsin, Ile-Ile-Ala-Glu-Lys (β-Lg, f: 71-75) and Ala-Leu-Pro-Met-His (β-Lg, f: 142-146), with proven action in vitro, in cell cultures and in animal model, both receiving the name of lactostatin. The β-lactostatin (Ile-Ile-Ala-Glu-Lys) caused decrease of micellar cholesterol of intestinal environment reducing its absorption. This lactostatin was also capable of inducing gene transcription for production of the human enzyme 7&alph;-hydroxylase, an enzyme that metabolizes cholesterol producing a hypocholesterolemiant effect. According to Cánovas et al., [30] hyperlipidemia, especially hypercholesterolemia, is one of the most important factors for the increased risk and the development of cardiovascular diseases. It has been shown that soy-derived peptides can be responsible for its hypocholesterolemic action, since it has been shown that soy protein hydrolysates promote greater decrease in plasma cholesterol than the intact protein. The hypocholesterolemiant effect of active peptides can be attributed to two different action mechanisms: (a) inhibiting cholesterol absorption, by decreasing micellar cholesterol in the intestine; (b) some peptides may activate LDL receptors, typically blocked by hypercholesterolemia or excessive intake of cholesterol by diet [104].
According to Undenigwe and Aluko [6] protein sources of hypocholesterolemic and hypolipidemic peptides include soy protein [17], milk proteins [105], egg white proteins [106], and fish proteins [107]. Enzymatic hydrolysis can also produce peptides capable of reducing TAG with reduction of lipidemia [108]. Most of the literature on peptides with hypolipidemic property has been obtained from soy proteinhydrolysates. The hypocholesterolemic and hypolipidemic properties of soy protein hydrolysates have been studied in animals [109] and in humans [110] and have been partially attributed to soy 7S globulin (β-conglycinin). This protein’s &alph;+&alph;' subunits strongly increase the expression of the Low Density Lipoprotein (LDL) receptor in hepatocyte cultures, resulting in increased LDL inactivation and degradation [111]. The peptide region responsible for this activity was identified and sequenced. With 24 amino acid residues this peptide corresponds to position 127-150 of the &alph;-subunit, of conglycinin, being responsible for modulating cholesterol homeostasis mediating LDL binding in Hepatocyte Cells (HepG2) [112]. On the other hand, Cho et al., [18] also identified an octopeptide (Phe-Val-Val-Asn-Ala-Tyr-Ser-Asn) of the enzymatic digesta of soy protein as a more potent stimulator of LDL receptor transcription in human liver cells, Hep T9A4. Therefore, the proteolytic digestion of soy protein was important in the release of small bioactive peptides with capacity to enhance the cardioprotective function. This property was also demonstrated in a study by Mockizuki et al., [113], who produced bioactive peptides from isoflavone-free 7S β-conglycinin, using bacterial proteases. The peptides derived from 7S β-conglycinin showed capacity of reducing triacylglycerols by altering the expressions of genes related to triacylglycerol synthesis, also decreasing Apo B-100 protein buildup in Hep G2 cells, partly due to increased expression of m-RNA for LDL receptor synthesis [113]. Apolipoprotein B (apo B-100) is a functional component of Very Low-Density Lipoprotein (VLDL) and its degradation reduces VLDL synthesis. These observations support previous studies showing that soy β-conglycinin provides beneficial effects related to plasma levels of triacylglycerols in humans [114].
In addition to alterations in gene expression, soy protein hydrolysates and their peptides also showed hypocholesterolemic function through binding with bile acids and neutral sterolsin the intestine, resulting in increased fecal elimination of these compounds that when pass to the systemic circulation will stimulate cholesterol synthesis in the organism [17,115]. This function shows that although bioactive peptides with high molecular mass cannot go through the intestinal epithelium and into the bloodstream, indirectly they will be exercising a beneficial action contributing to the lowering of lipidemia and cholesterolemia in hepatocytes and other types of cells, contributing to cholesterol homeostasis by increasing the removal of bile acids and cholesterol from the diet via fecal elimination, depending on their hydrophobic properties.
Lunasin, a polypeptide containing 43 AA res (MM 4.8kDa) – naturally present in soybeans, barley, rye, and wheat–, best known for its antitumor property, also exercises hypocholesterolemic activity by blocking the acetylation of histone H3 (Lys 14) reducing the production of the enzyme hydroxymethyl glutaryl-CoA reductase (HMG-CoA reductase), resulting in a reduction in cholesterol biosynthesis. Lunasin also increases the production of LDL receptors, resulting in reduction of circulating LDL-cholesterol (LDL-c) [116]. Structure-function studies are required for better understanding of the properties of bioactive peptides capable of interfering with the blood and liver cholesterol levels.
Several peptides with antithrombotic activity have been isolated and characterized, most derived from milk proteins (K-casein and lactoferrin). A peptide (His-Gly-Lys) was obtained from myofibrillar protein FK633, with antithrombotic property [117]. The main peptides with antithrombotic action, the casoplatelins, were obtained by hydrolysis of K-casein and are similar to the fibrinogen γ-chain [118]. The hypothesis is that the fibrinogen γ-chain and the K-casein must have originated from a common ancestor 450 million years ago [119]. Table 3 presents the characteristics of some peptides with antithrombotic activity isolated from K-casein and lactoferrin proteins, of bovine milk. It has been observed, in different animal models, that the fraction (39-42) of human lactoferrin has important antithrombotic effect; however, its application has not been recommended due to inducing endothelial cell detachment in vitro[120]. Hydrolysis of lamb casein also produced antithrombotic peptides [121]. Peptides with antithrombotic activity were isolated from K-casein (f: 113-116), and have also been found in fermented dairy products such as yogurt [122]. The AA sequence corresponding to the fraction (109-111) of K-casein was isolated from water extract of two fermented dairy drinks available at the Spanish market [123].
| Sequence of the AA | ProteinPrecursor | Reference |
| Leu-Ser-Phe-Met-Ala-Ile-Pro-Pro-Lys | k-cas (f: 103-111) | (Fiat et al.) [124] |
| Met-Ala-Ile-Pro-Pro-Lys-Lys-Asn-Gln-Asp-Asp-Lys | k-cas (f: 106-116) | (Fiat and Jolles) [9] |
| Met-Ala-Ile-Pro-Pro-Lys | k-cas (f: 106-112) | (Fiat and Jolles) [9] |
| Asn-Gln-Asp-Lys | k-cas (f: 113-116) | (Fiat and Jolles) [9] |
| Lys-Asp-Gln-Asp-Lys | k-cas (f: 112-116) | (Fosset and Tome) [121] |
| Pro-Pro-Leu | k-cas (f: 109-111) | (Hernández-Ledesma et al.) [123] |
| ND | k-cas (f: 152-160) | (Golbetti et al.) [125] |
| ND | k-cas (f: 155-160) | (Golbetti et al.) [125] |
| Leu-Arg-asp-Ser | Lactoferrin (LF) (f: 39-42) | (Rutherfurd and Gill) [123] |
| Lys-Arg-Asp-Pro-Ser | LF | (Mazoyer et al.) [126] |
| His-Gly-Lys | FK 633 (porcine myofibrillar) | (Chavakis et al.) [117] |
PROSPECTS AND POTENTIAL USE OF BIOACTIVE PEPTIDES AS INGREDIENTS, SUPPLEMENTS, IN FUNCTIONAL FOODS
The scientific literature has demonstrated, in food proteins, a large number of potentially bioactive peptide sequences, but that in the protein’s original primary structure shows no bioactivity. Bioactive peptides can be formed during protein hydrolysis, either in normal digestive process or in controlled proteolysis in vitro. Currently, very little is known about the quantities, bioavailability, and bioactivity of the peptides that are formed in the digestive process of foods, as components of the human diet. In order to quantify and measure the impact of the bioactivity of these peptides it is necessary to break the structure of the proteins, either as food component or as isolated, partial or totally purified protein and extract or concentrate the bioactive peptides of interest for health.
Technologies for extraction, hydrolysis, concentration, and purification as well as determination of the amino acid composition and sequence of the peptides are available, but are expensive. The cost-benefit balance will have to be evaluated on a case-by-case basis to justify the investment, considering both economic and human health aspects. It is expected that the attempt of using total hydrolysate and/or specific fractions of hydrolysate will be given greater priority in formulating nutritional supplements and developing foods with specific physiological functionality. Only the United States of North America, Canada, some European and Eastern countries have developed and used functional products with application of bioactive peptides.
Fish protein hydrolysates have potential applications as functional ingredients in different foods because they have numerous and characteristic properties such as: water retention capacity, oil absorption, protein solubility, jellification, ability to foaming and emulsification, in addition to combining numerous bioactivities with benefits for health [127,128].
Unlike the European and Asian countries, North America, Australia, and New Zealand, in Brazil and in South American countries, in general, little has been done with respect to the application of bioactive peptides and hydrolysates as food supplements and/or ingredients, in the formulation of specific functional foods or foods consumed by the general population. This gap as to effective utilization of our sources of bioactive peptides is due to the lack of greater integration between universities and research institutes and the food industry, for the development of integrated multidisciplinary projects, with funding shared between interested sectors: research, industry, and funding agencies for food research and technology.
From the 1980s there was a profound transformation of the concepts of healthy nutrition for the human species with the discovery of new components in foods, considered non-nutrients, but which for being bioactive contributed to improving human health in two complementary aspects: 1) acting as modulators of metabolism and possibly acting on the different metabolic cycles, also acting on the human genome influencing its expression to improve the effectiveness, benefiting the health of individuals and/or populations; 2) reducing the risks of chronic non-communicable diseases, thus contributing to improve the human health, well-being, and increase longevity. Although these concepts had their awakening in Japan [1], the original idea is attributed to Hippocrates (5th century B.C.), historically considered the father of medicine and supposed author of the phrase: “Let food be thy medicine and medicine be thy food.” Foods with these characteristics came to be called “functional foods” and their importance has been recognized not only by the Eastern countries, but also by all countries of Western culture [129-131]. In a simplified and concise way, a functional food is one that contains one or more bioactive substances in concentrations suitable for the quantity ingested with the diet to be enough to exercise in the metabolism the functions previously described in (1 and 2). On the other hand, for a diet to be considered functional (e.g., Mediterranean diet), it must provide a set of functional (bioactive) components that promote additively and/or synergistically the human health, well-being, and longevity.
Therefore, it is concluded that the last century (20th Century) was a time of great achievements in human nutrition and health due to advances in preventive and curative medicine with regard to contagious and communicable diseases, in addition to the major advancement in the science and technology of food and nutrition, providing food with better quality and more available to the population. The basic nutritional requirements were established and communicated to the population and health authorities. Measures were taken to eliminate, to a significant extent, diseases caused by lack or excess of certain nutrients in the diet. At the same time there was a significant increase in the life expectancy of the Brazilian population, which practically doubled in the course of the century. These changes occurred due to the improvement in nutrition and in conditions and lifestyle of part of the population. As a result of the increased longevity and decreased birthrate, there has been a predominant increase of the elderly population and an increasing incidence of chronic and/or degenerative diseases of multiple causes and much more complex treatment, such as cardiovascular diseases, cerebrovascular disease, diabetes, obstructive lung disease, inflammatory diseases in general, obesity, and osteoporosis. For these diseases, satisfactory solutions are still not provided by medicine and nutrition.
By the end of the last century the technologies for fractionation and purification of important food components such as: fractional precipitation, ultracentrifugation, selective extractions, membrane separation processes, different processes of dehydration, separation chromatography (affinity, molecular exclusion, ion exchange, and others), in addition to various forms of electrophoresis, were already available to industries in general and to the food industry.
IDEAL DIET: AN ATTAINABLE GOAL OR UTOPIA (?)
It has been documented throughout the human evolution history that primitive man would have developed their eating habits by a process of trial and error, probably seeking to satisfy their senses, notably the sensory senses involving the perception of color (vision), taste (sweet, sour, salty), and mouth feel (texture, softness, viscosity, chewiness). Considering the plasticity, flexibility, and adaptability, characteristics of the human metabolism, it is assumed that a set of environmental and metabolic stresses, on the primitive man, must have acted strongly in the fixation of metabolic events and phenotypic characteristics that came to be transmitted from generation to generation until the present times. Foods found in nature, not so abundant in variety and quantity, probably led our ancestors to prefer foods of greater hedonic appeal and high concentration of energy such as fat and protein (in animals), starch, soluble sugars, and salts (in roots and tubers, fruits and sea products, when available).
Not coincidentally, these are the favorite foods of modern civilization to date, with the difference that they are provided in great quantity and variety, because of the progress in agriculture and food industry, particularly throughout the past century. Paradoxically, the same foods that served as lifeline for our ancestors, in different parts of the planet, seem to have been and continue to be the villains largely responsible for what is known today as the Metabolic Syndrome, having as component the fat deposition, corpulence and obesity, which progress to diseases such as hypertension, diabetes, cardiovascular and neurological diseases, according to some reports in the current literature [132-138].
Considering the magnitude of the challenges that the food production sector had to face in the last 100 years, it is observed that there has been great evolution from simple demand for food for consumption to a complete range of accumulated properties that include complete product safety, sensory quality, nutritional value, and health promotion. However, this set of accomplishments seems to indicate only isolated successes. It is clear that the present state of the art has added to products currently found on supermarket shelves the result of a complex set of isolated events, rather than the result of a series of preplanned actions.
If we accept the premise that the human species is still evolving, the goal of achieving an ideal diet seems unattainable; we would be trying to reach a frontier that moves by evolutionary pressure. However, with the advances in genetics and human metabolism the concepts of nutrigenomics (genomics, proteomics, metabolomics) can now be applied, which enables the development of special diets for specific individuals and groups, in order to solve some dietary problems in the short and medium term.
According to Kremer et al., [139] there is growing concern that health is not merely the absence of disease but also involves continuous adaptation to environmental changes. A new definition of health emphasizes the ability of the organism to adapt to the constant challenges of physical, social, and emotional nature [140]. In the physiological domain, a healthy organism is able to maintain physiological homeostasis through changing circumstances, and that is called “allostasis” [141]. In the context of metabolic health this ability to adapt has been called “phenotypic flexibility” [142]. Chronic stress can induce adaptation processes that surpass the limits of normal phenotypic flexibility, leading to a progressive inflexibility, which may contribute to the establishment of diseases. Excess or lack of food components in diet induces changes in phenotypic flexibility. Micronutrients and bioactive compounds have key roles in the maintenance of phenotypic flexibility, while excess energy, high intake of glucose, sucrose and fructose or certain trans-fatty acids cause a decline in phenotypic flexibility. The micronutrients are involved in many metabolic processes with exclusive and interactive functions in the body, and which have been studied mainly in isolation.
Human health is based on a complete network of interactions between metabolic cycles, mechanisms, processes, and organs. Many of these processes have to work in a constantly changing environment (diet, infections, stress, temperature, exercise, etc.) and, therefore, in constant struggle to maintain internal homeostasis, adapting to these changes [142].
Also according to Kremer et al., [139], in order to progress in nutrition-related health, there is clear need to characterize the complexity that exists in the interactions between the nutrients and the metabolic pathways, mechanisms, processes, and organs that lead to human health. Moreover, it is clear that physical health is not the only aspect of human health related to nutrition, since mental and social health interact with physical health. The aforementioned authors suggest that, in order to advance the agenda of global nutrition, integrated nutritional research should be employed, using a system of health markers covering relevant aspects of the domains of physical, mental, and social health.
CHALLENGES FOR THE 2000S
Considering what has been presented here and the extensive literature available on what has been developed in food science and technology and the new concepts concerning nutrition and health, in the course of the last century to the present, it is unexpected the fact that at the level of the general population the knowledge and practice of nutrition still leave much to be desired. The question that has been asked fairly often is: why investments in food science and technology and the knowledge obtained in these areas have not translated into greater benefits for the health of the population?
FOOD SCIENCE AND TECHNOLOGY IN BRAZIL
Based on what has been presented in previous pages in this article and on the criticism – albeit veiled – of researchers and funding agencies’ analysts directed to the scientific and technological research in Brazil, the following can be inferred: 1) considering that Food Engineering is a multidisciplinary and applied field, whose ultimate purpose is to provide the consumer public with healthy, nutritious, and functional foods that are, preferably, affordable; 2) that such progress is only achieved through innovation and development of new foods that meet the rapid changes of consumers (such as the population’s age and health profile); 3) also considering that the resources for research and development have become increasingly scarce and scientific progress has become more complex; 4) it is clear today more and more the need for joint action – Food Science, Engineering, and Technology – as the only way so as to achieve the desired objectives and progress in food improvement, as part of the larger goal which is the welfare of the Brazilian people.
To better illustrate what would be a desirable effort for integration in food research and development network, we present the simplified scheme in (Figure 1).
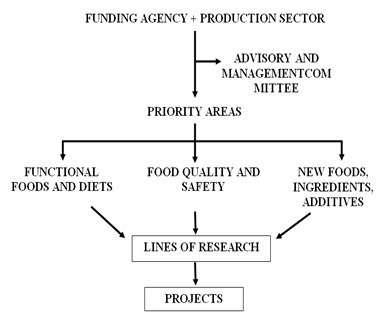 Figure 1: Food research network.
Figure 1: Food research network.
Table 4 suggests the main lines of action to improve the availability of functional diets and food for the population.
| 1. | Education |
| Introduction of food education at all levels of formal education | |
| Informal education by provision of information conducted by professionals from the areas of medicine, food science and technology, and media (properly guided) | |
| 2. | Science and Technology |
| Basic research | |
| Applied research | |
| Technological research | |
| Development of products for specific groups and purposes |
Table 4: Strategies to improve the availability of functional diets and food for the population.
FINAL CONSIDERATIONS
- The knowledge in the area of nutrition has had significant advances because of the related sciences: Genetics, Chemistry, Biochemistry, Physiology, Microanalysis, Computing, and Bioinformatics.
- Until the beginning of the second half of the last century were established the foundations of traditional nutrition, with respect to the mean needs of nearly all nutrients, with emphasis on those considered indispensable in the diet (essential) and that enabled the elimination of clinically manifest deficiency diseases, persisting, however, marginal deficiencies of various micronutrients, in various age groups of the population.
- The needs and adequacy of caloric intake are still objects of discussion, for different age groups and physiological conditions.
- Despite the possibility of elimination of deficiency diseases, chronic and degenerative diseases have become more prevalent from the second half of the last century to the present.
- The concept of functional foods and diets introduced from 1980 has opened new frontiers and challenges in deepening the knowledge of the causes and consequences of the so-called non-communicable chronic diseases.
- Bioactive compounds present in foods (nutrients including bioactive proteins and bioactive peptides, and non-nutrients) can have very important roles in reducing the risk and in controlling these diseases.
- The success achieved in human genomics and in techniques of microanalysis, structures, and statistics has enabled the emergence of the science of bioinformatics.
- Bioinformatics enables the evaluation of how genomic alterations (genotype) are reflected in metabolic alterations (phenotype) and the establishment of the roles of various compounds present in foods (nutrients and non-nutrients) in these transformations, which is conventionally called Nutrigenomics and/or Epigenetics.
- 9. The main objectives of Nutrigenomics and Epigenetics are the deepening of the knowledge of the causes and of the control of chronic non-communicable diseases and the possibility of development of more appropriate diets, considering individuals and groups with very similar genetic characteristics (genotype). This is the main challenge for nutrition research for the 21st century.
REFERENCES
- Barata S, Centeno M, Marques JP, Clode N, da Graça LM (2009) Hemorragia feto-materna grave Massive feto-maternal haemorrhage. Acta Obstet Ginecol Port 3: 169-172.
- Kliegman RM (2011) Nelson Textbook of Pediatrics, (19thedn). Elsevier/Saunders, USA.
- Kim YA, Makar RS (2012) Detection of fetomaternal hemorrhage. Am J Hematol 87: 417-423.
- Lobo AL, Tomás E, da Silva FP, Barbot J, Raposo T (2001) Anemia Neonatal Grave por Hemorragia Feto-Materna Caso Clínico. Acta Pediatr Port 32: 395-397.
- Zuppa AA, Scorrano A, Cota F, D’Andrea V, Francciolla A, et al. (2008) Massive fetomaternal hemorrhage and late-onset neutropenia: description of two cases. Turk J Pediatr 50: 400-404.
- Solomonia N, Playforth K, Reynolds EW (2012) Fetal-Maternal Hemorrhage: A Case and Literature Review. AJP Rep 2: 7-14.
- Dziegiel MH, Nielsen LK, Berkowicz A (2006) Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol 13: 490-495.
- Kecskes Z (2003) Large fetomaternal hemorrhage: clinical presentation and outcome. J Matern Fetal Neonatal Med 13:128-132.
- Arai S (1996) Studies on functional foods in Japan-state of the art. Biosci Biotechnol Biochem 60: 9-15.
- Aryoshi Y (1993) Angiotensin-converting enzyme inhibitors derived from food proteins. Trends in Food Science & Technology 4: 139-144.
- Kitts DD, Weiler K (2003) Bioactive proteins and peptides from food sources. Application of bioprocesses used in isolation and recovery. Curr Pharm Des 9: 1309-1323.
- Korhonen H, Philantro A (2006) Bioactive peptides: production and functionality. Int Dairy J 16: 945-960.
- Najafian L, Babji AS (2012) A review of fish-derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides 33: 178-185.
- Udenigwe CC, Aluko RE (2012) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77: 11-24.
- Undenigwe CC, Howard A (2013) Meat proteome as source of functional biopeptides. Food Res Int 54: 1021-1032.
- Cavazos A, Gonzales de Mejia E (2013) Identification of bioative peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compreh Rev Food Sci Food Safety 12: 364-380.
- Fiat AM, Jolles P (1989) Caseins of various origins and biologically active casein peptides and oligosaccharides: structural and physiological aspects. Mol Cell Biochem 87: 5-30.
- Gauthier SF, Pouliot Y, Saint Sauveur D (2006) Immunomodulatory peptides obtained by enzymatic hydrolysis of whey proteins. Int Dairy J 16: 1315-1323.
- Goulas A, Triplett EL, Taborsky G (1996) Oligophosphopeptides of varied structural complexity derived from the egg phosphoprotein, phosvitin. J Protein Chem 15: 1-9.
- Eckert E, Zambrowicz A, Pokora M, et al. (2013) Peptides derived from egg proteins. World’s Poultry Sci J 69: 375-386.
- Escudero E, Toldrá F, Sentendreu MA, Nishimura H, Arihara K (2012) Antihypertensive activity of peptides identified in the in vitro gastrointestinal digest of pork meat. Meat Sci 91: 382-384.
- Di Bernardini R, Mullen AM, Bolton D, Kerry J, O'Neill E, et al. (2012) Assessment of the angiotensin-I-converting enzyme (ACE-I) inhibitory and antioxidant activities of hydrolysates of bovine brisket sarcoplasmic proteins produced by papain and characterization of associated bioactive peptic fractions. Meat Sci 90: 226-235.
- Frong GW, Ragon LF, Niemann I (1986) Conalbuming as a antioxidant in turkey meat. Poultry Sci 65: 45.
- Fujii M, Matsumura N, Mito K, Shimizu T, Kuwahara M, et al. (1993) Antihypertensive Effects of Peptides in Autolysate of Bonito Bowels on Spontaneously Hipertensive Rats. Biosch Biotechnol Biochem 57: 2186-2188.
- Cho SJ, Juillerat MA, Lee CH (2007) Cholesterol lowering mechanism of soybean protein hydrolysate. J Agric Food Chem 55: 10599-10604.
- Cho SJ, Juillerat MA, Lee CH (2008) Identification of LDL-receptor transcription stimulating peptides from soybean hydrolysate in human hepatocytes. J Agric Food Chem 56: 4372-4376.
- Gu Y, Majunder K, Wu J (2011) QSAR-aided in sílico approach in evaluation of food proteins as precursors of ACE inhibitory peptides. Food Res Int 44: 2465-2474.
- Takano T (1998) Milk Derived Peptides and Hypertension Reduction. Int Dairy J 8: 375-381.
- Silva AV, Malcata FX (2005) Caseins as source of bioactive peptides. Int Dairy J 15: 1-15.
- Meisel H (1998) Overview on milk protein-derived peptides. Int Dairy J 8: 363-373.
- Schanbacher FL, Talhouk RS, Murray FA, Gherman LI, Willett LB, et al. (1998) Milk-born bioactive peptides. Int Dairy J 8: 393-403.
- Wu J, Ding X (2002) Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int 35: 367-375.
- Wu J, Aluko RE, Nakai S (2006) Structural requirements of Angiotensin I-converting enzyme inhibitory peptides: quantitative structure-activity relationship study of di- and tripeptides. J Agric Food Chem 54: 732-738.
- Wu J, Aluko RE, Nakai S (2006) Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Modeling of Peptides Containing 4-10 Amino Acid Residues. QSAR & Combinatorial Science 25: 873-880.
- Miguel M, Aleixandre A (2006) Antihypertensive peptides derived from egg proteins. J Nutr 136: 1457-1460.
- Sentendreu MA, Toldrá F (2007) Evaluation of ACE inhibitiory activity of dipeptides generated by the action of porcine muscle dipeptidyl peptidases. Food Chem 102: 511-515.
- De Leo F, Panarese S, Gallerani R, Ceci LR (2009) Angiotensin converting enzyme (ACE) inhibitory peptides: production and implementation of functional food. Curr Pharm Des 15: 3622-3643.
- Cánovas JM, Rentero PZ, Martínez AM-C, Hernández ML, Alemán JA (2011) Péptidos bioactivos. Clin Invest Arterioscl 23: 219-227.
- Tomita M, Bellamy W, Takase M, Yamauchi K, Wakabayashi H, et al. (1991) Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci 74: 4137-4142.
- Pellegrini A, Thomas U, Bramaz N, Hunziker P, von Fellenberg R (1999) Isolation and identification of three bactericidal domains in the bovine alpha-lactalbumin molecule. Biochim Biophys Acta 1426: 439-448.
- Pellegrini A, Detting, C, Thomas U, Hunziker P (2001) Isolation and characterization of four bactericidal domains in the bovine beta-lactoglobulin. Biochim Biophys Acta 1526: 131-140.
- Malkoski M, Dashper SG, O’brien-Simpson NM, Talbo GH, Macris M, et al. (2001) Kappacin, a novel antibacterial peptide from bovine milk. Antimicrob Agents Chymother 45: 2309-2315.
- Shamova OV, Orbov DS, Ovchinnikova TV et al. (2006) Antimicrobial peptides from leucocytes of Russian Sturgeon (Acipenser guldenstadti). Fundament Nauki 1: 10-13.
- Maksimyuk NN, Mar’ yanovskaya YV (2009) On the advances of enzymatic method on the synthesis of protein hydrolysates. Fundament Issled 1: 34-35.
- Kim SK, Wijiesekara I (2010) Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods 2: 1-9.
- Maksinova EM (2006) Elaboration of the technology of utilization of protein wastes using the method of enzymatic hydrolysis. Vestn MGTU 9: 875-879.
- Urakova IN, Pozhariskaya ON, Demchenko DV, Shikov AN, Makorav VG (2012) The biological activities of fish peptides and methods of their isolation. Russian J Mar Biol 38: 417-422.
- Cho JH, Park IY, Kim MS, Kim SC (2002) Matrix metalloproteinase 2 is involved in the regulation of the antimicrobial peptide Parasin I production in catfish skin mucosa. FEBS Lett 531: 459-463.
- Su Y (2011) Isolation and identification of pelteobagrin, a novel antimicrobial peptide from the skin mucus of yellow catfish (Pelteobagrus fulvidraco). Comp Biochem Physiol B Biochem Mol Biol 158: 149-154.
- Karaki H, Kuwahara M, Sugano S, Doi C, Doi K, et al. (1993) Oral administration of peptides derived from bonito bowels decreases blood pressure in spontaneously hypertensive rats by inhibiting angiotensin converting enzyme. Comp Biochem Physiol Ser C 104: 351-353.
- Hirono I, Hwang JY, Ono Y, Kurobe T, Ohira T, et al. (2005) Two different types of hepcidins obtained from the Japanese flounders Paralichthys olivaceus. FEBS J 272: 5257-5264.
- Fogaca AC, Da Silva PI, Miranda MT, Bianchi AG, Miranda A, et al.(1999) Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus microplus. J Biol Chem 274: 25330-25334.
- Froidevaux R, Krier F, Nedjar-Arroume N, Vercaigne-Marko D, Kosciarz E, et al. (2001) Antibacterial activity of a pepsin-derived bovine hemoglobin fragment. FEBS Lett 491: 159-163.
- Daoud R, Dubois V, Bors-Dodita L, Nedjar-Arroume N, Krier F, et al. (2005) New antibacterial peptide derived from bovine hemoglobin. Peptides 26: 713-719.
- Jang A, Jo C, Kang KS, Lee M (2008) Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem 107: 327-336.
- Ibrahim HR, Iwamori E, Sugimoto Y, Aoki T (1998) Identification of a distinct antibacterial domain within the N-Lobe of ovotransferrin. Biochim Biophys Acta (BBA) – Molecular Cell Research 1401: 289-303.
- Ibrahim HR (2000) Ovotrasferrin. In Naidu AS (ed.). Nutritional food antimicrobial systems, Boca Raton, CRC Press, Florida, USA.
- Wu J, Acero-Lopez A (2012) Ovotransferrin: Structure, bioactivities, and preparation. Food Res Int 46: 480-487.
- Di Bernardini R, Harnedy P, Bolton D, Kerry J, O’Neill E, et al. (2011) Antioxidant and antimicrobial peptic hydrolyzates from muscle protein sources and by-products. Food Chem 124: 1296-1307.
- Di Bernardini R, Rai DK, Bolton D, Kerry J, O'Neill E, et al. (2011) Isolation, purification and characterization of antioxidant peptidic fractions from a bovine liver sarcoplasmic proteins thermolysin hidrolyzate. Peptides 32: 388-400.
- Pihlanto A (2011) Milk protein products-Bioactive peptides. In: Bioactive Peptides. Elsevier, Amsterdam, Netherlands. Pg no: 879-886.
- Elias RJ, Kellerby SS, Decker FA (2008) Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 48: 430-441.
- Shen S, Chahal B, Majumder K, You SJ, Wu J (2010) Identification of novel antioxidative peptides derived from a thermolytic hydrolysate of ovotransferrin by LC-MS/MS. J Agric Food Chem 58: 7664-7672.
- Jang A, Lee M (2005) Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Sci 69: 653-661.
- Jae-Young Je, Zhong-Ji Qian, Hee-Guk Byun, Se-Kwon Kim (2007) Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Proc Biochem 42: 840-846.
- Samaranayaka AG, Kitts DD, Li-Chan EC (2010) Antioxidative and angiotensin-I-converting enzyme inhibitory potential of a pacific Hake (Merluccius productus) fish protein hydrolysate subjected to simulated gastrointestinal digestion and caco-2 cell permeation. J Agric Food Chem 58: 1535-1542.
- Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, et al. (2009) Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe Trevally (Selaroides leptolepsis). J Food Sci 74: 126-133.
- Ranathunga S, Rajapakse N, Kim SK (2006) Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur Food Res Technol 222: 310-315.
- Freiburghaus C, Janicke B, Lindmark-Mansson H, Oredsson SM, Paulsson MA (2009) Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J Dairy Sci 92: 2477-2484.
- Mader JS, Smyth D, Mashall J, Hoskin DW (2006) Bovine lactoferricin inhibits basic fibroblast growth factor- and vascular endothelial growth factor165-induced angiogenisis by competing for heparin-like binding sites on endothelial cells. Am J Pathol 169: 1753-1766.
- Hiroshi O, Hifumi O, Takashi Y (1993) Cytotoxic effect of sialoglycoprotein derived from avian egg white ovomucin on the cultured tumor cell. Medicine and Biology 126: 19-23.
- Watanabe K, Tsuge Y, Shimoyamada M, Ogama M, Ebina T (1998) Antitumor Effects of Pronase-Treated Fragments, Glycopeptides, from Ovomucin in Hen Egg White in a Double Grafted Tumor System. J Agric Food Chem 46: 3033-3038.
- Castro GA, Maria DA, Bouhallab S, Sgarbieri VC (2009) in vitro impact of whey protein isolate (WPI) and collagen hydrolysates (CHs) on B16F10 melanoma cells proliferation. J Dermatol Sci 56: 51-57.
- De Lumen BO (2005) Lunasin: a câncer preventive soy peptide. Nutr Rev 63: 16-21.
- Jeong HJ, Jeong JB, Kim DS, Park JH, Lee JB, et al. (2007) The cancer preventive peptide lunasin from wheat inhibits core histone acetylation. Cancer Lett 255: 42-48.
- Nakurte I, Kirhnere I, Manniece J, Saleniece K, Krigere L, et al. (2013) Detection of lunasin peptide in oats (Avena sativa L). J Cereal Sci 57: 319-324.
- Jeong HJ, Lam Y, de Lumen BO (2002) Barley lunasin suppresses ras- induced colony formation and inhibits core histone acetylation in mammalian cells. J Agric Food Chem 50: 5903-5908.
- Jeong HJ, Lee JR, Jeong JB, Park JH, Cheong YK, et al. (2009) The cancer preventive seed peptide lunasin from rye is bioavailable and bioactive. Nutr Cancer 61: 680-686.
- Freiburghaus C, Welinder C, Tjörnstad U, Lindmark-Månsson H, Paulsson M, et al. (2010) Identification of ubiquitin in bovine milk and its growth inhibitory effects on human cancer cell lines. J Dairy Sci 93: 3442-3452.
- Castellani O, Guérin-Dubiard C, David-Briand E, Anton M (2004) Influence of physicochemical condition and technological treatments on the ion binding capacity of egg yolk phosvitin. Food Chemistry 85: 569-577.
- Taborsky G, Mok CC (1967) Phosvitin. Homogeneity and molecular weight. J Biol Chem 242: 1495-1501.
- Dickinson E, Pinfield VJ, Horne DS (1997) On the “Anomalous” Adsorption Behavior of phosvitin. J Colloid Interface Sci 187: 539-541.
- Byrne BM, van Het Schip AD, van der Klundert JA, Arnberg AC, Gruber M, et al. (1984) Amino acid sequence of phosvitin derived from the nucleotide sequence of part of the chicken vitellogenin gene. Biochemistry 23: 4275-4279.
- Jiang B, Mine Y (2000) Preparation of novel functional oligophosphopeptides from hen egg yolk phosvitin. J Agric Food Chem 48: 990-994.
- Samaraweera H, Zang WG, Lee EJ, Ahn DU (2011) Egg yolk phosvitin and functional phosphopeptides--review. J Food Sci 76: 143-150.
- Fitzgerald RJ (1998) Potential uses of caseinophosphopeptides. Int Dairy J 8: 451-457.
- Scholz-Ahrens KE, Schrezenmeir J (2000) Effects of bioactive substances in milk on mineral and trace element metabolism with special reference to caseinphosphopeptides. Brit J Nutr 84: 147-153.
- Bouhallab S, Cinga V, Aít-Oukhatar N, Bureau F, Neuville D, et al. (2002) Influence of various phosphopeptides of caseins on iron absorption. J Agric Food Chem 50: 7127-7130.
- Schupbach P, Neeser JR, Golliard M, Rouvet M, Guggenheim B (1996) Incorporation of caseinoglycomacropeptide and caseinophosophopeptide into the salivary pellicle inhibits adherence of mutans Streptococci. J Dent Res 75: 1779-1788.
- Kitts DD (2005) Antioxidant properties of casein-phosphopeptides. Trends Food Sci Technol 16: 549-554.
- Hartman R, Meisel H (2007) Food-derived peptides with biological activity: from research to food application. Curr Opinion Biotechnol 18: 163-169.
- Bitri L (2004) Optimization study for the production of an opioid-like preparation from bovine casein by mild acidic hydrolysis. International Dairy Journal 14: 535-539.
- Shah NP (2000) Effects of milk-derived bioactives: an overview. Br J Nutr 84: 3-10.
- Meisel H, Schlimme E (1990) Milk proteins: precursors of bioactive peptides. Trends Food Sci Technol 1: 41-43.
- Korhonen H, Philanto-Läppäla A, Rantamäki P, Tupasela T (1998) Impact of processing on bioactive proteins and peptides. Trends Food Sci Technol 9: 307-319.
- Drewnowski A (1992) Food preferences and the opioid peptide system. Trends Food Sci Technol 3: 97-99.
- Kayser H, Meisel H (1996) Stimulation of human peripheral blood lymphocytes by bioactive peptides derived from bovine milk proteins. FEBS Lett 383: 18-20.
- Matar C, Le Blanc JG, Martin L, Perdigón G (2003) Biologically active peptides released in fermented milk: role and functions. In Farnworth ER (ed.), Handbook of Fermented Foods, Functional Food and Neutraceuticals Series, CRC Press, Florida, USA. Pg no: 177-201.
- Meisel H, FitzGerald RJ (2003) Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr Pharm Des 9: 1289-1295.
- Korhonen H, Pihlanto A (2003) Food-derived bioactive peptides--opportunities for designing future foods. Curr Pharm Des 9: 1297-1308.
- Monnai M, Horimoto Y, Otani H (1998) Immunomodificatory effect of dietary bovine kappa-caseinoglycopeptide on serum antibody levels and proliferative responses of lymphocytes in mice. Milchwissenschaft 53: 129-132.
- Manzo MA, López-Fandinõ R (2004) k-casein macropeptide from cheese whey: Physicochemcal, biological, nutritional, and technological features for possible uses. Food Rev Intern 20: 329-355.
- Li EW, Mine Y (2004) Immunoenhancing effects of bovine glycomacropeptide and its derivatives on the proliferative response and phagocytic activities of human macrophagelike cells, U937. J Agric Food Chem 52: 2704-2708.
- Shinoda I, Takase M, Fukuwatari Y, Shimamura S, Köller M, et al. (1996) Effects of lactoferrin and lactoferricin on the release of interleukin 8 from human polymorphonuclear leukocytes. Biosci Biotechnol Biochem 60: 521-523.
- Monnai H, Otani H (1997) Effect of bovine kappa-caseinoglycopeptide on serum antibody levels and proliferative responses of lymphocytes in mice. Milchwissenschaft 52: 192-196.
- Otani H, Monnai H (1995) Induction of an interleukin-1 receptor antagonist-like component produced from mouse spleen cells by bovine k-caseinoglycopeptide. Biosc Biotechnol Biochem 59: 1166-1168.
- Berthou J, Migliore-Samour D, Lifchitz A, Delettré J, Floc'h F, et al. (1987) Immunostimulating properties and three-dimensional structure of two tripeptides from human and cow caseins. FEBS Lett 218: 55-58.
- Min-Suk Ma, Bae IY, Lee HG, Yang CB (2006) Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench). FoodChem 96: 36-42.
- Honcock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24: 1551-1557.
- Biziulevicius GA, Kislukhina OV, Kazlauskaité J, Zuraité V (2006) Food-protein enzymatic hydrolysates possesses both antimicrobial and Immunostimulatory activities: a “cause and effect” theory of bifunctionality. FEMS Immunol Med Microbiol 46: 131-138.
- Ricci-Cabello I, Herrera MO, Artacho R (2012) Possible role of milk-derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr Rev 70: 241-255.
- Sirtori CR, Lovati MR, Manzoni C, Monetti M, Pazzucconi F, et al. (1995) Soy and cholesterol reduction: clinical experience. J Nutr 125: 598-605.
- Kirana C, Rogers PF, Bennett LE, Abeywardena MY, Patten GS (2005) Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactivities. J Agric Food Chem 53: 4623-4627.
- Manso MA, Miguel M, Even J, Hernández R, Aleixandre A, et al. (2008) Effects of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem 109: 361-367.
- Wergedahl H, Liaset B, Gudbrandsen OA, Lied E, Espe M, et al. (2004) Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol, and lowers acyl-CoA:cholesterol acyltransferase activity in liver of Zucker rats. J Nutr 134: 1320-1327.
- Kayashita J, Shimaoka I, Nakajoh M, Yamazaki M, Kato N (1997) Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-Fed rats because of its low digestibility. J Nutr 127: 1395-1400.
- Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T (2000) Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (Yellow KK). Nutrition 16: 349-354.
- Hori G, Wang M-F, Chan Y-C, Komtatsu T, Wong Y, et al. (2001) Soy protein hydrolysate with bound phospholipids reduces serum cholesterol levels in hypercholesterolemic adult male volunteers. Biosc Biotechnol Biochem 65: 72-78.
- Lovati MR, Manzoni C, Granazza E, Sirtori CR (1998) Soybean Protein Products As Regulators of Liver Low-density Lipoprotein Receptors. I. Identification of Active b-conglycinin subunits. J Agr Food Chem 46: 2474-2480.
- Lovati MR, Manzoni C, Granazza E, Arnoldi A, Kurowska E, et al. (2000) Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J Nutr 130: 2543-2549.
- Mockizuki Y, Macbuchi M, Kihno M, Hirotsuka M, Wadahama H, et al. (2009) Changes in lipid metabolism by soy beta-conglycinin-derived peptides in HepG2 cells. J Agric Food Chem 57: 1473-1480.
- Kohno M, Hirotsuka M, kito M, Matsuzawa Y (2006) Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean beta-conglycinin. J Atheroscler Thromb 13: 247-255.
- Yang S, Liu S, Yang H, Lin YH, Chen JR (2007) Soybean Protein Hydrolysate Improves Plasma and Liver Lipid Profiles in Rats Fed High-Cholesterol Diet. J Am Coll Nutr 26: 416-423.
- lunasin.com
- Chavakis T, Boeckel N, Santoso S, Voss R, Isordia-Salas I, et al. (2002) Inhibition of platelet adhesion and aggregation by a defined region (Gly-486-Lys-502) of high molecular weight kininogen. J Biol Chem 277: 23157-23164.
- Caen J, Jolles P, Bal dit, Soller C, et al. (1992) Anti-thrombotic activity of milk protein peptide sequences (in french). Cahiers Nutr Dietetique 27: 33-35.
- Jolles P, Levy-Toledano S, Fiata AM, Soria C, Gillessen D, et al. (1986) Analogy between fibrinogen and casein. Effect of an undecapeptide isolated from kappa-casein on platelet function. Eur J Biochem 158: 379-382.
- Rutherfurd KJ, Gill HS (2000) Peptides affecting coagulation. Br J Nutr 84: 99-102.
- Fosset S, Tome D (2000) Dietary protein – derived peptides with antithrombotic activity. Bull Int Dairy Fed 353: 65-68.
- Dionysius DA, Marschke RJ, Wood AJ, Milne J, Beattie TR, et al. (2000) Identification of physiologically functional peptides in dairy products. Aust J Dairy Technol.
- Hernández-Ledesma B, Amigo L, Ramos M, Recio I (2004) Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion. J Agric Food Chem 52: 1504-1510.
- Fiat AM, Miglione-Samour D, Jolles P, Drouet L, Bal dit Sollier C, et al. (1993) Biologically active peptides from milk proteins with emphasis on two examples concerning antithrombotic and immunomodulating activities. J Dairy Sci 76: 301-310.
- Golbetti M, Minervini F, Rizzello CG (2004) Angiotensin-I converting-enzyme inhibitory and antimicrobial bioactive peptides. Int J Dairy Technol 57: 173-188.
- Mazoyer EC, Bal dit Soller C, Drouet L, et al. (1992) Active peptides from human and cows milk proteins: effects on platelets function and vessel wall. In: Pubert-Branquet M, Dupont, CH, Paoletti R (eds). Foods, Nutrition, and Immunity, Karger, Basel, Switzerland. Pg no: 89-95.
- Chalamaiah M, Narsing RG, Rao DG, Jyothirmayi T (2010) Protein hydrolysates from meriga (Cirrhinus mrigala) egg and evoluation of their functional properties. Food Chem 120: 652-657.
- Chalamaiah M, Kumar BD, Hemalatha R, Jyothirmayi T (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: A review. Food chem 135: 3020-3038.
- Sgarbieri VC, Pacheco MTB, (1999) Alimentos funcionais fisiológicos. Braz. J. Food Technol 2: 7-19.
- Sgarbieri VC, (2012) Inovação nos processos de obtenção de Componentes do leite bovino. Editora Atheneu, São Paulo, Brazil. Pg no: 291.
- Urula N, Lahteenmaki L (2007) Consumers’ changing attitudes towards functional foods. Food Qual Prefer 18: 1-12.
- Rolls ET (2012) Taste, olfactory and food texture reward processing in the brain and control of appetite. Proc Nutr Soc 71: 488-501.
- Panek-Scarborough LM, Dewey AM, Temple JL (2012) Sensation and perception of sucrose and fat stimuli predict the reinforcing value of food. Physiol Behavior 105: 1242-1249.
- Naish KR, Harris G (2012) Food intake is influenced by sensory sensitivity. PlosOne 7: 43622.
- Monteleone P, Pisicitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, et al. (2012) Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glicerol in health humans: a pilot study. J Clin Endocrinol Metab 97: 917-924.
- Pontzer H, Raichlen DA, Wood BM, Mabulla AZP, Racette SB, et al. (2012) Hunter-Gatherer Energetics and Human Obesity. PlosOne 7: 40503.
- Wells CK (2012) The evolution of human adiposity and obesity: where did it all go wrong? Dis Model Mech 5: 595-607.
- Zhang F, Klebansky B, Fine RM, Liu H, Xu H, et al. (2010) Molecular mechanism of the sweet taste enhancers. PANS 107: 4752-4757.
- Kremer B, Van Wietmarschen H, Van Ommen B (2015) Getting a grip on complexity: systems nutrition. Sight and Life 29: 71-75.
- Huber M, Knottnerus JA, Green L, Jadad AR, Kromhout D, et al. (2011) How should we define health? Brit Med J 343: 4163.
- Schulkin J (2004) Allostasis, homeostasis and the costs of physiological adaptation. Cambridge University Press, UK.
- van Ommen B, van der Greef J, Ordovas JM, Daniel H (2014) Phenotypic flexibility as key factor in the human nutrition and health relationship. Genes Nutr 9: 423.
Citation: Sgarbieri VC (2017) Food Proteins and Bioactive Peptides, Functional Diets. J Food Sci Nut 3: 023.
Copyright: © 2017 Valdemiro Carlos Sgarbieri, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

