
Study on Aspergillus Species and Aflatoxin Levels in Sorghum (Sorghum bicolor L.) Stored for Different Period and Storage System in Kewet Districts, Northern Shewa, Ethiopia
*Corresponding Author(s):
Habtamu Fekadu GemedeDepartment Of Food Technology And Process Engineering, Center For Food Science & Nutrition, Addis Ababa University, College Of Natural Sciences, Wollega University, Nekemte, Ethiopia
Tel:+251 917036924,
Email:fekadu_habtamu@yahoo.com
Abstract
Sorghum serves as staple food for over 100 million people in Sub-Saharan African countries. It is the most important nutritional security crop and ranks third among major cereal crops in terms of area and production next to teff and maize in Ethiopia. However, Sorghum is susceptible to contamination by molds that produces aflatoxin that causes hepatotoxic and carcinogenic effects on humans and animals. This study was conducted to assess Aspergillus Species and aflatoxin level in Sorghum (Sorghum bicolor L.) stored under different storage system for different storage period. Thirty samples were analyzed for aflatoxin contamination using high performance liquid chromatography equipped with fluorescent detector and Aspergillus Species were isolated and identified using phenotypic features in a potato dextrose agar culture media. About 56.7%, 16.7%, and 23.3% of the Sorghum samples were found to be contaminated with Aspergillus flavus, Aspergillus niger and Aspergillus parasiticus, respectively. The level of total aflatoxin, aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2 were in the range of 11.44 to 344.26µg/kg, 3.95 to 153.72µg/kg, 1.17 to 91.82µg/kg, 9.87 to 139.64µg/kg, and 3.22 to 52.02µg/kg, respectively. The concentration of aflatoxin in all Sorghum samples surpassed the maximum level set by the European commission and therefore, deserves attention to control them across the Sorghum value-chain.
Keywords
INTRODUCTION
Sorghum (Sorghum bicolor L. Moench) is the world’s fifth most important cereal crop that is grown for grain and fodder in the semi-arid tropics, mainly in Asian and African countries [1]. Sorghum is used as a major food and nutritional security crop for more than 100 million people in the Horn of Africa [2]. Ethiopia is one of the major centers of origin and diversity for Sorghum cultivation [3]. Sorghum ranks third among major cereal crops in terms of area and production next to teff (Eragrostis teff) and maize (Zea mays) throughout the country [4]. It is estimated more than 1.6 million hectares of the land covered with Sorghum production [5]. The lives of millions of Ethiopians depend on Sorghum as a staple food crop [1]. However, Sorghum crops have been affected by numerous mould contaminations [6].
Aflatoxins are naturally occurring toxic secondary metabolites of the storage fungi (Aspergillus flavus and Aspergillus parasiticus) which are produced in most agricultural products stored at inappropriate places, temperatures and moisture level. It is extremely persistent under most conditions of storage, handling and processing [7]. These two species are common and widely distributed in tropical and sub-tropical parts of the world. Aflatoxin has been found as contaminant in agricultural food products especially in cereals and animal feeds. Aspergillus flavus is common and widespread in nature and is most often found when certain grains are grown under stressful conditions such as drought. Additionally, mould occurs in soil, decaying vegetation, hay and grains undergoing microbiological deterioration and invades all types of organic substrates whenever and wherever the conditions are favorable for its growth [8,9].
Aflatoxin contamination of food causes hepatotoxicity, carcinogenicity, immunosuppression, mutagenic and teratogenic [10] and associated with childhood stunting, which cause severe economical losses to the country [11]. It is a serious health problem because factors which encourage the production of these toxins by mycotoxin (Aspergillus flavus and Aspergillus parasiticus) abound in Africa. Their presence in food interferes with micronutrients absorption and status in the body and as a consequence, they affect immunity and development [10]. Aflatoxin B1 is one of the most potent naturally occurring animals carcinogen; and immunosuppressive to immunosuppressant [12]. The extent to which mycotoxins affect human health is difficult to investigate in countries where health system lack capacity and resources are limited. For instance, factors such as immune suppression contributing to the overall burden of infectious diseases is difficult to quantify, but is undoubtedly significant. However, food safety remains an important opportunity for addressing current health problems in developing countries [13].
Previous reports have indicated aflatoxin contamination of cereals and pulses such as Sorghum, teff, wheat, maize, peanut, legumes collected from silos, warehouses, shops and market places in Ethiopia [14]. Many investigators have detected aflatoxin and other mycotoxins in many agricultural foods such as maize, teff, broad bean, Sorghum, beriberi, traditional spices (mitten shiro) and wheat in Ethiopia [15-20].
Kewet woreda is one of the Sorghum producing districts in the country and farmers use both underground pit and above ground storage systems. The storage system “Gotera” are made from different plants, clay, grass, ash, and cow dung. In the underground pit storage, they wash the pit with water and put the Sorghum grain until they are full, then the cover with grass, clay and flatted stone. Some farmers are using cleaning, insecticides and fumigants to prevent insect damage and adding the Sorghum grain in to the pits. The grain is stored for long periods; especially, this is the case during times of food scarcity. These storage systems are believed to protect against insect damage and theft, fire, domestic and wild animals and improve the quality of Sorghum as well. These Sorghum grains are stored under unhygienic conditions and very often spoiled by moulds and may develop mycotoxin contamination. Therefore, this particular research was endeavored to analyze the occurrence of Aspergillus Species and aflatoxin content in Sorghum grain stored at different time periods and different storage system.
MATERIALS AND METHODS
Description of the study area
Chemicals and reagents
Survey questionnaire
Sampling and sample size
 Figure 1: About 1kg of Sorghum samples were collected from the aboveground storage and underground pit storage system from each selected role model farmer.
Figure 1: About 1kg of Sorghum samples were collected from the aboveground storage and underground pit storage system from each selected role model farmer.Determination of moisture content
Moisture content in (2)
Determination of aflatoxin
Method adaptation for analysis of aflatoxin
High performance liquid chromatography equipped with fluorescence detector was used for analyzing the aflatoxin level of the Sorghum samples. The column size was 250mm×4.6mm and the Rheodyne injector size was 20µL. Milli Q water and mixture of acetonitrile and methanol (in the ratio of 71.5/28.5) were used as a mobile phase. The Wavelength florescence detector was set at 440nm. The flow rate was limited to 1.0ml/mm. The parameters selected for method adaptation were linearity, specificity, and accuracy, limit of detection and quantification and precision.
Sample extraction and clean-up
The final extracts were filtered through a 0.45μm PTFE membrane, which is 150μl of the lower phase added in to vials and 20μL were injected into a high performance liquid chromatography column, using a Shimadzu high pressure liquid chromatography (Kyoto, Japan) with fluorescence detector (excitation at 365 nm and emission above 440 nm).
Isolation and identification of fungi
Isolates were identified to a species level based on morphological (phenotypic) features as described by [23]. For this purpose: isolates representing each pure culture were grown on PDA Agar at 25?C for 5-7 days. Fungal colonies that grow rapidly and produce colors of white, yellow, yellow-brown, brown to black or shades of green, mostly consisting of a dense felt of erect conidiophores were broadly classified as Aspergillus Species. while those that produce blue spores were considered as Pencillium species. Isolates with dark green colonies and rough conidia were considered as Aspergillus parasiticus. The major distinction currently separating Aspergillus niger from the other species of Aspergillus is the production of carbon black or very dark brown spores from Biseriate phialides [24].
Data analysis
RESULTS AND DISCUSSION
Information on farmer’s awareness of mould contamination
| Characteristics | Response | Frequency | Percent (%) |
| Age | 19-38 | 14 | 46.7 |
| 38-55 | 13 | 33.33 | |
| > 55 | 3 | 10 | |
| Education | Illiterate | 19 | 63.33 |
| Primary school | 9 | 30 | |
| Secondary school | 2 | 6.7 | |
| Sex | Female | 4 | 13.3 |
| Male | 26 | 86.7 | |
| Yes | Yes | 14 | 46.7 |
| No | 16 | 53.3 | |
| Color | Black | 8 | 26.6 |
| White | 6 | 20 | |
| Problem | Yes | 0 | 0 |
| No | 30 | 100 | |
| Critical problem | Insect | 15 | 50 |
| Rodent | 10 | 33.33 | |
| Mould | 5 | 16.7 | |
| Other | 0 | 0 | |
| Measure taken to control | Insect sides | 25 | 75 |
| Fire wood smoke | 5 | 25 | |
| Drying technology | Sun drying | 30 | 100 |
| Other technology | 0 | 0 | |
| Cleaning and sanitation | Yes | 30 | 100 |
| No | 0 | 0 | |
| Storage Location | Field | 15 | 50 |
| Inside house | 5 | 16.7 | |
| Courtyard | 10 | 33.3 | |
| Mixing | Yes | 0 | 0 |
| No | 30 | 100 | |
| Storage system | Above ground (Gotera) | 9 | 30 |
| Underground (pit) | 10 | 33.3 | |
| Both (Pit and Gotera) | 11 | 36.7 | |
| Duration of storage time periods | Six month | 11 | 36.7 |
| One year | 15 | 50 | |
| Two year and above | 4 | 13.3 | |
| More than four year | All | 100 |
Table 1: Socio demographic data and farmers’ awareness about mould contamination.
Aflatoxin level in Sorghum stored at different storage periods and storage system
| Storage system | Sample Code | B1 (µg/kg) | B2 (µg/kg) | G2 (µg/kg) | G1 (µg/kg) | Total Afs (µg/kg) | Moisture content (%) |
| < 12 month stored Sorghum | |||||||
| Aboveground storage | S01 | 28.82 | 11.17 | 3.1 | 16.62 | 75.57 | 11.4 |
| S02 | 3.96 | 4.29 | 50.2 | 111.25 | 16.59 | 10.4 | |
| S03 | 31.76 | 2.15 | 7.65 | 24.94 | 60.41 | 9.73 | |
| S04 | 71.52 | 22.81 | 26.25 | 139.64 | 176.49 | 9.53 | |
| S05 | 69.79 | 27.12 | 41.96 | 113.36 | 183.16 | 10.2 | |
| Underground pit storage | S06 | 82.34 | 14.77 | 8.44 | 16.98 | 177.33 | 11.3 |
| S07 | 31.49 | 20.38 | 7.46 | 56.39 | 100.97 | 10.33 | |
| S08 | 28.64 | 18.55 | 26.16 | 31.11 | 91.85 | 10.43 | |
| S09 | 28.8 | 19.11 | 23.07 | 50.31 | 93.4 | 8.77 | |
| SO10 | 5.3 | 16.54 | 8.47 | 17.24 | 46.48 | 11.53 | |
| 1-2 year stored Sorghum | |||||||
| Aboveground storage | SO11 | 21.75 | 2.76 | 21.02 | 101.79 | 44.27 | 11.86 |
| SO12 | 46.16 | 3.48 | 15.73 | 68.3 | 88.61 | 11.43 | |
| SO13 | 88.9 | 24.43 | 51.01 | ND | 210.53 | 10.78 | |
| SO14 | 33.17 | 52.05 | 26.28 | 18.96 | 175.16 | 9.82 | |
| SO15 | 31.06 | 40.56 | 4.65 | 23.87 | 145.61 | 9.33 | |
| Underground pit storage | SO16 | 8.86 | 35.01 | 12.65 | 116.13 | 94.28 | 11.05 |
| SO17 | 3.95 | 2.01 | 2.04 | 80.7 | 11.44 | 12.53 | |
| SO18 | 64.68 | 17.33 | 18.74 | 42.3 | 152.19 | 9.47 | |
| SO19 | 69.69 | 3.06 | 52.84 | 61.82 | 128.86 | 10.8 | |
| SO20 | 153.72 | 33.45 | 37.89 | ND | 344.26 | 9.4 | |
| ≥ 2 year stored Sorghum | |||||||
| Aboveground storage | SO21 | 26.27 | 6.58 | 19.4 | 12.92 | 65.17 | 9.4 |
| SO22 | 51.28 | 1.17 | 32.91 | 114.1 | 199.46 | 9.4 | |
| SO23 | 12.95 | 30.26 | 16.95 | 78.17 | 138.33 | 8.9 | |
| SO24 | 105.96 | 91.82 | 24.61 | 25.53 | 247.92 | 9.19 | |
| SO25 | 36.75 | 2.85 | 72.65 | 109.2 | 221.45 | 11.13 | |
| Underground pit storage | SO26 | 15.57 | 6.89 | 12.58 | 129.26 | 164.3 | 9.6 |
| SO27 | 9.6 | 21.35 | 15.62 | 51.79 | 98.36 | 8.8 | |
| SO28 | 4.59 | 10.08 | 3.22 | 9.87 | 17.68 | 11.75 | |
| SO29 | 7.25 | ND | 4.28 | 9.94 | 21.47 | 10.72 | |
| SO30 | ND | ND | ND | ND | ND | 9.4 | |
| % of contamination level | 96.66 | 93.33 | 96.66 | 90 | ----- | ----- | |
Table 2: Level of aflatoxin content in Sorghum sample stored for different period and storage system.
ND -means not detected.
Aflatoxin B1 and total aflatoxin content in Sorghum stored for less than 12 month
Aflatoxin B1 and total aflatoxin content in Sorghum stored for between one and two year
Aflatoxin B1 and total aflatoxin content in Sorghum stored for two or more than year
From the total 30 samples, 96.66%, 93.33%, 96.7%, and 90% were contaminated by aflatoxin B1, B2, G2 and G1, respectively. These showed total aflatoxin contamination by the level ranged from 11.44µg/kg to 344.26µg/kg and the mean total aflatoxin value of 123.85µg/kg. Aflatoxin contamination levels were detected Sorghum samples in range of 1.17-91.82µg/kg for AFB2, 3.22-139.64µg/kg for AFG2 and 9.87-139.64µg/kg for AFG1 (Table 2). Enormous variation of aflatoxin contamination was observed between 23 Sorghum samples and 7 samples had aflatoxin levels below detection limits (3 for AFG1, 2 for AFB2, 1 for AFB1 and AFG2). This may be due to the variation in fungal colonization, especially Aspergillus flavus, and spore density during the grain development stage. It had comparable to the variation of this fungus in developing Sorghum grain was studied earlier by Ratnavathi et al. [25].
About 96.66% of the total aflatoxin in the Sorghum samples was attributed to AFB1 which ranged from 3.95- 153.72µg/kg. Generally speaking, AFB1 is the most toxic aflatoxin among all types of aflatoxin and is considered to be hepatocarcinogenic and immunosuppressive as well [12]. Therefore, the high concentration of the AFB1 in Sorghum samples indicates that there could be a serious problem of aflatoxin contaminations in the study area.
The incidence of aflatoxin contamination in the examined Sorghum grain samples appear to be lower than those reported for Sorghum and maize [16] in which the aflatoxin contamination reached up to 1000µg/kg for Sorghum and 1388µg/kg for wheat, respectively. However, this study result showed a very high aflatoxin levels above the permissible limits. The maximum aflatoxin B1 content found in this research (153.72µg/kg) was below the highest amount reported by Habtam and Kelbesa (692µg/kg) [17]. On other hand, the presence of aflatoxin contamination (96.6%) reported in the present study was relatively higher than the previous study reported by Ayalew [26] aflatoxin infection (88%) in maize from Ethiopia.
The presence of aflatoxin B1 detected in the present study (153.72µg/kg) was generally higher than from the previous study by Chala et al., [15] on Sorghum in Ethiopia (29.5µg/kg). However, the amount of aflatoxin B1 (153.72µg/kg) in the Sorghum samples in this research was much relatively lower than the finding reported by Alpert et al., [27] which was as high as 1000µg/kg. The concentrations of aflatoxin G1 obtained in the present study were larger (139.64µg/kg) when compared with previous reports for Sorghum grain (29.65µg/kg) by Chala et al. [15].
On other hand, the percent of AFB1 level (96.66%) reported in the present study was relatively higher than the previous study reported in Brazil which was 39% for pre-fermented and 32% for post-fermented Sorghum samples [28] with maximum values of 5.10µg/kg and 30.05µg/kg, respectively. Therefore, the current study also showed aflatoxin contamination in Sorghum stored under different storage system and period. This indicates that there is high problem of aflatoxin contamination in the country.
Effects of storage periods on the level of aflatoxin contamination in Sorghum
| Aflatoxin content (µg/kg) | Aflatoxin content (µg/kg) | ||||
| AFG2 | AFG1 | AFB2 | AFB1 | Total aflatoxin | |
| < 12 months | 20.28a | 57.78a | 15.69a | 38.24ab | 64.31a |
| 1-2 years | 24.28a | 51.39a | 17.09a | 52.19a | 59.31a |
| > 2 years | 22.22a | 54.08a | 21.42a | 27.02b | 81.64a |
However, the aflatoxin content and mycotoxin development in Sorghum grain stored for different period may increase due to moisture migration from the surrounding and storage condition (temperature and humidity) reported by Mohamed et al., Mashilla et al., and Habtamu and Kelbessa [30,31,17]. The level of aflatoxin increment stated by Shephard [13], flood damage to grain (mainly Sorghum) in underground storage areas resulted in visible fungal contamination and these harsh realities; it is not surprising that fungal contamination of staple food. The current study showed that the study area has a suitable condition for mycotoxin development and farmers may not used sufficiently good handling, harvesting and storage practice. This practice may lead to elevated aflatoxin production.
Effects of storage system on the level of aflatoxin contamination in Sorghum
| Storage type | Average aflatoxin content (µg/kg) | |||
| AFG2 | AFG1 | AFB2 | AFB1 | |
| Above ground | 49.67a< | 63.91a | 21.57a | 44.1a |
| Underground pit | 30.05a | 44.92a | 14.56a | 34.3a |
HPLC chromatogram of the aflatoxin in Sorghum sample
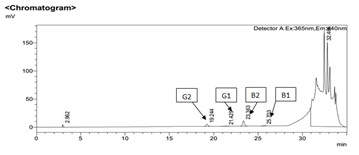 Figure 2: HPLC Chromatogram of 1 ppb standard mixture of four aflatoxin.
Figure 2: HPLC Chromatogram of 1 ppb standard mixture of four aflatoxin.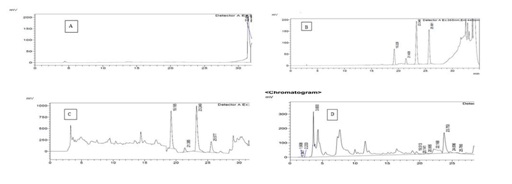 Figure 3: Chromatogram of blank sample (A), standard calibration curve (B) and naturally contaminated fungal sample (peanut) (C), Sorghum sample (D), (elution order AFG2, AFG1, AFB2 and AFB1).
Figure 3: Chromatogram of blank sample (A), standard calibration curve (B) and naturally contaminated fungal sample (peanut) (C), Sorghum sample (D), (elution order AFG2, AFG1, AFB2 and AFB1).Isolation and identification of Aspergillus Species from Sorghum sample
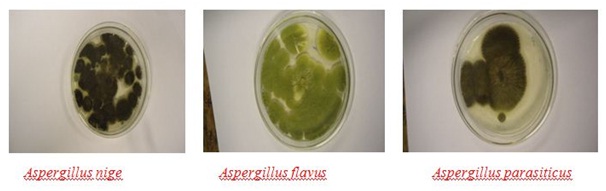 Figure 4: Morphological (phenotypic) feature of Aspergillus Species, (Photo by-Author).
Figure 4: Morphological (phenotypic) feature of Aspergillus Species, (Photo by-Author).In this study all Sorghum samples come from regions with temperatures ranging from 17 to 30?C which supports the growth of Aspergillus Species. Under tropical condition, stored products are more susceptible to Aspergillus Species than other fungi as many Aspergillus Species are favored by the combination of low water activity (aw) and relatively high storage temperature [17]. Although, cereal grains belong to corn, rice, barley; wheat and Sorghum are found susceptible to aflatoxin accumulation by aflatoxogenic fungus.
In table 5 indicated that the occurrence of Aspergillus spp. in Sorghum grain stored at different storage period and storage system; 56.7%, 16.7% and 23.33% of Aspergillus flavus, Aspergillus parasiticus and Aspergillus niger was found, respectively from the total 30 samples.
| Aspergillus species | Number of sample | % of Aspergillus species found |
| Aspergillus flavus | 17 | 56.66 |
| Aspergillus parasiticus | 5 | 16.66 |
| Aspergillus niger | 7 | 23.33 |
| Total | 29 | 96.65 |
However, the current results showed that Sorghum was more profoundly colonized by aflatoxin producing Aspergillus Species, with overall aflatoxin levels being correspondingly higher. The Sorghum grain contamination by Aspergillus Species. and the production of aflatoxin are highly influenced by the weather conditions prevailing during the grain development stage, i.e., seed set to physiological maturity stage [25]. In addition, this may be caused by the variations in cultivars, storage periods, and storage system and over all handling practices used.
Table 6 showed that the Aspergillus flavus (13.33% for < 12 months 23.33% for 1-2year and 20% for ≥ 2 year) and Aspergillus parasiticus (6.67% for < 12 months 6.67% for 1-2year and 3.33 % for ≥ 2 year) were occurred Sorghum stored at different periods. Aspergillus flavus and Aspergillus parasiticus stored at 1-2year was slightly larger than Sorghum grains stored with ≥ 2 year and < 12 month. This indicated that aflatoxin contamination in Sorghum grain was highly disposed from storage periods. The presence of Aspergillus niger (3.3%) in Sorghum stored between one and two year had lower than both 10 % of Aspergillus flavus and Aspergillus parasiticus. Sorghum stored with two or more years had relatively lower Aspergillus parasiticus (3.33%) than both 6.67% of Aspergillus parasiticus at Sorghum stored in less than 12 month and between one and two year.
| Duration of Storage period | Aspergillus species(%) | ||
| Aspergillus flavus | Aspergillus parasiticus | Aspergillus niger | |
| < 12 months | 13.33 | 6.67 | 10 |
| 1-2year | 23.33 | 6.67 | 3.33 |
| ≥ 2 year | 20 | 3.33 | 10 |
Table 6: The occurrence of Aspergillus Species in different storage periods.
Evaluation of aflatoxin results against different international standards
The aflatoxin content measured in all the Sorghum samples showed that natural aflatoxin was higher and 96.66 % of samples were contaminated by toxin as compared to highly susceptible crops like maize and groundnut [35]. However, the result also above the safety limit (20µg/kg) recommended by the Codex Alimentarius Committee.
In East African standard specification (CD-ARS 462:2012 (E) for Sorghum, it is stated that the Sorghumgrains shall comply with those maximum mycotoxin limits established by the Codex Alimentarius Commission. In particular, total aflatoxin levels in Sorghum grains for human consumption shall not exceed 10µg/kg with AFB1 not exceeding 5µg/kg when tested according to ISO 16050. However, the present study showed that the total aflatoxin concentration and AFB1 were surpassed the specified tolerable levels by the above mentioned regulations.
CONCLUSION
The level of total aflatoxin in Sorghum samples was above tolerable limits set by different organizations. This can be more hazardous to individuals who are more sensitive and prone to toxic effects of such highly carcinogenic food contaminants. Therefore, this situation clearly demands wider national or international programs for the control of aflatoxin contamination in Sorghum. In conclusion, aflatoxin control programs should focus on addressing all the factors that contribute to fungal growth across the value chain (i.e., pre-harvest to household practices). Hence, the concepts like HACCP “Farm to Fork” should be applied.
ACKNOWLEDGMENT
We are grateful to Center for Food Science and Nutrition of Addis Ababa University, Food, Medicine and Healthcare Administration and Control Authority of Ethiopia (EFMHACA) and Micronutrient Initiatives for funding this research.
REFERENCES
- Yoseph T, Sorsa Z (2014). Evaluation of Sorghum (Sorghum bicolor (L.) Moench) varieties, for yield and yield components at Kako, Southern Ethiopia. Journal of Plant Sciences, 2: 129-133.
- Katilé SO, Perumal R, Rooney WL, Prom LK, Magill CW (2010) Expression of pathogenesis-related protein PR-10 in Sorghum floral tissues in response to inoculation with Fusarium thapsinum and Curvularia lunata. Mol Plant Pathol 11: 93-103.
- Mekbib F (2009) Farmers’ breeding of Sorghum in the centre of diversity, ethiopia: i. socio-ecotype differentiation, varietal mixture and selection efficiency. Maydica 54: 25-37.
- Tigabu E, Andargie M, Tesfaye K (2012). Response of Sorghum (Sorghum bicolor (L.) Moench) genotypes to NaCl levels at early growth stages. African Journal of Agricultural Research Vol. 7: 5711 -5718.
- Central Statistical Authority, Federal Democratic Republic of Ethiopia (2010) Agricultural Sampling Survey Report on area and production of crops, Ethiopia.
- Pestka JJ, Smolinski AT (2005) Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev 8: 39-69.
- Ubwa ST, Abah J, Atu BO, Tyohemba RL, Yande JT (2014). Assessment of total aflatoxin s level of two major nuts consumed in Makurdi Benue State, Nigeria. International Journal of Nutrition and Food Sciences, 3: 397-403.
- Rajarajan PN, Rajasekaran KM, Asha Devi NK (2013) Aflatoxin Contamination in Agricultural Commodities. Indian J.Pharm.Biol.Res 1: 148-151.
- Rejiniemon TS, Hussain RR, Rajamani B (2015) In-vitro functional properties of Lactobacillus plantarum isolated from fermented ragi malt. South Indian Journal of Biological Sciences 1: 15-23.
- Iyanda AA, Anetor JI, Oparinde DP, Adeniyi FAA (2014) Aflatoxin contamination of foodstuffs: Its health implications in Sub-Sararan Africa. Annals of Experimental Biology 2: 63-73.
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, et al. (2013) Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382: 427-451.
- Tchana AN, Moundipa PF, Tchouanguep FM (2010) Aflatoxin contamination in food and body fluids in relation to malnutrition and cancer status in Cameroon. Int J Environ Res Public Health 7: 178-188.
- Shephard GS (2003) Aflatoxin and food safety: recent African perspectives. J Toxicol-Toxin Rev 22: 267-286.
- Kumeda Y, Asao T (2001) Heteroduplex panel analysis, a novel method for genetic identification of Aspergillus Section Flavi strains. Appl Environ Microbiol 67: 4084-4090.
- Chala A, Taye W, Ayalew A, Krska R, Sulyok M, et al. (2014). Multimycotoxin analysis of Sorghum(Sorghum bicolor L. Moench) and finger millet (Eleusine coracana L. Garten) from Ethiopia. Journal of Food Control 45: 29-35.
- Ayalew A, Fehrmann H, Lepschy J, Beck R, Abate D (2006) Natural occurrence of mycotoxins in staple cereals from Ethiopia. Mycopathologia 162: 57-63.
- Habtamu F, Kelbessa U (2001) Survey of Aflatoxin Contamination in Ethiopia. Ethiopian Journal of Health Science 11: 17-25.
- Besrat A, Gebre P (1981) A preliminary study on the aflatoxin content of selected Ethiopian foods. Ethiop Med J 19: 47-52.
- Geyid A, Maru A (1987) A survey of aflatoxin contents in maize, Sorghum and teff samples. Ethiopian Journal of Health Development 2: 59-70.
- Abate D, Gashe BA (1985) Prevalence of Aspergillus flavus in Ethiopian cereal grains: a preliminary survey. Ethiop Med J 23: 143-148.
- AOAC (2000) Association of official analytic chemists. Official method of analysis of the AOAC, (17thedn), Association of official analytic chemists, Rockville, MD, USA.
- GHENT university faculty of parasitical department of food laboratory (2014) Determination of aflatoxin in Maize Sorghum and peanut. Mytox, Belgium.
- Mohammed A, Chala A (2014) Incidence of Aspergillus contamination of groundnut (Arachis hypogaea L.) in Eastern Ethiopia. African Journal of Microbiology Research 8: 759-765.
- Klich MA (2002) Identification of common Aspergillus Species. Central bureauvoor Shimmel cultures, Utrecht, The Netherlands. pp.116.
- Ratnavathi CV, Sashidhar RB (2003) Substrate suitability of different genotypes of Sorghum in relation to Aspergillus infection and aflatoxin production. J Agric Food Chem 51: 3482-3492.
- Ayalew A (2010) Mycotoxins and surface and internal fungi of maize from Ethiopia. African Journal of Food Agriculture 9: 4109-4123
- Alpert ME, Hutt MS, Wogan GN, Davidson CS (1971) Association between aflatoxin content of food and hepatoma frequency in Uganda. Cancer 28: 253-260.
- Keller LAM, Pereyra CM, Cavaglieri LR, Keller KM, Almeida TX, et al. (2012) Fungi and Aflatoxin B1 in Pre and Post fermented Sorghum trench type silos destined to Bovine intensive-Rearing in Brazil. Julio 2: 81-91.
- Fufa H, Urga K (1996) Screening of aflatoxins in Shiro and ground red pepper in Addis Ababa. Ethiop Med J 34: 243-249.
- Mohamed AM, Al-Othman MR, Abeer R, Abd El-Aziz M (2013). Mycotoxigenic Fungi Contaminating Corn and Sorghum Grains in Saudi Arabia. Journal of Bot. 45: 1831-1839.
- Mashilla D, Yuen J, Sigvald R (2006) Effects of storage methods, storage time and different agro-ecological zones on chemical components of stored Sorghum grain in Hararghe, Ethiopia. Journal of Stored Products Research 42: 445-456.
- Sreenivasa MY, Dass RS, Janardhana GR (2010). Survey of postharvest fungi associated with Sorghum grains produced in karnataka (India). Journal of plant protection research 50: 335-339.
- European Food safety Aouthrity (2013) Aflatoxins (sum of B1, B2, G1, G2) in cereals and cereal-derived food products, European Food safety Aouthrity, Parma, Italy. Pg no: 1-11.
- Grybauskas AP, Thomison PR, Cassel EK (2000) Aflatoxins Maryland Cooperative Extension Fact Sheet 444: pg no: 88.
- RASFF (Rapid Alert System for Food and Feed) (2011) Determination of aflatoxin level and monitoring the effectiveness of the controls in place to limit consumer exposure these toxins.
Citation: weledesemayat GT, Gezmu TB, Woldegiorgis AZ, Gemede HF (2016) Study on Aspergillus Species and Aflatoxin Levels in Sorghum (Sorghum bicolor L.) Stored for Different Period and Storage System in Kewet Districts, Northern Shewa, Ethiopia. J Food Sci Nutr 3: 010.
Copyright: © 2016 Geremew Tassew weledesemayat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

