
Defining Methods to Create Consistent, Reproducible Impressions Deposited in Biofluids on a Variety of Substrates
*Corresponding Author(s):
Jessica ZarateForensic Science Research Facility, Madonna University, Livonia, Michigan, United States
Tel:+1 7344325523,
Email:jlzarate@madonna.edu
Abstract
In an effort to minimize human and environmental factors associated with the deposition of impression evidence researchers can utilize optimal deposition parameters to generate consistent reproducible impressions for analysis. The deposition parameters defined in this study provide a guideline for producing optimal fingerprints deposited in common biofluids (eccrine/sebaceous sweat, non-human oil, blood, semen and saliva) onto a variety of substrates encountered at crime scenes. Optimal quality impressions can be used as a control in depletion or dilution series to test the effectiveness of new products conduct chemical and physical enhancement trials as well as to validate enhancement methods for laboratory use.
Keywords
INTRODUCTION
A number of factors can affect the quality of deposited impressions making it difficult to generate consistent reproducible impressions for analysis and can potentially affect the results of impression-based research. In order to determine the effectiveness of chemical and physical enhancement methods for testing new products, comparing existing products, or validating methods and products for implementation in forensic laboratories, impression quality must be reproducible throughout the research trials. In creating consistency the substrate and biofluid in which the impressions are made must be controlled. It is important to maintain a constant laboratory temperature, as well as controlling the parameters associated with impression deposition, such as the ratio of friction skin surface area to the volume of biofluid pre-deposition waiting time, deposition pressure, angle and deposition pressure time. The ability to control for the impression deposition variables allows examiners to accurately assess enhancement methods based on optimal reproducible impressions. Variable impression quality prior to enhancement will influence the accuracy of results. Therefore deposition parameters should be standardized and adopted for each biofluid and substrate used in impression based research trials.
Variations in composition and viscosity of biofluids influence the ability of a fingerprint to hold impression details. The most common and most studied biofluid found within an impression is eccrine/sebaceous sweat. Friction skin contains eccrine pores embedded in skin, which secrete eccrine sweat; composed mainly of water with the remaining mixture containing organic and inorganic materials. In contrast sebaceous sweat is primarily composed of fatty acids, glycerides, cholesterol, squalene and lipid esters [1,2]. Environmental contaminants, such as cosmetics, hair products and tobacco use may also affect the chemical composition of deposited impressions [3]. Most fingerprints are deposited in a mixture of eccrine and sebaceous materials as sweat is constantly secreted from all types of skin pores. In fact most impressions will contain some amount of touch eccrine/sebaceous material, even if in combination with other biofluid such as non-human oil, blood, semen and saliva.
Non-human oil is the term used in this study to describe any food-based oil in which impression evidence could be deposited. Impressions deposited in oils can be transferred from food sources while eating or cooking to a variety of different substrates. The composition and viscosity of oils have the ability to create fragile yet valuable impressions that can be collected and enhanced as evidence. Some studies have analyzed oil or grease impressions in regard to determining suitable enhancement methods [4,5] but few studies have been published on this topic.
Blood is another biofluid frequently encountered at the scene of violent crimes, and it is therefore likely that impression evidence may be present. Blood is composed of erythrocytes, leukocytes, platelets and plasma which contain a mixture of amino acids, proteins, salts and other compounds [6]. Blood holds impressions well because it is highly viscous and once dried, impressions are very stable regardless of substrate porosity. A number of studies have conducted research trials associated with the enhancement of blood impressions [6-15] but deposition variability associated with the blood impressions or how this variability impacted the outcomes of the studies was often not discussed.
Semen is another biofluid commonly found in association with criminal cases. Composed of acid phosphatase, spermatozoa, citric, lactic acid, fructose and zinc [2], it is highly viscous and therefore likely to preserve impression evidence. In addition to semen, and saliva is also capable of holding impression evidence. Saliva is primarily composed of water, but also contains buccal epithelial cells, amylase, lysozyme, glucose [2] and possible contaminants such as food that can cling to the inner cheek and the teeth of an individual [16]. The high concentration of water has an effect on the stability of saliva impressions making them more fragile. Despite the possibility of these impressions being present at crime scenes there have only been a limited number of research studies that include semen [10] and saliva [7,10].
While many biofluids have the ability to hold impression evidence the substrate onto which the impression is deposited strongly affects the variables associated with deposition and ultimately will determine the appropriate chemical and physical enhancement method. The most important factor when considering substrate is porosity which is sub-divided into three main categories; non-porous, semi-porous and porous. Non-porous substrates do not absorb biofluid deposited with an impression leaving the impression on the substrate surface. Semi-porous substrates absorb some of the biofluid that is placed along with an impression onto the surface but some of the impression may still remain on the surface of the substrate. Porous substrates completely absorb the fluid in which the impression is deposited leaving little to no residue on the substrate surface. Other factors that should be considered when testing substrates, regardless of their porosity, are the background color(s), pattern(s) and degree of texture which can inhibit visualization of impression details and enhancement methods are often selected based on these factors. Most studies incorporate a wide range of substrates into impression trials to demonstrate the broad spectrum of evidentiary items associated with criminal cases since the influence of porosity is widely known and accepted in the field.
More recently, impression studies have begun to address the importance of standardizing protocols and creating consistent and comparable impressions for analysis [17,18], but as a whole the field has yet to adopt a systematic approach. Some variables that have garnered attention are the laboratory conditions depositor, skin temperature, volume, temperature of biofluid, pre-deposition waiting time, deposition pressure angle and deposition pressure time. Studies that have controlled some of these variables have mainly been associated with the deposition of eccrine/sebaceous [1,17,19-22] and blood impressions [7,11,13,15,23] but these guidelines should be considered essential for all fluids being used for research testing.
The importance of maintaining a constant laboratory temperature and humidity as well as minimizing air flow through the laboratory has been recognized [19,23]. Variability in laboratory temperature and air flow may alter dry times of deposited impressions creating difficulties in reproducibility. In addition, depositor friction skin temperature can increase or decrease eccrine/sebaceous sweat production which affects not only the eccrine/sebaceous impressions but impressions deposited in all biofluids given that there is a touch component to all impressions. However few studies made reference to the donor’s friction skin temperature [15,23] and its effect on the impression clarity [23]. The temperature of the biofluid has also been highlighted as a factor, specifically in the replication of blood impressions in order to mimic the fluid being secreted by a living subject [15,23]. Yet there were no references found that discuss the importance of temperature in relation to other biofluids used in impression studies.
As previously mentioned most impression studies are performed using eccrine/sebaceous sweat and blood though methods for application of these fluids to friction skin often vary. For the collection of eccrine/sebaceous impressions methods have been outlined for “loading” [24,25] or “grooming” [1,20,26] which have been generally accepted and incorporated into impression research trials. Common methodology includes the depositor rubbing their friction skin over the forehead, nose, body and/or hair of the donor from whom the material is being collected [4,20,22,26-33]. Some of these methods describe the depositors subsequently rubbing their hands and fingers together to evenly distribute the eccrine/sebaceous materials prior to deposition [1,5,17,20,26,34]. The latter is deemed a more realistic collection method for comparing laboratory impressions, because grooming or loading then directly depositing the impression will result in substantially more materials for creating impressions than if the materials are distributed more naturally by finger or hand rubbing [1,18,35]. Blood impression studies have also addressed some deposition parameters, including describing application of the fluid to friction skin such as dipping fingers into blood [11] and then blotting them on paper to remove excess [9,12] or shaking off the excess [10]. Others discussed placing a finger onto a blood-filled sponge [14] or paper towel [8].
The Langenburg study [23] took a more systematic approach to deposition variables, including laboratory temperature, donor skin temperature and surface area of the depositor’s friction skin, temperature, volume of blood, deposition pressure, pre-and post-deposition waiting intervals. These variables were incorporated into research trials to optimize blood impressions for comparable analysis [15]. Other studies have also indicated the volume of blood used when depositing blood impressions [7,13] but quantity is more difficult to address with other biofluids. This is especially problematic with eccrine/sebaceous sweat impressions as the eccrine/sebaceous sweat is collected directly from the donors skin creating difficulties in quantifying the amount of material used to make the impression [20]. To compensate one study [20] clearly defined the collection and deposition process for eccrine/sebaceous sweat impressions in a multi-step procedure.
Environmental contamination of friction skin prior to deposition of impressions will also alter the impression composition. To minimize this factor studies have utilized protocols to cleanse the friction skin of environmental contaminants such as lotions, shampoos, conditioners and hand soaps. The most frequently employed methods were hand washing [4,5,15,20,21,33] or alcohol wipes [20]. The use of hand soap for washing the friction skin surface is not recommended as it may contain fatty acids and cholesterol that in addition to altering the composition can interfere with chemical enhancement methods [20].
Prior to depositing impressions, the time in which the biofluid remains on the depositor’s friction skin should be controlled. This interval previously addressed in studies [11,13], defined as the pre-deposition waiting interval [15,23] varies based on the biofluid and the substrate onto which the impression is deposited. The pre-deposition waiting interval the amount of pressure and angle of contact, as well as the deposition contact time during the deposition of impressions will also affect the reproducibility of the impressions and can impact trial results.
The importance of controlling deposition pressure has been recognized and addressed in several studies [11,15,17, 19-21,23,30] some of which also addressed deposition angle [17,19] and duration of surface contact [1,11,17,19, 30,35]. To further address issues of deposition variability one study [17] designed an apparatus to control some of the variables that affect impression quality. Other studies have discussed the value of deposition pressure in creating impressions and attempted to control this variable by having the analyst physically place the depositor’s finger onto the substrate [1,19] but the deposition pressure was not recorded. Alternately a weigh scale has been used to more accurately quantify deposition pressure [15,21,23]. The variables associated with deposition pressure and angle are important to control in order to prevent visible distortion in impressions [17,36].
This study focused on setting guidelines to control the variables associated with impression depositions in order to yield consistent reproducible impressions on fifteen different substrates ranging in porosity. For the fifteen substrates deposition parameters were optimized for impressions deposited in eccrine/sebaceous sweat, non-human oil, blood, semen and saliva. The variables considered include laboratory temperature, biofluid temperature, friction skin temperature and the ratio of friction skin surface area to the volume of biofluid. In addition pre-deposition waiting time, deposition pressure and angle and deposition pressure time were controlled to allow researchers to improve the consistency of impressions deposited for use in the testing and analysis of products and protocols.
MATERIALS
Substrates
Substrates and any items that might come into contact with the impressions were treated with short wave UV light by exposure to germicidal mercury halide fluorescent lamps in a dedicated light box. The box utilized three GE G25T8 germicidal tubes arranged at 20 cm distance from the substrates to be treated. Substrates were exposed to 3 hours UV on each side at an intensity of 18 µW/cm2 to remove potential DNA contamination prior to downstream analysis. The substrate samples were then individually packaged prior to use to prevent cross-contamination.
Personal protective equipment
Biofluids
Prior to use the blood was refrigerated (approximately 36°F/2°C) the semen and saliva were frozen (approximately 32°F/0°C). The whole blood, semen and saliva samples were mixed thoroughly and heated to the average core body temperature (98.6°F/37°C) using a mini dry bath (Benchmark, USA) prior to depositions in order to simulate an impression deposited in whole samples of biofluids secreted from a living person. The non-human oil sample was stored and maintained at room temperature (70-75°F/21-24°C) to simulate an impression deposition being transferred from a food source while eating. The eccrine/sebaceous sweat was collected from the female donor’s forehead an hour after her face was cleaned with an individually packaged antibacterial moist towelette (Wet Ones, USA). The ingredients in the towelette were assessed to determine inherent fluorescent properties to ensure they did not adversely affect results in this study. The female donor’s forehead temperature (93-95°F/34-35°C) was maintained throughout the deposition process to simulate a subject’s average body temperature while depositing sweat impressions. An infrared digital thermometer (Cen-Tech, USA) was utilized to measure temperature throughout the trials. All biofluids were disposed of in accordance with institutional standards.
METHODS
Surface area calculations
Environmental control guidelines
The depositor then placed their thumb into a beaker of crushed ice with a thin plastic wrap (Saran, USA) barrier to prevent contact between the ice and friction skin. This minimized moisture accumulation and kept friction skin dry until the thumb temperature reached 70-71°F/21-22°C using an infrared thermometer. This temperature range provided ideal impressions with pronounced friction ridges while minimizing the production of eccrine sweat. Once the depositor’s skin temperature was in the target range (70-71°F/21-22°C) the non-eccrine/sebaceous biofluids were pipetted onto the depositor’s thumb using a P-10, 20 or 100 volume pipette (Gilson, USA) depending on the optimized volume of biofluid per substrate. Eccrine/sebaceous sweat however, could not be quantified as it was obtained directly from the female donor’s face and forehead. The friction ridge skin on the depositor’s thumb was loaded with sweat by rubbing the donor forehead in a circular motion for 5 seconds.
Pre-deposition waiting interval
Deposition pressure and pressure interval
The deposition pressure interval describes the time the depositor’s thumb remains in contact (while applying pressure) with the substrate during the deposition of the impression. These times vary based on the biofluid and the substrate in which the impression is being deposited; some may have an instant deposition pressure interval, whereas others may require extended time intervals to produce optimal impressions for analysis. The intervals in these trials were measured with a digital stop watch.
Visualizing and rating the impression to determine optimal parameters
The visual examination of blood impressions deposited on light colored substrates as well as the visualization of semen, saliva, eccrine/sebaceous sweat and non-human oil impressions on light colored substrates was conducted using oblique lighting under normal lighting conditions. However the visual examination of biofluids on dark colored or patterned substrates required visualization with an alternate light source or additional chemical enhancement methods; such as through the use of Zar-Pro™ Fluorescent Blood Lifters, Fluorescein and Methylene Blue (Tri-Tech Forensics, USA). The fluorogenically enhanced impressions were visualized and rated using a Rofin Polilight Flare Plus (Arrowhead Forensics, USA) with orange, yellow or red barrier filters (Arrowhead Forensics, USA).
Zar-Pro™ Fluorescent Blood Lifters (Tri-Tech Forensics, USA) were used according to the manufacturer’s directions to lift blood impressions from the substrate onto the white background of the lifter for visualization of blood impressions on dark or porous substrates, such as denim and cotton. The lifted impressions were rated under normal lighting and then fluoresced using alternate lighting at 505 nm wavelength with an orange barrier filter to visualize impression details.
Saliva and semen impressions deposited onto glass, aluminum, poster board and wall paper, were visualized under normal lighting without additional enhancement. Semen and saliva aliquots (2 mL) for finished wood were spiked with Methylene Blue (6 µL) and then vortexed (Vortex Genie 2, USA) to evenly distribute the Methylene Blue throughout the biofluid. The dyed impressions were visualized on the wood substrate under normal lighting conditions. For remaining substrates semen and saliva aliquots (2 mL) were spiked with Fluoresce in (2 µL) and then vortexed to evenly distribute the fluoresce in throughout the biofluid. The fluoresce in enhanced semen and saliva impressions were visualized with an alternate light source (Rofin Polilight Flare Plus, Arrowhead Forensics, USA) at 365 nm and 505 nm wavelengths with orange, yellow or red barrier filters. Neither dye used in color enhancement influenced the viscosity of the biofluids.
It is important to note, no additives were utilized for non-human oil because of insolubility and for eccrine/sebaceous sweat because it was coming directly from the skin of the female donor. Also all deposited impressions were replicated in triplicate for each substrate and biofluid using the thumbs of two male subjects.
Photography
RESULTS AND DISCUSSION
Optimal deposition parameters can be utilized to create consistent comparable and reproducible impressions for analysis only by setting deposition parameters for each biofluid on the various substrates selected for study. Variables that must be considered in order to generate optimal quality impressions include volume of biofluid, deposition pressure, pre-deposition waiting interval and deposition pressure interval. During this study, these parameters were optimized for fourteen of the fifteen substrates selected for impression deposition with all five biofluids utilized (Tables 1-5). The fifteenth substrate denim could only be optimized using blood and semen. The impression details for saliva, eccrine/sebaceous sweat and non-human oil impressions were very difficult to visualize on denim even with attempted dye enhancement of biofluids. Therefore, despite producing some visible impression details on the substrate, the standards for optimal quality were not met and cannot be considered optimized (Tables 1,2,5).
| Deposition Pressure (lbs/in2) | Pre-Deposition Waiting Interval/Deposition Pressure Interval (seconds) | |
| Nonporous Substrates: | ||
| Glass | 5-6 | 0/5 |
| Aluminum | 5-6 | 0/5 |
| Ceramic Tile | 8-10 | 0/5 |
| Stainless Steel | 8-10 | 0/5 |
| Vinyl Tile | 8-10 | 0/5 |
| Plastic | 8-10 | 0/5 |
| Semi-porous Substrates: | ||
| Glossy Paper | 8-10 | 0/1 |
| Painted Drywall | 7-8 | 0/5 |
| Polyvinyl Leather | 8-10 | 0/5 |
| Finished Wood | 8-10 | 0/5 |
| Wall Paper | 5-6 | 0/5 |
| Porous Substrates: | ||
| Poster Board | 5-6 | 0/5 |
| Untreated Wood | 3-4 | 0/1 |
| Cotton | 8-10 | 0/5 |
| Denim | 8-10 | 0/5 |
Table 1: Deposition parameters for eccrine/sebaceous sweat impressions.
| Oil Volume (µl) | Deposition Pressure (lbs/in2) | Pre-Deposition Waiting Interval/Deposition Pressure Interval (seconds) | |
| Nonporous Substrates: | |||
| Glass | 2 | 5-6 | 15/5 |
| Aluminum | 2 | 5-6 | 15/5 |
| Ceramic Tile | 3 | 8-10 | 15/5 |
| Stainless Steel | 1 | 8-10 | 15/5 |
| Vinyl Tile | 2 | 8-10 | 15/5 |
| Plastic | 1 | 5-6 | 15/5 |
| Semi-porous Substrates: | |||
| Glossy Paper | 2 | 8-10 | 5-Oct |
| Painted Drywall | 2 | 8-10 | 5-Oct |
| Polyvinyl Leather | 3 | 8-10 | 15/5 |
| Finished Wood | 3 | 8-10 | 5-Oct |
| Wall Paper | 2 | 8-10 | 15/5 |
| Porous Substrates: | |||
| Poster Board | 2 | 2-3 | 15/5 |
| Untreated Wood | 2 | 2-3 | 15/5 |
| Cotton | 10 | 8-10 | 15/5 |
| Denim | 15 | 8-10 | 5-Oct |
Table 2: Deposition Parameters for Non-human Oil Impressions.
| Blood Volume (µl) | Deposition Pressure (lbs/in2) | Pre-Deposition Waiting Interval/Deposition Pressure Interval (seconds) | |
| Nonporous Substrates: | |||
| Glass | 14 | 8-10 | 25/10 |
| Aluminum | 12 | 8-10 | 25/10 |
| Ceramic Tile | 12 | 8-10 | 25/10 |
| Stainless Steel | 14 | 8-10 | 25/10 |
| Vinyl Tile | 14 | 8-10 | 30/5 |
| Plastic | 12 | 8-10 | 20/5 |
| Semi-porous Substrates: | |||
| Glossy Paper | 17 | 8-10 | 20/5 |
| Painted Drywall | 18 | 8-10 | 25/5 |
| Polyvinyl Leather | 14 | 8-10 | 25/5 |
| Finished Wood | 18 | 8-10 | 30/5 |
| Wall Paper | 17 | 8-10 | 25/5 |
| Porous Substrates: | |||
| Poster Board | 20 | 8-10 | 45/5 |
| Untreated Wood | 18 | 8-10 | 20/5 |
| Cotton | 20 | 7-8 | 45/5 |
| Denim | 20 | 5-6 | 30/10 |
Table 3: Deposition parameters for blood impression.
| Semen Volume (µl) | Deposition Pressure (lbs/in2) | Pre-Deposition Waiting Interval/Deposition Pressure Interval (seconds) | |
| Nonporous Substrates: | |||
| Glass | 15 | 8-10 | 33/0 |
| Aluminum | 15 | 8-10 | 30/5 |
| Ceramic Tile | 12 | 8-10 | 20/10 |
| Stainless Steel | 16 | 8-10 | 20/10 |
| Vinyl Tile | 15 | 8-10 | 20/5 |
| Plastic | 12 | 8-10 | 25/5 |
| Semi-porous Substrates: | |||
| Glossy Paper | 17 | 8-10 | 20/5 |
| Painted Drywall | 18 | 8-10 | 25/5 |
| Polyvinyl Leather | 16 | 8-10 | 25/5 |
| Finished Wood | 18 | 8-10 | 30/5 |
| Wall Paper | 25 | 8-10 | 45/5 |
| Porous Substrates: | |||
| Poster Board | 30 | 8-10 | 45/5 |
| Untreated Wood | 18 | 8-10 | 20/5 |
| Cotton | 20 | 7-8 | 30/5 |
| Denim | 18 | 5-6 | 30/10 |
Table 4: Deposition parameters for semen impressions.
| Saliva Volume (µl) | Deposition Pressure (lbs/in2) | Pre-Deposition Waiting Interval/Deposition Pressure Interval (seconds) | |
| Nonporous Substrates: | |||
| Glass | 15 | 8-10 | 30/0 |
| Aluminum | 15 | 8-10 | 35/0 |
| Ceramic Tile | 14 | 8-10 | 20/5 |
| Stainless Steel | 14 | 8-10 | 20/5 |
| Vinyl Tile | 14 | 8-10 | 20/5 |
| Plastic | 14 | 8-10 | 20/5 |
| Semi-porous Substrates: | |||
| Glossy Paper | 15 | 8-10 | 20/5 |
| Painted Drywall | 16 | 8-10 | 15/5 |
| Polyvinyl Leather | 14 | 8-10 | 20/5 |
| Finished Wood | 20 | 8-10 | 25/5 |
| Wall Paper | 14 | 8-10 | 20/5 |
| Porous Substrates: | |||
| Poster Board | 14 | 8-10 | 20/5 |
| Untreated Wood | 14 | 8-10 | 20/5 |
| Cotton | 18 | 5-6 | 25/5 |
| Denim | 25 | 1-2 | 45/0 |
Table 5: Deposition parameters for saliva impressions.
The biofluid in which the impression was deposited was the most relevant factor in ensuring consistency of impressions due to variations in composition and viscosity. This strongly affected the volume of fluid as well as the pre-deposition waiting and deposition pressure intervals. Pre-deposition waiting intervals set in this study ranged from 0 to 45 seconds whereas the deposition pressure interval ranged from 0-10 seconds (Tables 1-5). It is important to note that as the volume of biofluid increased it was necessary to increase the pre-deposition waiting interval to achieve proper drying prior to deposition. In addition the non-human oil and eccrine/sebaceous sweat impressions were never dried to a fixed state even after the pressure deposition waiting interval (Tables 1-2). This made the impressions more susceptible to being altered or destroyed post deposition through handling and packaging.
Another variable that strongly affected the reproducibility of the impressions in all biofluids was the porosity of the substrate (Tables 1-5). As porosity increased, visualization of impression details was impaired by absorption of the biofluids. This was a general trend overall but also a trend within each individual porosity grouping (non-porous, semi-porous and porous). In all cases, the volume and deposition pressure were closely tied to the varying degree of substrate porosity and texture.
Deposition parameters were deemed specific to each biofluid and therefore were not directly comparable (Tables 1-5). In fact fluid volume, deposition pressure, pre-deposition waiting interval and deposition pressure interval were variable when assessing impressions deposited in eccrine/sebaceous sweat, non-human oil, blood, semen and saliva on a non-porous substrate such as glass.
Parameters for porous substrates were particularly problematic due to increased absorption of biofluids into the substrate itself. This touch absorption requires lighter deposition pressures to avoid dissipation of the ridge details into surface voids present in more porous substrates. Eccrine/sebaceous sweat and non-human oil impressions generally required less deposition pressure than the other biofluids (Tables 1-2). This variant seemed to be due to the dispersant properties of the oil-based biofluids which often resulted in impression distortion as deposition pressure increased. In decreasing the deposition pressure, this problem was alleviated and optimal impressions were deposited. It was observed in this study that the volume of biofluids, as well as deposition pressure did create some tonally reversed impressions. This phenomenon was primarily observed in the deposition of blood impressions deposited on glossy paper.
Substrate degree of porosity is also highly variable even within the subcategories studied; this was further influenced by the texture of the particular substrates. Texture and porosity greatly influenced the volume of biofluid and the deposition pressure necessary to create optimal impressions regardless of the porosity subcategory. In addition, increasing the volume for some biofluids affected the pre-deposition waiting interval because it was necessary to allow the biofluid to dry just enough to transfer an optimal impression to the various substrates (Tables 2-5). A longer pre-deposition waiting interval was preferred over a longer deposition pressure interval as optimized impressions were more easily achieved when the biofluid was allowed to dry on the friction skin as opposed to during the contact with the substrate. This is most evident in the porous/textured substrates, with cotton and denim showing the necessity of reducing deposition pressure as well.
One of the challenges encountered during optimization of deposition parameters was the visualization of latent impressions on substrates with problematic background colors or patterns impacting the ability to visualize the biofluid (Figures 1-10). Impressions deposited on non-porous light colored substrates were easily visible for analysis (Figures 1,2,5,6) while porous and dark colored and patterned or textured substrates (Figures 3,4,7-10) were the most difficult to assess. In porous substrates this was partly due to the biofluid being absorbed rather than merely sitting on the surface of the substrate making the impression harder to visualize with oblique lighting under normal lighting conditions. In dark colored and patterned or textured substrates, the impression details were often obscured due to the background pattern and color which in some cases concealed or mimicked the ridge details of the impression (Figures 1,4,5,7-10). Therefore some of the optimal deposition parameters were set by using enhancement methods to visualize impressions and assess impression quality (Figures 11-14).
 Figure 1: Eccrine/Sebaceous impression on stainless steel.
Figure 1: Eccrine/Sebaceous impression on stainless steel.
 Figure 2: Eccrine/Sebaceous impression on glass.
Figure 2: Eccrine/Sebaceous impression on glass.
 Figure 3: Non-human oil impression on painted drywall.
Figure 3: Non-human oil impression on painted drywall.
 Figure 4: Non-human oil impression on polyvinyl leather.
Figure 4: Non-human oil impression on polyvinyl leather.
 Figure 5: Saliva impression on aluminum.
Figure 5: Saliva impression on aluminum.
 Figure 6: Semen impression on plastic.
Figure 6: Semen impression on plastic.
 Figure 7: Blood Impression on Vinyl Tile.
Figure 7: Blood Impression on Vinyl Tile.
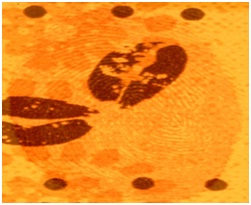 Figure 8: Blood Impression on Wall Paper.
Figure 8: Blood Impression on Wall Paper.
 Figure 9: Blood Impression on Poster Board.
Figure 9: Blood Impression on Poster Board.
 Figure 10: Blood impression on cotton.
Figure 10: Blood impression on cotton.
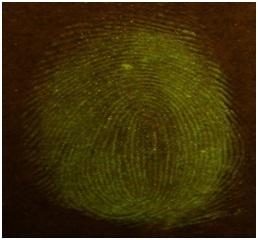 Figure 11: Fluorescein spiked saliva impression on poster board.
Figure 11: Fluorescein spiked saliva impression on poster board.
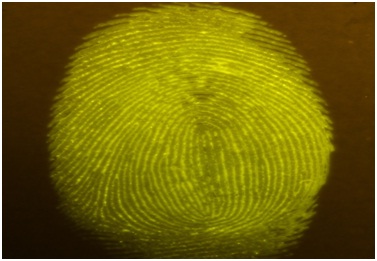 Figure 12: Fluorescein spiked saliva impression on glossy paper.
Figure 12: Fluorescein spiked saliva impression on glossy paper.
 Figure 13: Zar-Pro lifted blood impression from cotton normal lighting.
Figure 13: Zar-Pro lifted blood impression from cotton normal lighting.
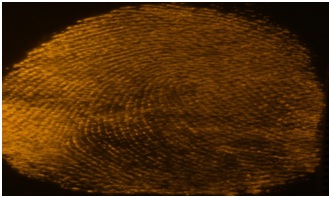 Figure 14: Zar-Pro lifted blood impression from cotton alternate lighting.
Figure 14: Zar-Pro lifted blood impression from cotton alternate lighting.
Denim was a problematic substrate in this study as it was difficult to visualize impression details deposited in saliva, eccrine/sebaceous sweat and non-human oil. The porosity of the denim as well as the dark colored and textured weave-patterned background made the visualization of impression details on the substrate surface difficult even with the addition of Fluorescein or Methylene Blue. The eccrine/sebaceous sweat and non-human oil samples could not be spiked with dye due to solubility issues with the biological materials. Finished (polyurethane coated) wood was another difficult substrate for deposition of semen and saliva impressions as the biofluid was quickly absorbed into the substrate and the ridge detail was obscured possibly due to the reflective properties of the polyurethane.
The guidelines for optimal impressions being proposed are meant for research purposes and are recognized as being unrealistic for deposition conditions under which actual evidence is submitted for analysis in association with criminal cases. However optimal quality impressions are necessary as a benchmark for the creation of depletion series, thus serving as a study control. A depletion series starting with an optimal (control) impression provides a comprehensive series ranging from optimal to faint impressions. This method is best suited to test the effectiveness of new products, conduct chemical and physical enhancement trials for the comparison of existing methods, as well as to validate enhancement methods for laboratory use. Furthermore, the depletion series impressions can also be cut in half after deposition (depending on the substrate) to conduct a side by side split series comparison of techniques on impressions that can be considered equal. Dilution series can also be conducted by diluting some biofluids with water to replicate crime scene clean-up situations, while still maintaining consistency of impression quality throughout the trials.
Optimizing these parameters across a broad range of biofluids and substrates demonstrated the interaction amongst many variables and highlighted that no single variable can be separated from the influence of the others examined. Deposition trials were replicated in triplicate with two male donors showing consistency across all results. Given that the volume of biofluid necessary to create an optimal impression is closely tied to the surface area of the friction skin of the donor it is especially important to recognize that to change the volume of biofluid used will require that all other parameters be adjusted accordingly. Therefore to implement these guidelines, changes can be calculated by keeping the relationship between the substrates and parameters consistent with those provided in tables 1 - 5.
Conclusion
Guidelines produced here can help control the multiple factors associated with depositing consistent, reproducible impressions on multiple substrates, which is necessary to assess the effectiveness of physical and chemical enhancement methods. During the deposition phase of research trials it is often not possible to visualize deposited impressions making optimal parameters essential to producing consistent impressions for research. Optimized parameters for some difficult to visualize biofluids in this study were generated using enhancement methods to ensure that these biofluids can be confidently used unenhanced even when visualization is difficult. Subsequent research trials to test and expand new and existing products have confirmed the utility of these parameters and we encourage other researchers to consider utilizing these guidelines in an effort to standardized protocols for producing consistent and comparable impressions during analysis and independent replication.
ACKNOWLEDGEMENT
We would like to thank members of the Madonna University Forensic Science Research Facility and especially Dr. Wilson Muse III, Christine Siress and Jodi Campo for their assistance with this study. Funding was provided by the National Institute of Justice (NIJ) grant 2013 DN-BX-K026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Justice.
REFERENCES
- Croxton RS, Baron MG, Butler D, Kent T, Sears VG (2010) Variation in amino acid and lipid composition of latent fingerprints. Forensic Sci Int 199: 93-102.
- Virkler K, Lednev IK (2009) Analysis of bodily fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int 188: 1-17.
- Ramotowski RS (2001) Composition of Latent Print Residue. In: Lee HC, Gaensslen RE (eds.). Advances in Fingerprint Technology (2ndedn). CRC Press, Boca Raton, Florida, USA. Pg no: 63-104.
- Gaskell C, Bleay SM, Ramadani J (2013) Natural yellow 3: A novel fluorescent reagent for use on grease-contaminated fingermarks on nonporous dark surfaces. J For Ident 63: 274-285.
- Gaskell C, Bleay SM, Wilson H, Park S (2013) The enhancement of fingermarks on grease-contaminated, non-porous surfaces: A comparative assessment of processes for light and dark surfaces. J For Ident 63: 289-319.
- Bossers LC, Roux C, Bell M, McDonagh AM (2011) Methods for the enhancement of fingermarks in blood. Forensic Sci Int 210: 1-11.
- Grubwieser P, Thaler A, Köchl S, Teissl R, Rabl W, et al. (2003) Systematic study on STR profiling on blood and saliva traces after visualization of fingerprint marks. J Forensic Sci 48: 733-741.
- Marchant B, Tague C (2007) Developing fingerprints in blood: A comparison of several chemical techniques. J For Ident 57: 76-93.
- Moore J, Bleay S, Deans J, NicDaeid N (2008) Recovery of fingerprints from arson scenes: Part 2- fingerprints in blood. J For Ident 58: 83-108.
- Parkin BH, Hartley K (1987) The detection of fingerprints and other marks in body fluids by the use of agar gels. For Sci Int 35: 267-275.
- Petretei D, Angyal M (2015) Recovering bloody fingerprints from skin. J For Ident 65: 813-827.
- Sears VG, Butcher CP, Fitzgerald LA (2005) Enhancement of fingerprints in blood part 3: Reactive techniques, acid yellow 7 and process sequences. J For Ident 55: 741-763.
- Strongin RM, Sibrian-Vazquez M (2009) Developing fluorogenic reagents for detecting and enhancing bloody fingerprints. In NIJ Award (2007-DN-BX-K171) Pg no: 1-38.
- Yapping L, Wang Y (2004) Bloody latent fingerprint detection using LeuR6G. J For Ident 54: 542-546.
- Zarate J, Morden C (2011) A fluorogenic method for lifting, enhancing, and preserving bloody impression evidence. J For Ident 61: 260-280.
- Babu NA, Masthan KMK, Bhattacharjee T, Elumalai M (2014) Saliva- the key regulator of oral changes in diabetes patients. International Journal of Pharmaceutical Sciences & Research 5: 2579-2583.
- Fieldhouse S (2011) Consistency and reproducibility in fingermark deposition. For Sci Int 207: 96-100.
- Kent T (2010) Standardizing protocols for fingerprint reagent testing. J For Ident 60: 371-379.
- De Alcaraz-Fossoul J, Mestres Patris C, Balaciart Muntaner A, Barrot Feixat C, Gené Badia M (2013) Determination of latent fingerprint degradation patterns - a real fieldwork study. Int J Legal Med 127: 857-870.
- Archer NE, Charles Y, Elliott JA, Jickells S (2005) Changes in the lipid composition of latent fingerprint residue with time after deposition on a surface. For Sci Int 154: 224-239.
- Jasuja OP, Toofany MA, Singh G, Sodhi GS (2009) Dynamics of latent fingerprints: The effect of physical factors on quality of ninhydrin developed prints-- a preliminary study. Sci Justice 49: 8-11.
- Merritt D, Morgan JP, Houlgrave S, Ramotowski R, Brock A, et al. (2015) Development of Latent Prints on Tyvek Large Pak and Padded Pak Shipping Envelopes. J For Ident 65: 828-849.
- Langenburg G (2008) Deposition of bloody friction ridge impressions. J For Ident 58: 355-389.
- Sanders N (2011) Recovery of fingerprint evidence from post-blast device materials. J For Ident 61: 281-289.
- Williams NH, Elliott KT (2005) Development of latent prints using titanium dioxide (TiO2) in small particle reagent, white (SPR-W) on adhesives. J For Ident 55: 292-305.
- Perry H, Sears VG (2015) The use of natural yellow 3 (curcumin) for the chemical enhancement of latent friction ridge detail on naturally weathered materials. J For Ident 65: 45-66.
- Beaudoin A (2012) Fingerprint staining technique on dark and wetted porous surfaces: Oil red O and rhodamine 6G. Journal of Forensic Identification 62: 315-329.
- Cohen D, Cohen EH (2010) A significant improvement to the SPR process: More latent prints were revealed after thorough wiping of small particle reagent - treated surface. J For Ident 60: 152-162.
- Frick A, Fritz P, Lewis SW, van Bronswijk W (2012) A modified oil red O formulation for the detection of latent fingermarks on porous substrates. J For Ident 62: 634-641.
- Goel TL (2015) Developing latent fingermarks on thermal paper: Comparison of the 1,2-indanedione-zinc chloride dry contact method to the hot print system. J For Ident 65: 34-43.
- Ma R, Bullock E, Maynard P, Reedy B, Shimmon R, et al. (2011) Fingermark detection on non-porous and semi-porous surfaces using NaYF4:Er,Yb up-converter particles. For Sci Int 207: 145-149.
- Schiemer C, Lennard C, Maynard P, Roux CP (2005) Evaluation of techniques for the detection and enhancement of latent fingermarks on black electrical tape. J For Ident 55: 214-238.
- Song DF, Sommerville D, Brown AG, Shimmon RG, Reedy BJ, et al. (2011) Thermal development of latent fingermarks on porous surfaces -- further observations and refinements. For Sci Int 204: 97-110.
- Marriott C, Lee R, Wilkes Z, Comber B, Spindler X, et al. (2014) Evaluation of fingermark detection sequences on paper substrates. For Sci Int 236: 30-37.
- Fritz P, van Bronswijk W, Dorakumbura B, Hackshaw B, Lewis SW (2015) Evaluation of a solvent-free p-dimethylaminobenzaldehyde method for fingermark visualization with a low-cost light source suitable for remote locations. J For Ident 65: 67-90.
- Rodriguez CM, de Jongh A, Meuwly D (2012) Introducing a semi-automatic method to simulate large numbers of forensic fingermarks for research on fingerprint identification. J Forensic Sci 57: 334-342.
Citation: Zarate J, Nasser-Beydoun N, Hulscher A, Jones N, Barta JL (2016) Defining Methods to Create Consistent, Reproducible Impressions Deposited in Biofluids on a Variety of Substrates. J Forensic Leg Investig Sci 2: 013.
Copyright: © 2016 Jessica Zarate, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

