
Lifestyle Intervention to Increase Physical Activity and Social Participation among Older Adults with Diabetes: Result of a Feasibility Study
*Corresponding Author(s):
Pearl G LeeGeriatric And Palliative Medicine, University Of Michigan, 2215 Fuller Road 11G, Ann Arbor, MI 48105, United States
Tel:+1 7348453182,
Email:pearllee@med.umich.edu
Abstract
Purpose: To assess the impact of lifestyle intervention on physical activity and participation in social roles and activities among sedentary older adults with Type 2 Diabetes (T2DM).
Materials and Methods: We conducted a pilot study involving an 18-week tailored lifestyle intervention, led by an Occupational Therapist (OT), among community-dwelling adults who were 65 years and older, sedentary, and had T2DM. The intervention included 6 sessions (2 in-person and 4 phone calls) of individually tailored consultation to modify lifestyle based on participants’ baseline 1-week home accelerometry-derived PA and social participation.
Results: Twenty-three individuals completed baseline home monitoring activities, 87% completed the study. At the end of the study, participants reported significant improvements in the 4-week PA using the Community Healthy Activities Model Program for Seniors questionnaire: +1689 Kcal/week mean total PA and +1533 Kcal/week moderate-vigorous intensity PA. Participants also reported improvement in the Ability to Participate in Social Roles and Activities (+2.97, p=0.01) and Satisfaction with Participation in Discretionary Social Activities (+3.51, p=0.02).
Conclusion: Among sedentary older adults with T2DM, a brief OT-delivered lifestyle modification intervention improved self-reported PA and social participation, two important factors of disability, suggesting that such intervention may contribute to preventing disability in a high risk population.
Keywords
INTRODUCTION
Type 2 Diabetes Mellitus (T2DM) affects over 25% of older adults in the USA [1], and is associated with multiple co-morbidities with increased risk of disability [2]. Engaging in regular physical activity is a key part of the management of T2DM [3]; yet older adults are especially likely to be sedentary. Only 12% of adults aged 75 or older engage in 30 minutes of moderate PA for 5 or more days per week and 65% report no leisure PA. Current PA interventions have limited impact among older adults with T2DM because of modest short-term effects and high drop-out rates over time [4]. Potential barriers for older patients with T2DM to reach PA goals include co-existing geriatric syndromes and physical function impairments that can limit their participation in moderate-intensity PA [5], such as brisk walking for 150 minutes/week [3,6].
Participation or social participation, defined as an individual’s involvement in daily activities in home, community, and societal environment, such as volunteering, caregiving and leisure activities [7,8], may be another important factor in promoting PA among older adults. A previous PA intervention aimed to delay mobility disability found that individuals with a higher level of participation were more successful in achieving the intervention goals [9]. Lower level of participation in daily life and social activities has been shown to be associated with higher risk for physical inactivity [10]. Therefore, we hypothesized that a PA intervention that also involves social participation may improve PA and mobility disability.
We present the results of a proof-of-concept study testing the feasibility of a lifestyle intervention among adults aged ≥65 years with T2DM based on their daily PA patterns at home. The intervention was based on social cognitive theory [11] and the World Health Organization’s International Classification of Functioning, Disability and Health (ICF) model on health and disability [12], which links an individual’s functioning at body function/structure, activity, and participation levels with personal and environmental factors. Lifestyle-based PA has been shown to improve physical functioning of older adults with T2DM [13], but has not been shown to also improve participation [14]. In this study, we hypothesized that a brief Occupational Therapist (OT) - led lifestyle-based PA intervention, tailored for the individual’s functioning in the context of personal and environmental factors, is feasible to deliver and can result in improvement in PA, participation and physical functioning among older adults with T2DM. OT are ideal choices for this intervention as they are trained to use strategies to promote participation of individuals with functional impairment in daily activities while adapting to their environment [15].
METHODS
Study design
The study was a pre- and post- one-group intervention trial conducted at an academic medical center from March 2016 to February 2017. Figure 1 and appendix 1 represented the CONSORT diagram and study flowchart, respectively. The study was approved by the University of Michigan Institution Review Board and all participants signed the consent prior to study activities.
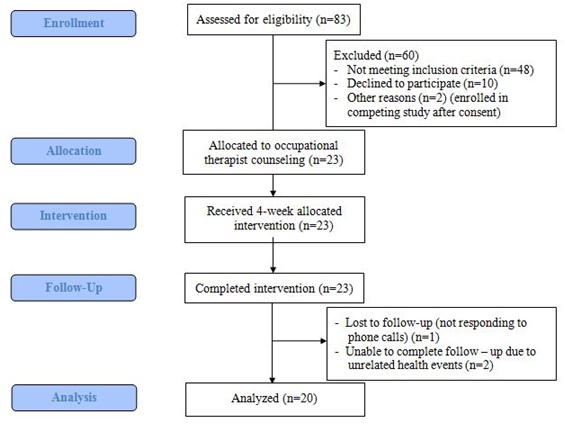 Figure 1: CONSORT diagram of the study.
Figure 1: CONSORT diagram of the study.
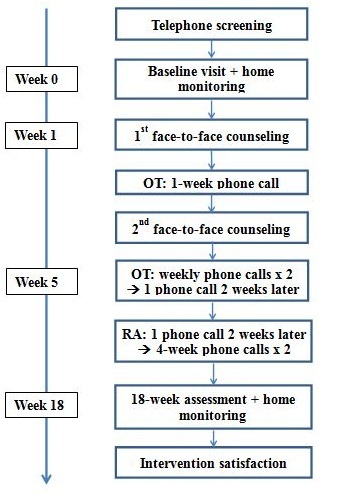 Appendix 1: Flowchart of the intervention. OT = Occupational Therapist; RA = Research Assistant.
Appendix 1: Flowchart of the intervention. OT = Occupational Therapist; RA = Research Assistant.
Study participants
Community-dwelling adults aged ≥65 years with T2DM were recruited from a diabetes research registry maintained by the Michigan Diabetes Research Center, and through advertisement around the medical center campus. Phone and in-person screening were conducted with additional inclusion criteria: being sedentary (current performance of
Intervention
The research team, including a licensed OT, developed the intervention manual. The manual provides a guide for the OT to apply the three self-regulation components when counseling the participants [17]: self-observation, self-evaluation and self-reaction. The manual includes information on exercise safely with diabetes, description and examples of four types of exercises (i.e., aerobic, strength, balance and flexibility training) [18], and a variety of lifestyle-based PA categorized based on individual’s social roles (i.e., participate in family activities such as shopping and fishing, or participate in community activities such as charity walks) [19]. As an incentive, all participants were offered to use a Fitbit Zip during the study period.
After obtaining consent, participants were asked to complete 7-day homework, recording detailed PA and participation activities, while wearing an accelerometer -ActiWatch [Respironics, Bend OR] on their non-dominant wrist. The homework and accelerometer were then mailed back to the study team in a pre-stamped envelope. Participants were excluded from the intervention if they did not adhere to the study procedures to a level that the team felt was appropriate to participate in the intervention (i.e., did not enter 75% of responses into the accelerometer, or did not wear the accelerometer for at least 5 out of 7 days with three consecutive days, or if the accelerometer was worn less than 70% of the wake time).
Using the participants’ baseline home activities, an OT counseled them on how to improve physical and participation activities in 30-60-minute face-to-face sessions (Appendix 2 and 3). Using techniques grounded in social cognitive theory, the OT counseled the participants to set weekly physical and participation activity goals and strategies to achieve the goals, and utilizing the three steps of self-regulation to overcome barriers to reach their individual goals. Participants were encouraged to keep a weekly physical and participation activity log. Additional OT follow-ups included a phone call one week following the first face-to-face counseling session, a second face-to-face counseling session, followed by 2 weekly phone calls, and final call2 weeks later. At the subsequent phone and face-to-face encounters, the OT worked with the participants to complete the activity logs, further encouraged them to work toward their individual goals, and helped them to problem solve any barriers.
|
Physical activity goals |
|
- Walking for 30 minutes every day |
|
- Sitting exercises for 20 minutes three times a week |
|
- Light weight lifting twice a week |
|
- Less time in the recliner |
|
Participation goals |
|
- Go to movies with neighbor |
|
- Visit grandkids |
|
- Watch soccer games, with grandkids |
|
- Go to a lecture sponsored by the Alzheimer’s Association |
Appendix 2: Examples of physical activity goals and participation goals.
|
Roles |
Strategies |
|
|
|
|
Self |
Incorporate some extra walking into your daily routine |
|
Add a 10-15-minute walk after each meal, you can walk inside the house, around the house, around the block, or walk in the mall |
|
|
Walk to meetings with friends or family, when going to the letter box walk down to the end of the street and back |
|
|
Stand up while talking on the telephone |
|
|
Family |
Invite a friend or family to help you be more active |
|
join an exercise class together |
|
|
Watch a good TV show or movie with family or friend |
|
|
When watching TV at home, plan to get up and NOT sit during each commercial breaks |
|
|
Community |
Tell friends and family about the study so they can remind you to get up and move or maybe motivate others to be more active as you are trying to |
|
(Neighbors, |
Join local community walk / run events (Turkey Trout, 1K, 5K, etc.) |
|
Friends) |
Stand up aftereachh and of bridge, or playing Solitaire, or when cutting fabric for quilting, or standing at an easel to paint |
|
Domestic activities |
Do household chores with yours pouse/kids/grandkids |
|
Do some exercises (e.g., walk around the kitchen) while waiting for meal to cook |
|
|
When lifting the milk or something heavier, lift it 5 times or more up and down, before setting it down. |
Appendix 3: Examples of physical activities recommended by occupational therapist based on social roles.
Then a research assistant called the participants in a tapering fashion over the next 10 weeks, just asking if they have achieved their weekly PA and participation goals, no additional counseling was provided. At 18-weeks, all participants were assessed, then given the ActiWatch to wear for 7 days, and a stamped envelope to mail it back to the study team. After completion of the study, all participants were invited to attend a semi-structured interview session to share their experiences with the study team for the purpose of refining the intervention. Subsidiary prompts ensured that all relevant issues could be addressed. Interview questions explored the participants’ opinion on specific components of the intervention, motivation and barriers to PA and participation. The interviews took up to 30 minutes and were audio recorded.
Feasibility measures
The feasibility measures included participant satisfaction with the intervention, intervention adherence and time to deliver the intervention. Satisfaction was measured by a brief survey of a series of Likert scale items and semi-structured interviews. Throughout the study, participants were encouraged to contact the study team for any adverse events and research staff routinely inquired about their well-being during all interactions. Semi-structured interviews assessed participant’s feedback in two broad areas: key components of the study, and potential motivators and barriers to PA and participation. Questions on the study were more targeted and specific, whereas questions on PA and participation were more general and broad. All questions were followed by the interviewer prompting the participant to provide more in-depth feedback.
Outcome measures
The primary outcomes were changes in PA and participation over 18-weeks. PA was measured by the Community Healthy Activities Model Program for Seniors (CHAMPS) surveys and ActiWatch counts. The CHAMPS survey is a commonly utilized and validated 41-item questionnaire to evaluate PA in community-dwelling older adults [20]. The CHAMPS assesses physical activities over the past 4 weeks, including the number of times and hours per week involved in each activity. Each of these activities is assigned a metabolic equivalence task value consistent with the intensity of effort usually associated with performing the activity, thus allowing calculation of caloric expenditure per week [21]. ActiWatch is a validated wrist-worn accelerometer that generates objectively measured PA counts [22]. Participants wore the accelerometer during the 2 weekly home-monitoring periods (at baseline and at final week). Two measures were used to examine activity level and sedentariness 1) average counts/minute; 2) percent immobility time, or the percentage of epochs in the given interval scored as immobile by the ActiWatch [23].
Participation was assessed by three validated questionnaires from the Patient-Reported Outcomes Measurement Information System (PROMIS) assessment center [24]. Each questionnaire was standardized with a USA adult population with mean of 50 and standard deviation 10. The Ability to Participate in Social Roles and Activities (APSRA, PROMIS Item Bank V2.0) assesses participation restriction, including problems experienced in social interaction, employment, transportation, community, social and civic life. The remaining two questionnaires were Satisfaction with Participation in Social Roles (SPSR, PROMIS Item Bank V1.0) (e.g., I am satisfied with my ability to do things for my family), and Satisfaction with Participation in Discretionary Social Activities (SPDSA, PROMIS Item Bank V1.0) (e.g., I am satisfied with my ability to do things for fun at home (like reading, listening to music, etc.)).
Secondary outcomes were changes in physical function as measured by Timed Up and Go (TUG; time to rise from a chair, walk 3 meters and return to the chair); Six Minute Walk (6MW); and Comfortable Gait Speed over a 10-meter walk. Blood samples were analyzed for glycosylated hemoglobin A1c (A1c) by the University of Michigan’s Diabetes Research Center Chemistry Laboratory.
Data analysis
We did not power this proof-of-concept study as our aim was to test the feasibility of the intervention, and no previous studies have investigated the effect of PA intervention on participation changes. We also intentionally kept the overall sample size small (under 25) to allow more comprehensive evaluation and refinement of the intervention. The feedback from the interviews will be used to inform design of future larger randomized-controlled trial. Descriptive statistics (means and standard deviations) were used to estimate time spent in face-to-face counseling and phone calls by the OT. Mean satisfaction and percentage of sessions attended were also calculated. Percent intervention adherence was calculated based on the attendance at the 2 OT face-to-face visits and the completion of the 4 phone calls from the OT.
Data from the interviews were transcribed and read by two research team members. Initially the transcribed data were separated into participants who had improved PA (CHAMPS results and /or ActiWatch scores) versus those who did not improve. Data in each group were then examined for themes reported by previous studies [25-27], such as internal factors involved the individual’s own decision-making, and external factors involved an individual’s contextual environment, which are independent of an individual’s decision-making [25-28,]. Data regarding participation were separately reviewed to identify for any common themes. Other co-authors read the relevant data to establish consensus on the interpretation.
For pre-post comparisons, we performed paired t tests comparing CHAMPS scores for all activities and for moderate-intensity activities, accelerometer mean daily activity counts and immobile time from baseline versus the final 7 days of the study. Similarly, paired t tests were used to test for statistically significant scores in participation surveys and physical function. Effect sizes for PA and participation changes were estimated using the ratio of the mean change to the standard deviation of that change [29]. All statistical analyses were performed using Stata14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
RESULTS
A total of 35 individuals consented for the study. Ten were excluded by the study team because they did not complete home monitoring activities. Two were excluded prior to the intervention because they also enrolled in another PA training program. The remaining 23 individuals all completed the intervention; 2 later had to withdraw for unrelated health events, and 1 was lost to follow-up, leading to a retention rate of 87% (n=20) among those who completed the baseline activities (Figure 1).
Among the 20 participants who completed the study, mean (± standard deviation) age was 73 ± 5.95 years, 50% were female, 85% White, 75% completed college, and had a mean of 2.9 ± 1.88 chronic diseases in addition to diabetes. The mean BMI was 31.7 ± 6.08 kg/m2, A1c 7.2% ± 1.17%, diabetes duration 13.9 ± 7.57 years and 33% were on insulin. Half of the participants reported difficulty walking up several flights of stairs. With respect to baseline characteristics, there was no statistical difference between the individuals who completed the study and the 15 individuals who consented but did not complete the study (all p>0.05).
Feasibility results
Adherence to the intervention was good: 100% participants completed the 2 face-to-face counseling sessions and at least 1 of the 4 OT phone calls; 87% completed at least 2 OT phone calls. The total mean (± standard deviation) OT counseling time was 112 ± 24 min, including 92 ± 13.8 min face-to-face time, 21 ± 15 minutes for the 4 phone calls in total. Sixteen participants completed the feedback survey (Table 1). Overall they were satisfied with the intervention and gained knowledge on how to improve PA. No adverse events related to the intervention were reported.
|
Satisfaction of the study |
Participants responded “Yes” |
|
Willing to volunteer for this study again |
81% |
|
Would recommend this study to a good friend |
94% |
|
Improved understanding of the current level of physical activity |
88% |
|
Improved understanding of ways to improve physical activity |
75% |
|
Satisfaction of the Intervention Components* |
Participants “Satisfied / very satisfied” |
|
Weekly activity logs |
75% |
|
Face-to-face counseling by the OT |
88% |
|
Phone calls from the OT |
75% |
|
Strategies recommended by the OT |
81% |
Table 1: Survey of 16 participants who completed the study.
*The answers in the survey on intervention components had 5 choices: very dissatisfied, dissatisfied, neither satisfied nor dissatisfied, satisfied, and very satisfied.
Intervention results
Based on the 4-week recalled CHAMPS activities, the participants had significant improvement in total PA (+1689 Kcal/week, p<0.01; effect size 0.71) and moderate-vigorous intensity PA (+1533 Kcal/week, p<0.01; effect size 0.78) (Table 2). The moderate-vigorous activity more than doubled from the baseline. Based on the accelerometer, the counts per minute and percentage of immobile time were unchanged (p>0.05). Two of the three measures of participation improved significantly: APSRA (+2.97, p=0.01; effect size 0.40) and SPDSA (+3.51, p=0.02; effect size 0.41); SPSR remained unchanged (p=0.41). Eighty percent of participant had improved or stable SPDSA score, 60% had improved APSRA and 62% had improved SPSR. There was substantial variability in individual participant’s response to these three measures of participation: 1 participant had a decline in all three measures, 10 participants improved in all three measures, 6 participants declined in both APSRA and SPSR (r=0.78, p=0.0001). Finally, comfortable gait speed improved by 0.05 meters/second (p=0.04); results from TUG, 6MW test and A1c were unchanged (p>0.05).
|
Participant characteristics (N=20) |
Baseline (mean ± SD) |
Outcome (mean ± SD) |
Change from baseline (mean ± SD) |
P value |
|
A1c, % |
7.20 ± 1.18 |
6.97 ± 0.76 |
-0.23 ± 0.88 |
0.25 |
|
BMI, kg/m2 |
31.69 ± 6.08 |
31.76 ± 5.82 |
0.08 ± 0.99 |
0.73 |
|
TUG, sec (n=19) |
10.06 ± 1.87 |
10.32 ± 2.32 |
0.26 ± 0.95 |
0.26 |
|
Gait speed, m/s (n=19) |
1.17 ± 0.18 |
1.22 ± 0.19 |
0.05 ± 0.10 |
0.04 |
|
6MW, m (n=19) |
347.70 ± 53.05 |
357.35 ± 60.32 |
9.66 ± 27.79 |
0.14 |
|
CHAMPS Total (Kcal/wk) |
2433.85 ± 1605.19 |
4122.99 ± 2954.94 |
1689.14 ± 2333.16 |
0.004 |
|
CHAMPS: Any physical activities per week, times |
13.30 ± 8.46 |
19.15 ± 11.85 |
5.85 ± 8.93 |
0.009 |
|
CHAMPS moderate activity (Kcal/wk) |
1055.43 ± 1168.40 |
2588.57 ± 2526.40 |
1533.14 ± 1900.50 |
0.002 |
|
CHAMPS: Moderate-vigorous physical activities per week, times |
3.70 ± 3.88 |
8.1 ± 6.58 |
4.4 ± 4.53 |
0.0004 |
|
ActiWatch Counts/min (n=18) |
236.65 ± 90.48 |
242.18 ± 90.86 |
5.52 ± 42.90 |
0.59 |
|
ActiWatch immobile time per day; % |
24.25 ± 9.57 |
24.63 ± 10.08 |
0.37 ± 6.96 |
0.82 |
|
*Ability to participate in social roles & activities |
54.51 ± 7.37 |
57.48 ± 7.64 |
2.97 ± 4.90 |
0.01 |
|
*Satisfaction with participation in Social Roles |
54.66 ± 7.50 |
55.90 ± 7.10 |
1.24 ± 6.64 |
0.41 |
|
*Satisfaction with participation in discretionary social activities |
55.42 ± 8.16 |
58.43 ± 6.23 |
3.01 ± 5.43 |
0.02 |
Table 2: Intervention results on participants who completed the study.
P-value: Paired t-test comparing the means before and after intervention. A1c = Hemoglobin A1c; BMI = Body Mass Index; TUG= Timed Up and Go test; 6MW = 6-minute walk test; CHAMPS = Community Healthy Activities Model Program for Seniors.
*Patient-Reported Outcomes Measurement Information System (PROMIS) survey scores are ranged from 0-100, with population mean 50. Higher scores meant better ability to participate or more satisfaction with participation.
Participant input from interviews
Several general themes on PA engagement emerged from the semi-structured interviews (n=10). The first one was that as a result of the study, the participants in general gained “more awareness” of the opportunities to be more physically active throughout the day, and became “more knowledgeable” about how to be physically active. Another theme was that the participants liked the intervention, particularly the goal setting portion of the study. While most individuals were motivated to be more active because they knew PA can improve their health, barriers to activities differed among the 5 individuals who improved their PA based on CHAMPS and accelerometer measures (i.e., responders) versus those who did not improve (i.e., non-responders). The responders reported barriers that are related to the environment and motivation, including bad weather, lack of time and lack of motivation. In addition to these barriers, the non-responders also reported barriers that are health related: knee pain, fatigue and exhaustion due to emphysema.
The motivators to participate socially included learning new knowledge, setting a goal and joy of spending time with family or neighbors:
- “[Motivation to be more interactive is] want to learn more about Alzheimer’s”
- “I’ve not talked to neighbor for a long time and now I visit her frequently”
- “With this study, I am spending more time with the grandkids. I can move better, and not hurting”
Barriers for participation included distance from family/neighbors and physical health concerns:
- “Most of the people take off during the winter.... I don’t have family in the area....no longer have a dog”
- “Colds (sickness)”
DISCUSSION
This proof-of-concept study demonstrated feasibility and improvements in self-reported physical activity and participation among sedentary older adults with T2DM. Eighty-seven percent of the participants who completed the baseline home monitoring activities completed the study. For the participants who completed the intervention, our program was well accepted; over 90% of those surveyed were willing to recommend the study to a good friend. The intervention was successful in improving PA based on a validated self-report PA measure (CHAMPS), comfortable gait speed, and two of the three participation outcomes, but not the accelerometer counts. The brief counseling time, averaging less than 2 hours per person, strengthens the feasibility of the intervention and can be beneficial for wide dissemination if the results are further confirmed in larger randomized-controlled studies. The interview response suggested that PA interventions for older adults with diabetes may be more effective if they were implemented in conjunction with medical management of other symptoms.
While previous studies have shown that lifestyle - based PA can improve the management of T2DM and prevent physical function disability [13], our study is the first to find that such intervention also improves the measures of participation. In fact, an effect size of 0.40-0.41 is likely clinically meaningful as 0.30 is generally considered as a useful criterion for a minimally important difference in patient-reported outcome measures [29]. Our results contradict the findings from a systematic review of 18 randomized-controlled trials, which concluded that exercise interventions do not improve participation in life roles in older adults [14], perhaps because these exercise programs focused on functional improvement and did not consider environmental and personal factors. Our intervention, on the other hand, delivered by occupational therapist, focused on helping individuals to adapt to their environment based on their capacity [15]. Indeed, our positive results in participation may be in part be due to the tailored counseling based on each individual’s environmental and personal factors, and helping the participants set their own participation goals. Consistent with previous literature, the interview responses suggested that educational activities, social network and physical health were all important factors in social participation [7]. The response from one interviewee suggested that social participation can be improved through self-observation and goal setting: “Actually seeing it in writing. I’ve not talked to neighbor for a long time and now I visit her frequently.” Results from our study need to be confirmed in larger studies and the roles of environment and personal factors in participation will need to be further assessed.
For older adults, maintaining social participation or reducing participation restriction are considered highly important [30], and participation is associated with good quality of life [31] and faster gait speed [32]. Maintaining participation is associated with lower levels of morbidity, physical disability and mortality [12,33-35]. Participation allows fulfilment of valued life activities, aspects of identity and social roles (e.g., being a worker, caregiver or community member) [12,30].
Diabetes was a leading driver of the population attributable risk for social participation restriction when compared with arthritis, ischemic heart disease and cognitive impairment among older Canadians [36]. Chronic diseases such as diabetes impact daily routines, as the individuals need to allocate time and effort to manage their medical conditions (i.e., more physician visits) which may lead to less time for participation [37]. More patients with diabetes reported at least one social participation restriction than those without diabetes (32% vs. 23%, p=0.003) [37]. As older age is associated with higher prevalence of multiple chronic conditions [38], future intervention studies involving older adults should consider including participation as an outcome because it transcends different chronic conditions; measurement of participation also captures the individual's capacity to fulfil basic tasks of life as well as the interaction between his/her capabilities, environment and needs [12,33,39,40].
Among the three objective physical function measures, gait speed showed statistically significant improvement, albeit not clinically significant [41]; improvement of gait speed by 0.1m/s is associated with improved survival [42] and reduced risk for disability [43]. Nevertheless, our finding is promising given the brief intervention, and that the natural course of the physical functioning is to decline over time, even with intense PA and weight loss [13]. Therefore, the fact that our intervention led to slightly improved or unchanged physical function over 18 weeks suggests that it may be beneficial for physical function.
Our study has several strengths. The intervention is innovative because it: 1) was tailored based on each individual’s own baseline PA performed within his/her environment, consistent with the ICF model; 2) used an accelerometer-assisted, field-based method to address the individual’s unique physical and social environment [44]; 3) was delivered by an OT to guide individuals with functional impairment to adapt to their environment [45]; and 4) incorporated both PA goals as well as participation goals. The study provided preliminary effect size estimation for future intervention and supports a run-in period in the future to improve participant retention from enrollment as 29% of the enrolled participants had difficulty fulfilling the protocol requirement [46].
This study has a number of limitations, including small sample size, short duration of the follow up and a lack of a control group. Despite these limitations, our study procedure was generally feasible and well-accepted and there were some preliminary effects of the intervention, including improvement in self-reported PA and stable physical functioning. The self-reported CHAMPS results were susceptible to recall bias and social desirability, thus the effectiveness of the intervention on PA should be interpreted with caution. However, CHAMPS is a well validated questionnaire with evidence of reliability [47], and provides an estimate of 4-weeks activity, whereas the accelerometer only recorded activities in 1 week. Participants enrolled in a clinical trial may have symptomatic improvement even before the intervention [48]. Some of our participants were likely to have started changing their behavior and became more active, which would be captured by the accelerometer at baseline assessment. In fact, such behavior led to two individuals being excluded from the remaining of the study as they joined another PA program right after enrollment into the study. The CHAMPS results would more likely reflect true baseline activities prior to enrollment. Additionally, older adults with multiple chronic medical conditions are at risk to have an acute or unexpected health event that can interfere with their activity level. Therefore, one week’s accelerometer measurement may not fully reflect the overall usual activity level of the individuals [49]. At least two of our participants reported having a “bad week” due to unrelated and unexpected health events during the week when they wore the accelerometers.
There is an urgent need for successful and sustainable interventions to improve PA among sedentary older adults with diabetes. Due to the heterogeneous health status of this population, interventions that are tailored based on the individual’s functional status in the context of their environment may be effective in leading to long-term benefits [45]. This study provides preliminary support for a feasible, OT-facilitated lifestyle intervention to improve self-reported PA and participation in a sample of sedentary older adults with T2DM. Future randomized controlled trials will assess long-term outcomes of such a tailored-activity program in diverse populations.
ACKNOWLEDGEMENT
Funding: This work was supported by VA Rehabilitation Research and Development Career Development Award (5-IK2-RX001190 to P.G.L.), the National Institute on Aging Claude Pepper Center (AG024824 to P.G.L.), and the National Institute of Diabetes and Digestive and Kidney Diseases (MDRC, P30DK020572). The sponsors had no role in the project beyond funding.
Trial Registration: ClinicalTrials.gov Identifier: NCT02699307; registered 2/10/2016.
DECLARATION OF INTERESTS
The authors declare that they have no competing interests. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
REFERENCES
- CDC (2017) National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States. CDC, Georgia, USA. 1-20.
- Songer TJ (1995) Disability in diabetes. In: Harris MI, Cowie CC, Stern MP (Eds.). Diabetes in America (2ndedn). National Institutes of Health, Maryland, USA.
- Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, et al. (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42: 2282-2303.
- Hillsdon M, Foster C, Thorogood M (2005) Interventions for promoting physical activity. Cochrane Database Syst Rev: CD003180.
- Lee PG, Cigolle CT, Ha J, Min L, Murphy SL, et al. (2013) Physical function limitations among middle-aged and older adults with prediabetes: One exercise prescription may not fit all. Diabetes Care 36: 3076-3083.
- Kluding PM, Bareiss SK, Hastings M, Marcus RL, Sinacore DR, et al. (2017) Physical Training and Activity in People With Diabetic Peripheral Neuropathy: Paradigm Shift. Phys Ther 97: 31-43.
- Galenkamp H, Deeg DJH (2016) Increasing social participation of older people: Are there different barriers for those in poor health? Introduction to the special section. Eur J Ageing 13: 87-90.
- Mallinson T, Hammel J (2010) Measurement of participation: Intersecting person, task, and environment. Arch Phys Med Rehabil 91: 29-33.
- Corbett DB, Rejeski WJ, Tudor-Locke C, Glynn NW, Kritchevsky SB, et al. (2018) Social Participation Modifies the Effect of a Structured Physical Activity Program on Major Mobility Disability Among Older Adults: Results From the LIFE Study. J Gerontol B Psychol Sci Soc Sci 73: 1501-1513.
- Kim D, Subramanian SV, Gortmaker SL, Kawachi I (2006) US state- and county-level social capital in relation to obesity and physical inactivity: A multilevel, multivariable analysis. Soc Sci Med 63: 1045-1059.
- Conn VS, Minor MA, Burks KJ, Rantz MJ, Pomeroy SH (2003) Integrative review of physical activity intervention research with aging adults. J Am Geriatr Soc 51: 1159-1168.
- World Health Organization (2001) International classification of functioning, disability and health: ICF. WHO, Geneva, Switzerland.
- Rejeski WJ, Bray GA, Chen SH, Clark JM, Evans M, et al. (2015) Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci 70: 345-353.
- Beauchamp MK, Lee A, Ward RF, Harrison SL, Bain PA, et al. (2017) Do Exercise Interventions Improve Participation in Life Roles in Older Adults? A Systematic Review and Meta-Analysis. Phys Ther 97: 964-974.
- American Occupational Therapy Association (2014) Occupational therapy practice framework: domain & process (3rdedn). AOTA Press, Maryland, USA.
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC (2002) Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 40: 771-781.
- Clark NM, Gong M, Kaciroti N (2014) A model of self-regulation for control of chronic disease. Health Educ Behav 41: 499-508.
- Rodgers AB, Pocinki KM, National Institute on Aging (2009) Exercise & physical activity your everyday guide from the National Institute on Aging at NIH. NIA, Maryland, United States.
- Gardiner PA, Eakin EG, Healy GN, Owen N (2011) Feasibility of reducing older adults' sedentary time. Am J Prev Med 41: 174-177.
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D (2001) CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc 33: 1126-1141.
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, et al. (2003) Table B1: Revised Codebook for CHAMPS Physical Activity Measures.
- Gironda RJ, Lloyd J, Clark ME, Walker RL (2007) Preliminary evaluation of reliability and criterion validity of Actiwatch-Score. J Rehabil Res Dev 44: 223-230.
- Murphy SL, Kratz AL (2014) Activity pacing in daily life: A within-day analysis. Pain 155: 2630-2637.
- Hahn EA, DeWalt DA, Bode RK, Garcia SF, DeVellis RF, et al. (2014) New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol 33: 490-499.
- Costello E, Kafchinski M, Vrazel J, Sullivan P (2011) Motivators, barriers, and beliefs regarding physical activity in an older adult population. J Geriatr Phys Ther 34: 138-147.
- Korkiakangas EE, Alahuhta MA, Husman PM, Keinänen-Kiukaanniemi S, Taanila AM, et al. (2011) Motivators and barriers to exercise among adults with a high risk of type 2 diabetes--a qualitative study. Scand J Caring Sci 25: 62-69.
- Schutzer KA, Graves BS (2004) Barriers and motivations to exercise in older adults. Prev Me 39: 1056-1061.
- Korkiakangas EE, Alahuhta MA, Laitinen JH (2009) Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: A systematic review. Health Promot Int 24: 416-427.
- Cohen J (1988) Statistical power analysis for the behavioral sciences (2ndedn) Lawrence Erlbaum Associates, New Jersey, USA.
- Grime J, Richardson JC, Ong BN (2010) Perceptions of joint pain and feeling well in older people who reported being healthy: A qualitative study. Br J Gen Pract 60: 597-603.
- Netuveli G, Wiggins RD, Hildon Z, Montgomery SM, Blane D (2006) Quality of life at older ages: Evidence from the English longitudinal study of aging (wave 1). J Epidemiol Community Health 60: 357-363.
- Warren M, Ganley KJ, Pohl PS (2016) The association between social participation and lower extremity muscle strength, balance, and gait speed in US adults. Prev Med Rep 4: 142-147.
- Holmes WR, Joseph J (2011) Social participation and healthy ageing: A neglected, significant protective factor for chronic non communicable conditions. Globalization and Health 7: 43.
- Levasseur M, Desrosiers J, Noreau L (2004) Is social participation associated with quality of life of older adults with physical disabilities? Disabil Rehabil 26: 1206-1213.
- Levasseur M, Desrosiers J, Whiteneck G (2010) Accomplishment level and satisfaction with social participation of older adults: association with quality of life and best correlates. Qual Life Res 19: 665-675.
- Griffith LE, Raina P, Levasseur M, Sohel N, Payette H, et al. (2017) Functional disability and social participation restriction associated with chronic conditions in middle-aged and older adults. J Epidemiol Community Health 71: 381-389.
- Meek KP, Bergeron CD, Towne SD, Ahn S, Ory MG, et al. (2018) Restricted Social Engagement among Adults Living with Chronic Conditions. Int J Environ Res Public Health 15: 158.
- Lochner KA, Cox CS (2013) Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis 10: 120137.
- Dale C, Prieto-Merino D, Kuper H, Adamson J, Bowling A, et al. (2012) Modelling the association of disability according to the WHO International Classification of Functioning, Disability and Health (ICF) with mortality in the British Women's Heart and Health Study. J Epidemiol Community Health 66: 170-175.
- Resnik L, Plow MA (2009) Measuring participation as defined by the international classification of functioning, disability and health: an evaluation of existing measures. Arch Phys Med Rehabil 90: 856-866.
- Bohannon RW, Glenney SS (2014) Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J Eval Clin Pract 20: 295-300.
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, et al. (2011) Gait speed and survival in older adults. JAMA 305: 50-58.
- Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, et al. (2016) Gait Speed Predicts Incident Disability: A Pooled Analysis J Gerontol A Biol Sci Med Sci 71: 63-71.
- Murphy SL, Lyden AK, Clary M, Geisser ME, Yung RL, et al. (2011) Activity pacing for osteoarthritis symptom management: study design and methodology of a randomized trial testing a tailored clinical approach using accelerometers for veterans and non-veterans. BMC Musculoskelet Disord 12: 177.
- Zubala A, MacGillivray S, Frost H, Kroll T, Skelton DA, et al. (2017) Promotion of physical activity interventions for community dwelling older adults: A systematic review of reviews. PLoS One 12: 0180902.
- Pablos-Méndez A, Barr RG, Shea S (1998) Run-in periods in randomized trials: Implications for the application of results in clinical practice. JAMA 279: 222-225.
- Falck RS, McDonald SM, Beets MW, Brazendale K, Liu-Ambrose T (2016) Measurement of physical activity in older adult interventions: A systematic review. Br J Sports Med 50: 464-470.
- Targum SD (2017) Early symptomatic improvement affects treatment outcome in a study of major depressive disorder. J Psychiatr Res 95: 276-281.
- Provencher V, Mortenson WB, Tanguay-Garneau L, Bélanger K, Dagenais M (2014) Challenges and strategies pertaining to recruitment and retention of frail elderly in research studies: a systematic review. Arch Gerontol Geriatr 59: 18-24.
Citation: Lee PG, Connell CM, Alexander NB, Murphy SL (2019) Lifestyle Intervention to Increase Physical Activity and Social Participation among Older Adults with Diabetes: Result of a Feasibility Study. J Gerontol Geriatr Med 5: 036.
Copyright: © 2019 Pearl G Lee, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

