
The Cognition-exercise Interaction in Parkinson's Disease: A Perspective on Current Rehabilitative Approaches with Promise to Impact the Whole Disease Sequelae
*Corresponding Author(s):
Madeleine E HackneyDepartment Of Medicine, Division Of General Medicine And Geriatrics, Atlanta Veterans Affairs Rehabilitation R&D Center Of Excellence For Visual And Neurocognitive Rehabilitation, Emory University School Of Medicine, United States
Tel:+1 4043216111, 5006
Email:mehackn@emory.edu; madeleine.hackney@gmail.com
INTRODUCTION
In the last few decades, growing interest has been generated in better understanding motor-cognitive integration and the effects exercise has upon cognition in healthy older adults as well as adults with Parkinson's Disease (PD), a disorder with currently intractable cognitive and motor symptoms. In this perspective piece we will briefly summarize the current research landscape and explore possible mechanisms by which exercise may affect cognition in those with PD. We will then present suggestions on methodological issues that should be addressed to clarify the impact of exercise on function in individuals with PD as well as potential areas for future research aimed at determining ways to limit the motor and non-motor disease burden.
PD is the second most common neurodegenerative disorder of later life and is related to lost dopamine cells in the substantia nigra of the basal ganglia. Current estimates suggest 7 to 10 million people worldwide are living with PD. Due to progressive worsening of PD-related symptoms, management and treatment of this disease can be costly, with estimates exceeding $100,000 per patient [1].
The cardinal features of PD include bradykinesia (i.e., extreme slowness of moving), tremor, rigidity, and gait/postural impairment. These features manifest into altered neuromuscular control of the upper extremities and poor lower extremity function, leading to reduced independence and decreased health-related Quality of Life (QOL) for affected individuals. Difficulty turning and dual-tasking, balance challenges, and gait dysfunction are also present and worsen as the disease progresses, escalating the risk of falls [2,3].
NON-MOTOR SYMPTOMOLOGY
While PD has traditionally been considered a “movement” disorder, multiple systems are affected, compromising neurotransmitter systems and also encompassing cognitive, mood, and motivational systems. Nearly 80% of individuals with PD at some point in the disease process develop cognitive impairment [4], which interacts with the compromised motor system. Historically, movement and cognition have been thought to be directed by isolated, proprietary, cortical regions. However, overlapping neural systems likely serve both cognitive and motor function [5]. In PD the link between motor and cognitive function is especially clear. For example, increased bradykinesia is associated with the presence of mild cognitive impairment and impaired executive performance [6]. Also, the magnitude of the center of pressure displacement, a reliable measure of balance performance, is negatively correlated with performance on cognitive tests known to activate dorsolateral frontal regions [7]. Further, standard clinical tests involving multidirectional stepping over obstacles have a strong relationship with executive function performance in individuals with mild-moderate PD [8] (Figure 1). Additionally, people with PD often experience exaggerated dual task impairments relative to their age-matched peers [9]. The magnitude of dual task costs, or decrement in performance during concurrent tasks, is highly dependent on the task difficulty: as the secondary cognitive task increases in difficulty, so does the impairment in the primary motor task [10]. Findings from the dual task literature are consistent with the idea that motor and cognitive processing share cortical processing resources.
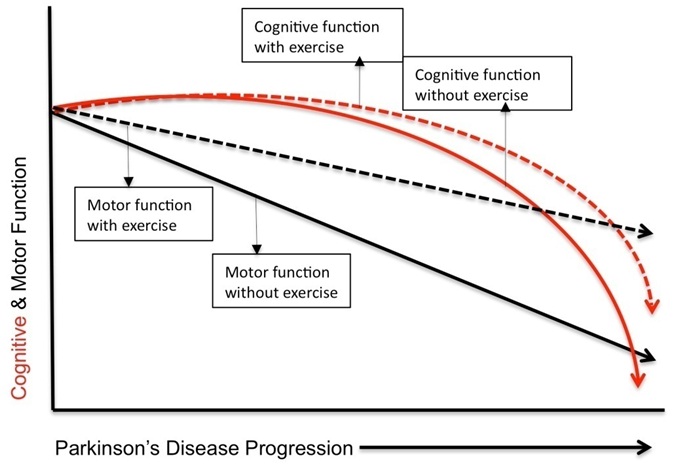 Figure 1: Theoretical illustration demonstrating the relative impact of exercise on motor (Black) and cognitive (Red) function on disease progression in Parkinson's disease.
Figure 1: Theoretical illustration demonstrating the relative impact of exercise on motor (Black) and cognitive (Red) function on disease progression in Parkinson's disease.
A spectrum of cognitive impairments affects individuals with PD over the course of the disease. While most individuals with PD show executive difficulties, others have co-occurring, distinct memory difficulties. Other, less studied, cognitive domains that appear to be impacted by PD include language outcomes, and more specifically, verbal fluency. Verbal fluency tasks are complex tasks requiring the development of strategies for searching either semantic memory in the case of category fluency or phonemic memory in the case of letter fluency. In fact, verbal fluency performance has been found to be predictive of QOL in PD [11]. Importantly, language outcomes and verbal fluency have a significant impact on activities of daily living and QOL, which often leads to interference with efficient and effective communication (Table 1).
| Motor |
| Bradykinesia (slowness of movement) |
| Tremor |
| Rigidity |
| Stride length shortening |
| Backward walking difficulty |
| Deregulation of cadence |
| Difficulty with internal generation of movement |
| Freezing (stopping of gait) |
| Movement initiation impairment |
| Cognitive |
| Psychomotor slowing |
| Declines in processing speed |
| Executive dysfunction |
| Set-shifting difficulty |
| Difficulty multitasking |
| Working memory impairment |
| Forgetfulness/memory impairment |
| Verbal Fluency impairment |
| Lost integrity of visuo-spatial cognition |
Table 1: Motor and cognitive impairments found in many individuals with Parkinson's disease.
EXERCISE AS THERAPY FOR COGNITIVE DECLINE
Recently, interest in using exercise as a treatment for PD motor and cognitive function has increased substantially. Below we highlight progress that has been made in this field, we clarify the research areas that must be resolved to elucidate the role of exercise in PD for improving both motor and cognitive function, and set the stage for future research directions.
In the last 10 years alone the number of publications addressing exercise for PD has more than tripled [12]. A 2012 study examining a 10-month community-based group exercise program for people with PD [13] found that the group exercise program resulted in improved endurance with maintained walking speed and balance. Another 16-month community exercise program investigated three exercise approaches: functional exercise addressing flexibility and balance, supervised aerobic exercises, and home-based exercises [14]. At four months, the functional exercise program improved overall function. The aerobic program was superior for improving walking specifically.
Despite these encouraging data that have shown the benefits of exercise for the motor system, their impact on cognitive function was not explored. However, evidence has recently emerged demonstrating there could be multiple cognitive subtypes of PD with different trajectories of cognitive decline [15], all of which desperately need treatments. Given the cognitive breadth of PD, the emergent interest in investigating the effects of exercise upon cognitive abilities is not surprising. Abundant data have begun to demonstrate that higher levels of habitual exercise can blunt cognitive decline associated with aging and neurological disease. Further, higher levels of habitual exercise enhance functioning in specific cognitive domains in aging and age-related disease.
CHANGES POTENTIALLY RELATED TO NEUROPLASTICITY
Briefly, we now highlight the purported neurobiological mechanisms responsible for protecting and enhancing brain health and related outcomes. We focus on functionally relevant, neuroplasticity-related changes attributed to physical activity, which are mechanisms that may account for exercise benefits on cognitive function.
Neuroplasticity is changes in structure or function of the nervous system on the basis of experience. Plastic changes can occur on anatomical, molecular, genetic, structural and functional levels within the nervous system. A possible neurobiological mechanism underlying the positive effects of exercise is the increased synthesis and release of neurotransmitters and neurotrophins, which could enhance neurogenesis, angiogenesis and thus neuroplasticity [16]. Additionally, reorganization of cortical representations, synaptogenesis, and synaptic potentiation, likely play a role.
Several small studies have examined the benefits of exercise for those with PD on various aspects of cognition. Six months of generalized moderate-intensity, multimodal physical training (consisting of aerobic, resistance, coordination and balance elements) led to improvements in the capacity for abstraction and mental flexibility as measured by the Wisconsin Card Sorting Task in 10 older individuals with PD compared to a non-exercising control group [17]. Fifteen individuals with PD who participated in programs of anabolic and aerobic exercise two times weekly for 12 weeks showed improvements in verbal fluency [18]. Low intensity passive cycling on a tandem bike, in a ‘forced exercise' situation one time per week over 4 weeks led to improvements by 19 people with PD on the Trails Making Test A&B, an executive function measure [19]. Dos Santos Mendes et al., assigned 16 individuals with early PD to Wii Fit training, to evaluate the motor and cognitive demands of the games on people with PD, in comparison to 11 healthy older adults. Compared to healthy controls, those with PD had no deficit in motor learning or retention on seven of the ten games, but had marked learning deficits on three games. However, the PD cohort was able to transfer motor ability gained from the games to a similar, but untrained task [20].
SUGGESTIONS AND FUTURE DIRECTIONS
These recent studies are encouraging and provide preliminary evidence supporting exercise effects on a variety of aspects of cognition for those with PD. However these findings must be replicated in phase III clinical trials. Further, all of these studies employed a range of cognitive outcome tasks, and improvements were limited to one or two executive function measures. Additionally, the number and size of the published studies of exercise effects in PD are limited, rendering definitive conclusions difficult to make. Based on evidence presented here, evaluating exercise's effects on particular aspects of cognitive function that are implicated in PD is recommended. Such investigation should include tests of well-defined executive functions and language outcomes. Examining the effects of exercise over both longer and shorter intervals and doses, and more randomized clinical trials are necessary to elucidate which symptoms of PD are amenable to change. In particular, if the goal is to examine neural mechanisms of plasticity and improvement, observing changes in a range of cognitive and motor abilities in a group of PD participants over 6 month, or better, 12 month intervals is necessary. These recommendations, if observed, will begin to bring the research on exercise in PD in line with previous exercise research completed in older adults (Table 2).
| Evaluation of dose response in exercise trials |
| Comparative effectiveness of modes of exercise |
| Multicomponent exercise interventions |
| Exercise intervention in conjunction with pharmacological aids |
| Exercise intervention in conjunction with other behavioral interventions (eg: cognitive training) |
| Interventions to optimize multitasking |
| Mechanistic studies to better under neural underpinnings of exercise adaptation: |
| (Neural imaging, neurostimulation with Transcranial magnetic stimulation, Electromyography) |
| Evaluation of mediating variables impacted in exercise (eg: mood, stress, apathy, depression, sleep) |
Table 2: Future research directions and clinical trial suggestions utilizing exercise to address cognitive, motor and motor-cognitive impairments in Parkinson's disease.
While significant progress has been made to understand and develop effective behavioral interventions for individuals with PD, many methodological issues should be addressed to clarify the impact of exercise on PD. Many studies do not ensure that all participants receive the same amount of treatment, which makes inference regarding the effects of one dose versus another difficult. Voss et al., demonstrated behaviorally relevant changes in functional connectivity, measured via fMRI, were correlated to improved executive function in older adults who participated in 12 months of a walking program. This cohort was also examined at 6 months but there were only non-significant trends towards improvement [21]. Therefore, ongoing exercise over a substantial period of time is likely necessary to detect significant functional benefits garnered through neuroplastic improvement mechanisms. Obtaining better monitoring of studies' success with exercise compliance and actual dosing achieved per participant, (i.e., with remote, instrumented telehealth means of assessment, instead of traditional self-completed patients' logs) would be helpful. Beyond laboratory, controlled experiments investigating dose-responsiveness and attrition rates, comparative effectiveness studies evaluating the potential of practical, real-life exercise interventions with community participants with PD are necessary. Such studies would shed light on the practicality of disseminating and implementing evidence-based exercise programs and further expose the challenges to adherence found within this difficult to treat population.
In the future, uncovering neural mechanisms of improvement may be the most important area of discovery for enhancing non-pharmacological interventions that address cognitive and motor issues as well as the cognitive-motor interaction in PD. The knowledge and principles gained could impact not only exercise disciplines but also pharmacological and surgical treatments for older adults with PD and related neurological disorders. The neuroprotection and neurorestoration that might be derived from consistent, task specific and frequent aerobic exercise, may extend into improved mood and functional activity performance, thereby impacting cognition. Investigating mechanisms of mediating variables, e.g., mood capability to accomplish daily activities, to impact cognition via exercise must be examined. Further work related to neurobiological mechanisms will be crucial to informing rehabilitative interventions as well as specifying pharmacological and surgical treatments. Imaging with positron emission tomography, functional magnetic resonance imaging and transcranial magnetic stimulation hold promise as well, given that lower limb neural pathways, implicated in exercise, are virtually unexplored in connection with rehabilitative efforts in PD. To address the needs of those with PD, motor symptom treatment cannot be the sole concern in care. As pharmacological and surgical methods remain only partially effective in treating symptoms of those with PD, and fall risk and related injury are prevalent among older adults, non-pharmacological approaches that address cognitive, motor and motor-cognitive impairments can provide some measure of amelioration for an otherwise intractable condition.
ACKNOWLEDGEMENT
We would like to gratefully acknowledge Dr. Steven Wolf for reviewing the manuscript and providing constructive input and valuable insight. Department of Veterans Affairs Career Development awards N0780-W and B8034-W supported ME Hackney and JR Nocera, respectively.
REFERENCES
- Noyes K, Liu H, Li Y, Holloway R, Dick AW (2006) Economic burden associated with Parkinson's disease on elderly Medicare beneficiaries. Mov Disord 21: 362-372.
- Bloem BR, van Vugt JP, Beckley DJ (2001) Postural instability and falls in Parkinson's disease. Adv Neurol 87: 209-223.
- Bloem BR, Hausdorff JM, Visser JE, Giladi N (2004) Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord 19: 871-884.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P (2003) Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 60: 387-392.
- Domellöf ME, Elgh E, Forsgren L (2011) The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson's disease. Mov Disord 26: 2183-2189.
- Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, et al. (2012) Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 83: 601-606.
- Nocera JR, Price C, Fernandez HH, Amano S, Vallabhajosula S, et al. (2010) Tests of dorsolateral frontal function correlate with objective tests of postural stability in early to moderate stage Parkinson's disease. Parkinsonism Relat Disord 16: 590-594.
- McKee KE, Hackney ME (2014) The Four Square Step Test in individuals with Parkinson's disease: association with executive function and comparison with older adults. NeuroRehabilitation 35: 279-289.
- O'Shea S, Morris ME, Iansek R (2002) Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther 82: 888-897.
- Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, et al. (2011) Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev 35: 715-728.
- Stegemöller EL, Nocera J, Malaty I, Shelley M, Okun MS, et al. (2014) Timed up and go, cognitive, and quality-of-life correlates in Parkinson's disease. Arch Phys Med Rehabil 95: 649-655.
- van der Kolk NM, King LA (2013) Effects of exercise on mobility in people with Parkinson's disease. Mov Disord 28: 1587-1596.
- Steffen T, Petersen C, Dvorak L (2012) Community-based exercise and wellness program for people diagnosed with Parkinson disease: experiences from a 10-month trial. J Geriatr Phys Ther 35: 173-180.
- Schenkman M, Hall DA, Barón AE, Schwartz RS, Mettler P, et al. (2012) Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther 92: 1395-1410.
- Tröster AI (2011) A précis of recent advances in the neuropsychology of mild cognitive impairment(s) in Parkinson's disease and a proposal of preliminary research criteria. J Int Neuropsychol Soc 17: 393-406.
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, et al. (2013) Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun 28: 90-99.
- Tanaka K, Quadros AC Jr, Santos RF, Stella F, Gobbi LT, et al. (2009) Benefits of physical exercise on executive functions in older people with Parkinson's disease. Brain Cogn 69: 435-441.
- Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, et al. (2011) Exercise and Parkinson's: benefits for cognition and quality of life. Acta Neurol Scand 123: 13-19.
- Ridgel AL, Kim CH, Fickes EJ, Muller MD, Alberts JL (2011) Changes in executive function after acute bouts of passive cycling in Parkinson's disease. J Aging Phys Act 19: 87-98.
- dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, Guedes da Silva K, Oliveira Tde P, et al. (2012) Motor learning, retention and transfer after virtual-reality-based training in Parkinson's disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy 98: 217-223.
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, et al. (2010) Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2: 32.
Citation: Nocera JR, Hackney ME (2015) The Cognition-exercise Interaction in Parkinson's Disease: A Perspective on Current Rehabilitative Approaches with Promise to Impact the Whole Disease Sequelae. J Gerontol Geriatr Med 1: 003.
Copyright: © 2015 Joe Robert Nocera, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

