
Low-Molecular Weight Bacterial Metabolites in Host-Microbial Interaction
*Corresponding Author(s):
Beloborodova NVVa Negovsky Research Institute Of General Reanimatology, Russian Academy Of Medical Sciences, 25-2, Petrovka Street, 107031 Moscow, Russian Federation
Tel:+7 9161317454,
Email:nvbeloborodova@yandex.ru, alchemist755@yandex.ru
Abstract
The review gives an insight into inherent biological properties of bacterial metabolites - low-molecular weight Phenylcarboxylic Acids (PCAs), including Benzoic Acid (BA), p-Hydroxyphenyllactic Acid (HPLA), Phenyllactic Acid (PLA), p-Hydroxyphenylacetic Acid (HPAA), Phenylacetic Acid (PAA), and Phenylpropionic Acid (PPA). It has been demonstrated that bacteria from human microflora - predominantly anaerobes - can metabolize aromatic amino acids into PCAs, and PCAs are capable to suppress the growth and propagation of other bacteria, entering competitive interactions within microbial associations. The authors suggest that in the human colon, where concentrations of microbial metabolites reach biologically active level, PCAs may exert not only local, but also systemic effects, thus any deviation from existing composition of microbial associations may potentially result in the breakdown of habitual PCAs balance and emergence of PCAs with opposite biological properties. Available published data as well as findings from own research allowed us to substantiate a novel approach directed at the development of new therapeutic strategies based on regulation of local and systemic balance of microbial aromatic metabolites in the human body.
Keywords
ABBREVIATIONS
PCAs - Phenylcarboxylic Acids
BA - Benzoic Acid
HPLA - p-Hydroxyphenyllactic Acid
PLA - Phenyllactic Acid
HPAA - p-Hydroxyphenylacetic Acid
PAA - Phenylacetic Acid
PPA - Phenylpropionic Acid
BAA - Benzamino-Acetic Acid
AMM - Aromatic Microbial Metabolites
ATP - Adenosine Three-Phosphate
SB - Sodium Benzoate
ROS - Reactive Oxygen Species
LPS - Lipopolysaccharide
iNOS - Inducible NO-Synthase
MCTs - Monocarboxylate Transporters
MFS - Major Facilitator Super Family
HA - Hippuric Acid
NOAEL - No Observed Adverse Effect Level
MIC - Minimal Inhibitory Concentration
MBC - Minimal Bactericidal Concentration
MFC - Minimal Fungicidal Concentration
INTRODUCTION
Well established and well balanced biological intercommunication between macro and microorganisms has been formed in the process of evolution. Meanwhile this phenomenon is hardly taken into account in the context of clinical research, as traditionally both the research and descriptions of biochemical and signaling processes are done separately for the macroorganism and inhabiting it microflora. It’s due mostly to the inertness of medical science which keep replicating erroneous perception of diverged biochemical regulatory pathways of pro and eukaryotic organisms, neglecting their continuous coevolution.
We believe that further progress in clinical science is impossible without considering the role of human microflora vital activities, habitability and intercommunication with human metabolism, without discovering common signal pathways envisaging key roles of microbial metabolites in pathogenesis of both infectious and non-infectious (such as oncologic, endocrine, mental, etc.,) diseases. Clinical microecology, a novel domain in medical science, is the most appropriate term, encompassing all aspects listed above.
This deficit in knowledge of microecology is most painfully evident in anesthesiology and critical & emergency care medicine. Sepsis remains the leading direct cause of death in intensive care units, despite intensive use of multilevel and multi-component monitoring, most potent antimicrobials and hitech organ replacement technologies [1-5]. Active research of aromatic microbial metabolites and their potential role in tanatogenesis is being carried out in the Lab of human Metabolism in Critical States (MCS), Negovsky Scientific Research Institute of General Reanimatology [6].
It has been shown that simple chemical compounds act as signal molecules and bio-regulators in microbial community, representing the most archaic autoregulation and intercellular communication mechanism, so called quorum sensing [7]. In the process of evolution low molecular weight compounds have secured their principal role in the human metabolism, it’s suffice to mention some hormones (such as endogenous catecholamines, thyroid hormones), neurotransmitters (serotonin, γ-aminobutyric acid), tissue and mitochondrial metabolism autocrine regulators (NO), etc.
Simple chemical compounds may play important bridging role in the intercommunication between bacterial and human metabolism. For example, adrenaline and other catecholamines turned out to be involved into interbacterial communication, as well as into bacterial interaction with macro-organism [8,9]. Preliminary data on established profiles of live microorganisms exometabolites in human serum are already published [6,10,11]. Comprehensive studies of microbial metabolites in human biological liquids & tissues seem to be the most promising approach for future deeper insights into potential impact of micro-ecological derangements on human organism that instantly manifests via impaired balance of exometabolites.
CLINICALLY RELEVANT PHENYLCARBOXYLIC ACIDS
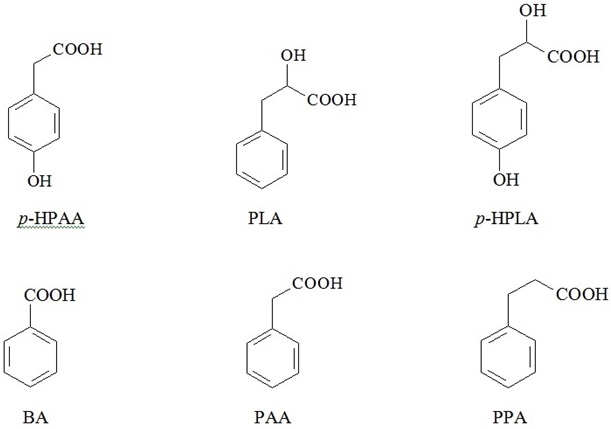
Moreover, we established direct correlation between cumulative serum content of PCAs and the severity of disease [10]. Quantification of some PCAs was successfully used in clinical practice for verification of sepsis (invention patent No 2423704 RU), although deeper insights and better understanding of specific roles and mechanisms of action of microbial exometabolites in human metabolism are still lying ahead [14-17].
Benzoic acid - represented to the fullest in available publications - has been chosen as a model for theoretic analysis of diverse biological PCAs properties.
Benzoic acid
BA is naturally synthesized by bacteria, plants and fungi. High BA concentrations are found in fermented dairy foods, considerable amounts of BA are produced by Lactobacilli from hippuric acid and accumulated as the final product of phenylalanine biodegradation (Figure 2) [15,18,19].
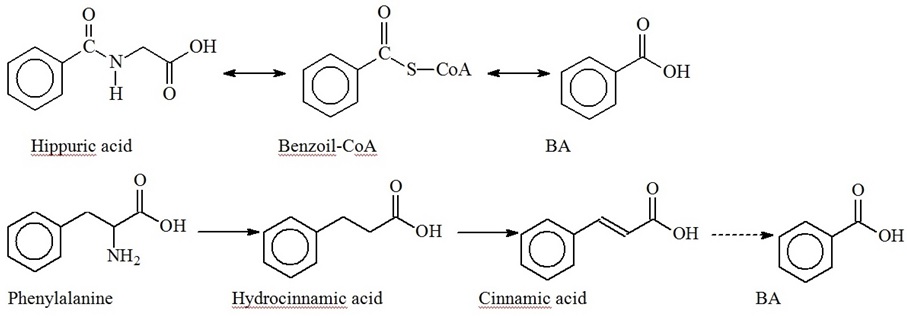
BA concentration in yogurt varies from 9 to 56 mg/kg, in cheese it amounts up to 200 mg/kg and more [15]. BA is naturally found in tomatoes, beans, cereals, nuts, fruits, honey and mushrooms [20,21]. High BA concentrations up to 0.05% of total weight are found in different berries, in particular up to 4500 mg/kg in arctic cranberries and blueberries [22]. Instant increase of BA production in response to infection was established in plants [23]. Salicylic (ortho-hydroxybenzoic) acid is most common and best studied among herbal low-molecular weight PCAs. Ortho-hydroxybenzoic acid was shown to interfere with the expression of genes encoding mitochondrial proteins, and it’s concentration was fluctuating proportionally to BA levels [24]. Fungi produce BA via biodegradation of phenylalanine [25]. In herbivorous BA was found in soft tissues and milk [19]. There’s no published data or any reference in Human Metabolome Database (HMDB; www.hmdb.ca), indicating that BA is produced in human body, either as the final product of phenylalanine biodegradation or as a result of de novo synthesis from aliphatic compounds. Nevertheless, BA is also produced in the human body via benzaldehyde (present in food stuff) oxidation or benzyl alcohol (present in many medicinal drugs) oxidation [26,27], or polyphenolic products oxidation [28,29]. Knoop F was the first to demonstrate that BA is formed via β-oxidation of lateral PCAs chains with uneven number of carbon atoms (phenylpropionic and phenylvaleric acids), meanwhile β-oxidation of lateral PCAs chains with even number of carbon atoms (phenylbutyric, phenylcaproic acid) yields Phenylacetic Acid (PAA) [30]. Higher concentrations of BA, as compared to other PCAs, were found both in feces - 6.2 mg/L [31], and blood serum - 0.079 mg/L - of healthy volunteers [6,10]. BA found in human biological matrices & fluids originates predominantly from ingested food, as well as from the production by GIT microflora and from oxidation of polyphenoles [28,30]. Figure 3 shows production pathways of some PCAs in the human body from both, human and bacterial phenylalanine and tyrosine metabolism [14].
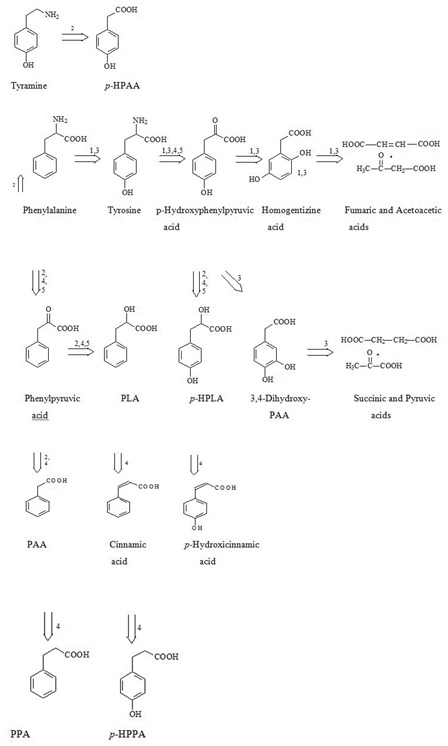 Figure 3: Interrelation between Endogenous and Microbial Catabolic Pathways of PCAS Synthesis from Phenylalanine and Tyrosyne in Humans [14].
Figure 3: Interrelation between Endogenous and Microbial Catabolic Pathways of PCAS Synthesis from Phenylalanine and Tyrosyne in Humans [14].Although, bacteria utilize some PCAs, in particular Hydroxyphenylpropionic Acid (HPAA) and p-hydroxyphenylpropionic Acid (p-HPPA) as precursors for phenylalanine, tyrosine and tryptophan synthesis [32].
Higher organisms were shown to lose their capacity to produce some metabolites in close co-habitation with microflora in the process of co-evolution. Figure 3 shows the example of anaerobic formation of cinnamic, hydroxicinnamic, phenylpropionic and hydroxyphenylpropionic acids exclusively by bacteria.
antimicrobial effects
| Microorganism | pH | MIC, mg/L |
| Esherichia coli [33] | 6.0 | 100-200 |
| Lactobacillus spp [15] | 4.3-6.0 | 300-1800 |
| Klebsiella pneumonia [33] | 6.0 | 100-200 |
| Pseudomonas aeruginosa [34] | 5.0/7.0 | 250/1000 |
| Pseudomonas aeruginosa [33] | 6.0 | 200-500 |
| Staphylococcus aureus [34] | 5.0/7.0 | 500/1000 |
| Staphylococcus aureus [33] | 6.0 | 50-100 |
| Streptococcus spp [15] | 5.2-5.6 | 200- 400 |
| Candida albicans [34] | 5.0/7.0 | 130/>1000 |
| Zygosaccharomyces bailii [33] | 4.8 | 4500 |
| Zygosaccharomyces bailii [33] | 4.0 | 1200 |
Table 1: Minimal Inhibitory BA Concentrations for Some Bacterial and Fungal Species, mg/L.
Due to inherent antiseptic properties BA and its’ salts are commonly used as preservatives (E210-?213) in food and cosmetic industry.
BA potential bacteriostatic and bactericidal effect on upper GIT tract microflora was demonstrated in experimental studies on piglets. Bacteriological studies of PCAs’ potential to inhibit pure cultures of clinically significant strains showed that BA, as well as Phenylacetic Acid (PAA) and Phenylpropionic Acid (PPA), were inhibiting to greatest extent the growth of ?. coli, with non-pathogenic ATCC 25992 ?. colistrain being more resistant to BA/PCAs, than enteropathogenic ?157:H7 E. coli. A negligible effect of PCAs with one or two Hydroxyl Groups (3-hydroxy- 4-hydroxyl-3, 4-dihydroxy-substituted PCAs) in aromatic ring was documented with enteropathogenic strain, and no effect with non-pathogenic ATCC 25992 strain at ≤ 1000 mg/L concentrations. Hydroxybenzoic acids turned out to be more potent than BA in Lactobacilli inhibition, while BA and derivatives were most effective inhibitors of pathogenic Staphylococcus aureus (strain EP167). PAA and PPA seem to be the supreme - as compared to their hydroxyl-derivates - inhibitors of Lactobacilli and S. aureus [35].
Pseudomonas aeruginosa PAO1 from gram -ve family showed resistance to BA and other PCAs at 1000 mg/L concentration. BA and PPA at 1000 mg/L only partially (by 16% and 29%, respectively) inhibited the ?andida albicans MY1055. The authors suggested that different microorganism’s sensitivity to PCAs depends predominantly on specific structure of cellular wall [36]. It’s important to mention that all PCAs in this review inhibited propagation of microorganisms at concentration’s values ranging within one order, which implies similar mechanisms of their action, according to the theory of weak organic acids (see below).
It has been suggested that BA belongs to so called allelochemicals, expressing allelopathy i.e., suppressing or inhibiting growth of other organisms in the environment [37]. This can be well applicable to other PCAs. We suggest that PCAs regulate to certain extent the diversity and propagation tempos of human microflora [36].
Jenner AM et al., identified significant amounts of different PCAs in human fecal waters with predominating PAA- 479 µ?, PPA -166 µ?, p- HPPA - 68 µ?, 3,4-dihydroxy- cinnamic acid - 52 µ?; BA - 51 µ?, 3-hydrohy-phenylacetic acid- 46 µM; p-HPAA - 19 µ? and 3,4- dihydroxy-PAA - 7 µ?. Of importance, long-term monitoring of colonic PCAs’ profile in participating volunteers showed consistent persistence of BA levels, varying within 23-25 µ? in consecutive daily samples [31].
The results from own studies also confirm the potential of anaerobic bacteria derived from human microflora to produce PCAs [38]. Moreover, the identified PCAs profile was consistent with that of Jenner AM et al. (Table 2). Of importance, some PCAs in anaerobic cultures were accumulated up to the levels, stated by other authors as concentrations inhibiting microbial propagation.
| PCAs | Lactobacillus fermentum, 24 hour culture | Bifidobacterium bifidum, 24 hour culture | Lactobacillus plantarum, 72 hour culture | Lactobacillus fermentum, 72 hour culture |
| PPA |
155.7 mg/L (1036.8 µ?) |
|||
| p-HPPA | 123.2 mg/L (795.6 µ?), Jenner AM [31] | |||
| p-HPLA | 13.7 mg/L (72.3 µ?) Beloborodova N et al. [38] |
(39.4 mg/L (216.28 µ?) Beloborodova N et al. [36] |
260 ± 13 µ?, Francesca Valerio [40] | Undeterminable, Francesca Valerio [40] |
| PLA |
35.4 mg/L (213 µ?) |
64.9 mg/L (390 µ?)Beloborodova NV et al. [38] | 310 ± 19 µM, Francesca Valerio [40] | Insignificant amounts, Francesca Valerio [40] |
Mechanisms of intracellular PCAs penetration
| Microorganism | BA | Acetic acid | Lactic acid |
| ?. cereus ATCC11778 | 296 | 2020 | 3480 |
| B. subtilis ATCC6633 | 192 | 105 | 8320 |
| E. coli ATCC25922 | 316 | 1550 | 3720 |
| L. fermentum ATCC14931 | 2500 | 26300 | 25300 |
| L. plantarum EH22G | 2610 | 27500 | 30700 |
“Theory of weak organic acids” has been proposed to explain the antimicrobial potential of BA and other low-molecular weight organic acids [23]. This theory says that the proportion of non-dissociated BA molecules increases at low pH values of the solution/environment, making it possible for lipophylic BA molecules to penetrate through plasmatic cell membrane. Intracellular pH value is usually close to neutral, thus BA would dissociate in neutral environment with the release of H+ ion which would lead to intracellular acidification and inevitable impairment of cell functions. Similar penetration pattern is applicable to other weak organic acids [17,47]. Table 4 presents data on correlation between Minimal Inhibitory (MIC), Minimal Bactericidal (MBC) and Minimal Fungicidal (MFC) BA concentrations values and environmental pH values.
|
Microorganisms and pH values |
S. aureus NCTC 4163 |
P.aeruginosa NCTC 6749 |
B. subtilis NCTC 10400 |
C. albicans ATCC 10231 |
|||||
| pH=5 | pH=7 | pH=5 | pH=7 | pH=5 | pH=7 | pH=5 | pH=7 | ||
| BA | MIC | 500 | 1000 | 250 | 1000 | 130 | 1000 | 130 | >1000 |
| MBC/MFC | 500 | 1000 | 250 | >1000 | 130 | 1000 | 250 | >1000 | |
| SB | MIC | 390 | 6250 | 1560 | 25000 | 190 | 6250 | 12500 | 25000 |
| MBC/MFC | >50000 | >50000 | 6250 | 25000 | 25000 | 50000 | 25000 | >50000 | |
MBC - Minimal Bactericidal Concentration
MFC - Minimal Fungicidal Concentration
Note: Effect of pH values on antimicrobial potential of BA and sodium benzoate. Lower BA and SB concentrations are required to suppress bacteria in acidic environment, which is consistent with weak organic acids theory [11].
Effects on cell metabolism
In earlier study on murine mitochondrial culture BA at 0.1 µM concentration was shown to reduce membrane potential and calcium content to a significant extent, suppressing mitochondrial respiration (I complex of respiratory chain) and inhibiting oxidation of pyruvate presumably due to pyruvate dehydrogenase blockage. These effects of benzoate, viewed as toxic, were attenuated by menadione and dithiothreitole due to oxidation of thiol groups [38,54]. It was also found that BA and other PCAs inhibit production of Reactive Oxygen Species (ROS) in neutrophils, while ROS are known to impair phagocytic activity [38]. These results are consistent with other published data [55,56].
Sodium benzoate at 0.5-2 µ? was reported to markedly suppress Lipopolysaccharide (LPS) - induced production of some cytokines (TNF-α, IL-1β), NF-κB and iNOS (inducible NO-Synthase) by microglia. Although exposure time to Sodium Benzoate (SB) (i.e., duration of microglia cells incubation with SB) before LPS addition to the culture medium was of critical importance for accomplishment of SB effects [57]. Spanish group also reported the inhibitory effect of other bacterial metabolites 3,4-dihydroxy-phenylpropinic and 3,4-dihydroxy-phenylacetic acids on production of pro-inflammatory cytokines (TNF-a, IL-1b and IL-6) in mononuclear cells [58].
Addition of SB to microglia cell culture was associated with down-regulated expression of superficial ?D-markers and class II Major Histocompatibility Complex (M?? II). Similar phenomena were reported in experiments with human astrocytes [57].
Marked inhibition of fatty acids oxidation was induced in experimental setting by SB added to homogenized murine liver at 0.5-2 µ?, meanwhile parenteral administration of 5-10 mmol/kg (1220-2440 mg/kg) SB to rats resulted in significant reduction of ATP, ?oA and acetyl-?oA and increased levels of ammonium in liver tissue [59].
Membrane transport of low-molecular weight metabolites
Lin J et al., reported glutamate-induced bacterial resistance to acidic environment as more effective mechanism than arginine-dependent resistance used by ?. coli [60]. These mechanisms have not yet been clarified, but experiments with E. coli established enhanced expression of more than 30 proteins in response to BA challenge [61].
Membrane ?+-ATPase is responsible for proton removal from intracellular space in Saccharomyces cerevisiae. This process is instantly intensified after BA addition to culture media [50]. Saccharomyces cerevisiae, in contrast to Zygosaccharomyces bailii, can’t metabolize BA anions, thus they expel BA anions from the cell by transport carriers. Induction of Pdr12p- transporter synthesis is considered to be the principal mechanism of Saccharomyces cerevisiae adaptation to BA, providing benzoate removal by active transport mechanism [51].
Membrane carrier Pdr12p belongs to ABC-transporters super-family (ATP-binding cassette) and, apart from benzoate it also transports other anions of weak organic acids, including p-HPAA ? PAA anions [17]. ???-transporters were found in both prokaryotes and eukaryotes, including humans [62,63]. ABC-transporters play key role in bacterial resistance to antimicrobials in prokaryotic organisms, while in humans - in resistance to anticancer drugs [64].
BA anion transport in mammals and humans is executed by proton-dependent Monocarboxylate Transporters (MCTs), and Sodium-dependent Monocarboxylate Transporters (SMCTs) from MFS super family (Major Facilitator Super Family). MCTs family is represented by at least 14 membrane proteins, responsible for transportation of low-molecular weight monocarbonic acids, thyroid hormones, and such important for basal metabolism monocarboxylates as lactate, pyruvate and acetoacetate. SMCTs, harnessing sodium gradient, transport lactate, pyruvate and ketone bodies from extracellular environment in intestinal and renal epithelium and brain [65-67].
MCT1 is a universal transporter for the majority of tissues and organs, including BBB (blood-brain barrier), while some other MCTs are characterized by organ specificity. MCTs maintain intracellular pH by removal from cytosol of organic acids, produced via glycolysis and other metabolic processes. Muscular cells, erythrocytes and cancer cells are highly MCTs-dependent due to active glycolysis and intensive production of organic acids [68]. Liver and kidneys can utilize lactate for gluconeogenesis, while smooth cardiac and stripped skeleton muscles use lactate for “breathing” [69,70]. Depending on the tissue and it’s functional activity MCTs either remove monocarboxylate organic acids from the cell, or transport them into the cell. In general, specific transport of mono- and ?4-dicarboxylates plays key role in energy metabolism of eukaryotic cell, linking intracellular and systemic metabolic processes in the entire organism [71]. It has been demonstrated by now, that aromatic acids, such as BA and phenylpyruvic, can inhibit MCTs and interfere with cell capacity to maintain optimal intracellular pH value under elevated intracellular concentration of PCAs, thus changing enzymatic pathways inside the cell [68,72-76].
BA metabolism by microorganisms
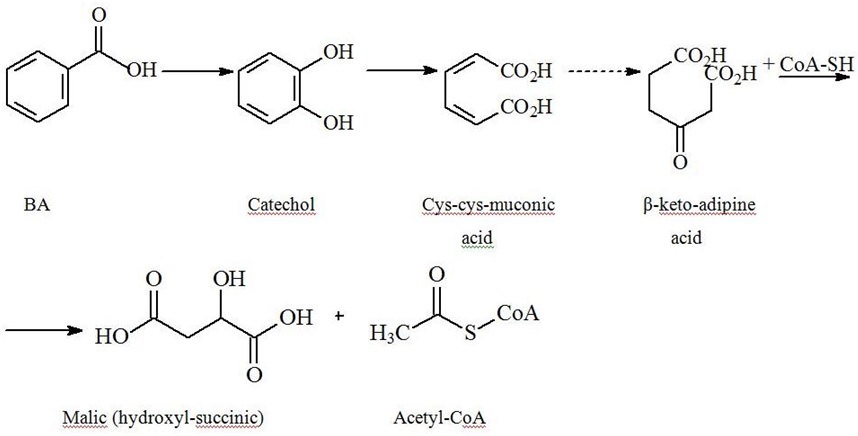
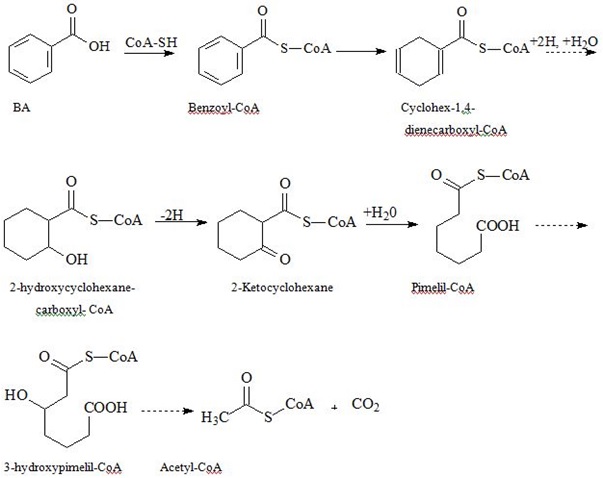
| Pathway/Key Enzyme | Intermediary products | Final products |
| Aerobic | ||
|
β-keto-adipine pathway/dioxygenase intrinsic for both - bacteria and fungi. [6,78-80]. |
Catechol (pyrocatechine)→ ortho-disintegration |
→ pyruvate and acetaldehyde [77]. |
| Monooxygenase |
4-hydroxybenzoic, 3,4- dihydroxybenzoic (protocatechuic), 2,5- dihydroxybenzoic (gentisine) acids [48,77,81]. Benzoyl-???® |
Acetyl-CoA and succinyl-CoA [82]. |
| Anaerobic | ||
| Benzoyl-C?? sequential reduction of double bounds in the ring and its’ ultimate breakdown | Acetyl-CoA and carbon dioxide | |
Pyrocatechin-as the key BA aerobic disintegration intermediate - is similar to endogenous catecholamines with inherent potential to raise blood pressure and dilate airways [83-86].
Fermenting bacteria do not get any energy benefit from aromatic ring breakdown. While nitrate-reducing bacteria and aerobes yet to greater extent utilize Acetyl-CoA in tri-carbonic acids cycle producing considerable amounts of ATP, thus over-compensating energy losses for BA metabolism. Formation of benzoyl-CoA also occurs in anaerobic degradation of PAA, phenol, p-cresol, aniline, BA precursors, and p-hydroxy BA [87-89].
In current study sepsis associated gram +ve and gram -ve bacteria produced a range of PCAs (predominantly PLA and p-HPLA, with the exception of Pseudomonas aeruginosa and Acinetobacter baumanii producing p-HPAA predominantly), although their potential to synthesize PCAs in pure culture is much lower than capacity of obligate anaerobes. As anaerobic bacteria do not get any energy benefits from aromatic ring breakdown, they had to develop some mechanisms of resistance to PCAs (biochemical or symbiotic) in order to tolerate accumulated amounts of PCAs in the environment.
PCAs excretion from human organism
Besides, part of BA (20%) is excreted via conjugation with glucuronic acid [93]. Based on published data from human tissue cultures study average BA of glycine conjugation rate in human liver is equal to 254±90.5 nmol/min per 1 gram liver tissue (range 94.4 - 564), in renal cortex homogenates it is somewhat higher i.e., 321±99.3 nmol/min per 1 gram renal tissue (range 63.3 ? 542) [94]. In humans PAA and p-HPAA are known to form conjugates with glycine and glutamine, PPA with glycine, although international metabolome databases give no references on p-HPAA and PAA.
In a healthy individual 70% of resulting Hippuric Acid (HA) after ingestion of a single BA test dose is excreted with urine within first 6 hours [84]. This is actually a description of the Quick’s (Hippuric Acid) test used for liver failure assessment.
HA concentration will be considerably elevated in individuals with renal failure, moreover, HA is commonly recognized as a uremic toxin [95,96]. Of importance, ?. jejuni from Campylobacter genus was recently identified as a pathogen causing gastroenteritis, and it differs from non-pathogenic E. coli by its’ capacity to degrade HA to BA and glycine [97].
Results from our most recent experimental in-vitro study indicate that Bacteroides spp. are able to consume sepsis-associated p-HPLA and p-HPAA. This finding suggest that Bacteroides spp. the most predominant bacterial species in human gut microbiota may be involved in elimination of alternative tyrosine metabolic pathways products from the human body in parallel with endogenous mechanisms of detoxification [98]. This study was awarded the Sepsis Forum 2014 prize (Institute Pasteur, France).
PCAs dose-dependent effects in humans and animals
Biotransformation rate of BA and of it’s salts in humans varies within 17-29 mg/kg/h and does not seem to be dose-dependent [18]. BA peak plasma concentration is reached within 1-2 hours [18], and metabolic acidosis inevitably occurs after ingestion of BA 1000 mg/kg/day, usually associated with hypokalemia and hypocalcaemia. In a volunteer study incremental increase of BA doses up to 2500 mg/day was associated with nausea, headache, fatigue and heartburn. Manifestations of BA toxicities were documented when serum concentrations exceeded 800 mg/L (6.55 mM) [84].
In our cohort of patients with sepsis the documented average cumulative serum concentration of clinically significant PCAs was equal to 25.7 µ? (with 25% and 75% inter-quartile range 13 µ? and 59.2 µ?, respectively) [10]. Published data indicate that in phenylketonuria cases cumulative levels of PLA can cross the threshold of 50 µ? [100].
Sodium benzoate is administered as therapeutic agent perorally or as i/v infusions at 250-500 mg/kg/day to treat hyperammonemia in individuals with urea cycle disorders [101], and therapy is often associated with nausea [18]. SB brakes bilirubin-albumin bond, releasing bilirubin into circulation and increasing serum concentration of free bilirubin with inevitable manifestation of it’s toxicity [102,103]. Therapy with SB is also associated with elevated levels of blood tryptophan and cerebral serotonin, behaving as hunger suppressants. In lab experiments trypsin benzoate induced modification of three-dimensional protein configuration [104]. Impaired HA synthesis in schizophrenia has been documented by some authors [105]. Impaired mental state and severe acidosis resulting in 2 deaths have been reported in 3 published cases of SB overdose [106].
FDA classifies BA and sodium benzoate as substances that are Generally Recognized as Safe (GRAS-listing). JECFA Committee (The Joint Fao/Who Committee on Food Additives) considers as Acceptable the Daily Intake (ADI) of BA and sodium benzoate equal to 0-5 mg/kg body weight [107].
CONCLUSION
Low-molecular weight aromatic acids such as benzoic and other phenylcarboxylic acids, known as intermediate and final products of bacterial metabolism, demonstrate bioregulatory activity directed not only at micro but also at macro-organism (i.e., human body as a whole).
It has been stated that the severity of disease correlates with cumulative Aromatic Microbial Metabolites (AMM) load i.e., summative AMM concentrations in patient’s blood serum [10,11]. Following the universal theory of weak organic acids, PCAs’ mechanism of action is also universal and uniform: they cause acidification of intracellular medium, inhibit ATP synthesis and/or deplete intracellular ATP deposits, i.e., make certain input into development of cytopatic hypoxia in sepsis [108,109]. Microorganism’s sensitivity to PCAs varies considerably, as it has been shown above, but there’s a general trend to enhancing PCAs’ toxicity with growing acidification of the environment (acidosis).
Summarizing published and own data, we outline the following statements and proposals for future research to better substantiate the hypothesis on two integrated (microbial and host’s) metabolisms, and the role of microbial exometabolites in host’s homeostasis [6]:
1. In periepithelial layers of human natural biocenoses, and at the borders of any infectious tissue lesion (in pericapillary and periendothelial space) PCAs levels reach the values, sufficient for local and/or systemic manifestation of their biological effects, leading to modified composition and biological activity of the microbiota, and modified reactivity of immune-competent cells and tissue-specific functions, etc.
2. In some clinical conditions (such as sepsis, shock, hypoxia, severe renal/hepatic failure, mitochondrial dysfunction and oths.) distribution patterns of PCAs’ precursors, PCAs and their metabolites would considerably differ from the patterns found in a healthy individual. Any deviation from healthy pattern can results in modified biological activity of metabolites, also due to changes in their intracellular concentrations.
3. Accumulation of new data on PCAs would provide a background for future development of novel therapeutic strategies, such as: regulation of the composition and metabolic activity in natural microbiocenoses and pathologic biotopes (foci of infectious lesions) in the human body; stewardship of human metabolism; regulation of immune-cell reactivity via correction of metabolic profile, etc.
We need deeper insight into natural regulation of microbiocenosis, into signal pathways and mechanisms, which provide integration of two metabolisms, i.e., of the host and of it’s microbiota. The main intention of this review is to draw attention of professionals to the development of new therapeutic approaches based on regulation of local and systemic balance of aromatic microbial metabolites in human organism, so that to improve clinical outcomes in most severe conditions, such as sepsis.
ACKNOWLEDGEMENT
This article was supported by Russian Scientific Foundation, Grant No 15-15-00110.
REFERENCES
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580-637.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303-1310.
- Khubutia MS, Shabanov AK, Chernenkaya TV, Godkov MA, Dorfman AG (2011) Infectious pulmonary complications in reanimation and intensive care in patients with multiple trauma. General Reanimatology 7: 24.
- Moroz VV, Lukatch VN, Shifman EM, Dolgikh VT, Yakovleva II (2004) Sepsis: Clinical and patho-physiological aspects of intensive therapy: manual for physicians. IntelTech, Petrozavodsk, Russia.
- Rudnov VA, Belsky DV, Dekhnitch AV (2011) Infections in intensive care units in Russia: results of national multicenter study. Klyn Microbiol Antimicrob Chemother 13: 294-303.
- Beloborodova NV (2012) Integration of human microbiome and metabolism in critical conditions. General Reanimatology 8: 42.
- Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2.
- Shpakov AO (2009) [Bacterial nonpeptide quorum-sensing signal molecules]. Mikrobiologiia 78: 163-175.
- Lyte M, Vulchanova L, Brown DR (2011) Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 343: 23-32.
- Beloborodova NV, Yu OA, Khodakova AS, Chernevskaya EA, Khabib ON (2012) Origin and clinical relevance of low-molecular-weight phenol metabolites in human blood serum. Anesthesiology and Reanimatology 5: 65-72.
- Beloborodova NV, Yu VA, Osipov AA (2012) Laboratory diagnostics of bacterial intoxication by blood gas-chromatography. Clinical Laboratory Diagnostics 9: 79.
- Beloborodova NV, Arkhipova AS, Beloborodov DM, Boyko NB, Melko AI, et al. (2006) Chromato-mass-spectrometry detection of low-molecular-weight aromatic compounds of microbial origin in blood serum of patients with sepsis. Clinical Laboratory Diagnostics 2: 3-6.
- Khodakova A, Beloborodova NV (2007) Microbial metabolites in the blood of patients with sepsis. Critical Care 11: 5.
- Beloborodova NV, Khodakova AS, Bairamov IT, Olenin AY (2009) Microbial origin of phenylcarboxylic acids in the human body. Biochemistry (Mosc) 74: 1350-1355.
- Davidson PM, Sofos JN, Branen AL (2005) Antimicrobials in food. (3rdedn), Taylor & Francis Group, UK.
- Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, et al. (2000) Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl Environ Microbiol 66: 4084-4090.
- Hazelwood LA, Tai SL, Boer VM, de Winde JH, Pronk JT, et al. (2006) A new physiological role for Pdr12p in Saccharomyces cerevisiae: export of aromatic and branched-chain organic acids produced in amino acid catabolism. FEMS Yeast Res 6: 937-945.
- World Health Organization (2000) Concise International Chemical Assessment Document 26. Benzoic acid and sodium benzoate. World Health Organization, Geneva, Switzerland.
- Sieber R, Biitikofer U, Bosse JO (1995) Benzoic acid as a natural compound in cultured dairy products and cheese. Int Dairy J 5: 227-246.
- Kaškoniene V, Maruška A, Kornyšova O, Charczun N, Ligor M, et al. (2009) Quantitative and qualitative determination of phenolic compounds in honey. Chemine Technologija 3: 74-80.
- Stijve T, Amazonas MAL, Giller V (2004) Characterisation of flavour and taste compounds in Agaricus blazei Murrill sensu Heinem, the cultivated almond mushroom. Australasian Mycologist 22: 116-122.
- Pappas E, Schaich KM (2009) Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr 49: 741-781.
- Russel NJ, Could GW (2003) Food preservatives. (2ndedn), Springer, Newyork, USA.
- Belozerova NS Impact of cytokines and salicylic acid on expression of genes encoding mitochondrial proteins Moscow: SFBI «IFR»RAS; 1-150.
- Moore K, Rao PV, Towers GH (1968) Degradation of phenylalanine and tyrosine by Sporobolomyces roseus. Biochem J 106: 507-514.
- Andersen A (2006) Final report on the safety assessment of benzaldehyde. Int J Toxicol 25: 11-27.
- Health & Consumer Protection Directorate-General (2002) Opinion of the Scientific Committee on Food on Benzyl alcohol. European Commission Health & Consumer Protection Directorate-General, Brussels, Belgium.
- Lord RS, Bralley JA (2008) Clinical applications of urinary organic acids. Part 2. Dysbiosis markers. Altern Med Rev 13: 292-306.
- Grün CH, van Dorsten FA, Jacobs DM, Le Belleguic M, van Velzen EJ, et al. (2008) GC-MS methods for metabolic profiling of microbial fermentation products of dietary polyphenols in human and in vitro intervention studies. J Chromatogr B Analyt Technol Biomed Life Sci 871: 212-219
- Knoop F (1904) Der Abbau aromatischer Fettsäuren im Tierkörper. Beitr Chem Physiol Pathol, Kuttruff, Breisgau, Germany.
- Jenner AM, Rafter J, Halliwell B (2005) Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med 38: 763-772.
- Khan RI, Onodera R, Amin MR, Mohammed N (2002) Aromatic amino acid biosynthesis and production of related compounds from p-hydroxyphenylpyruvic acid by rumen bacteria, protozoa and their mixture. Amino Acids 22: 167-177.
- Russell AD (1991) Mechanisms of bacterial resistance to non-antibiotics: food additives and food and pharmaceutical preservatives. J Appl Bacteriol 71: 191-201.
- Borawska MH, Czechowska SK, Markiewicz R, Palka J, Swislocka R et.al. (2008) Antimicrobial activity and cytotoxicity of picolinic acid and selected picolinates as new potential food preservatives. Polish Journal of Food and Nutrition Sciences 58: 415-418.
- Knarreborg A, Miquel N, Granli T, Jensen BB (2002) Establishment and application of an in vitro methodology to study the effects of organic acids on coliform and lactic acid bacteria in the proximal part of the gastrointestinal tract of piglets. Animal Feed Science and Technology 99: 131-140.
- Cueva C, Moreno-Arribas MV, Martín-Alvarez PJ, Bills G, Vicente MF, et al. (2010) Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res Microbiol 161: 372-382.
- Glass AD (1974) Influence of Phenolic Acids on Ion Uptake: IV. Depolarization of Membrane Potentials. Plant Physiol 54: 855-858.
- Beloborodova N, Bairamov I, Olenin A, Shubina V, Teplova V, et al. (2012) Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J Biomed Sci 19: 89.
- Gerez CL, Torres MJ, Font de Valdez G, Rollán G (2012) Control of spoilage fungi by lactic acid bacteria. Biological Control 64: 231-237.
- Valerio F, Lavermicocca P, Pascale M, Visconti A (2004) Production of phenyllactic acid by lactic acid bacteria: an approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol Lett 233: 289-295.
- Suskovic J, Kos B, Beganovic J, Pavunc AL, Habjanic K, et al. (2010) Antimicrobial Activity of Lactic Acid Bacteria. Food Technol Biotechnol 48: 296-307.
- Lavermicocca P, Valerio F, Visconti A (2003) Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl Environ Microbiol 69: 634-640.
- Dieuleveux V, Lemarinier S, Guéguen M (1998) Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int J Food Microbiol 40: 177-183.
- Gabel LF (1921) The relative action of preservatives in pharmaceutical preparations. Journal of the American Pharmaceutical Association 10: 767-768.
- Cruess WV, Richert PH (1929) Effect of Hydrogen ion concentration on The toxicity of sodium benzoate to microorganisms. J Bacteriol 17: 363-371.
- Hsiao CP, Siebert KJ (1999) Modeling the inhibitory effects of organic acids on bacteria. Int J Food Microbiol 47: 189-201.
- Warth AD (1989) Transport of Benzoic and Propanoic Acids by Zygosaccharomyces bailii. Journal of General Microbiology 135: 1383-1390
- Mollapour M, Piper PW (2001) The ZbYME2 gene from the food spoilage yeast Zygosaccharomyces bailii confers not only YME2 functions in Saccharomyces cerevisiae, but also the capacity for catabolism of sorbate and benzoate, two major weak organic acid preservatives. Mol Microbiol 42: 919-930.
- Verduyn C, Postma E, Scheffers WA, van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8: 501-517.
- Holyoak CD, Stratford M, McMullin Z, Cole MB, Crimmins K, et al. (1996) Activity of the plasma membrane H(+)-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl Environ Microbiol 62: 3158-3164
- Holyoak CD, Bracey D, Piper PW, Kuchler K, Coote PJ (1999) The Saccharomyces cerevisiae weak-acid-inducible ABC transporter Pdr12 transports fluorescein and preservative anions from the cytosol by an energy-dependent mechanism. J Bacteriol 181: 4644-4652.
- Krebs HA, Wiggins D, Stubbs M, Sols A, Bedoya F (1983) Studies on the mechanism of the antifungal action of benzoate. Biochem J 214: 657-663.
- Pearce AK, Booth IR, Brown AJ (2001) Genetic manipulation of 6-phosphofructo-1-kinase and fructose 2,6-bisphosphate levels affects the extent to which benzoic acid inhibits the growth of Saccharomyces cerevisiae. Microbiology 147: 403-410.
- Fedotcheva NI, Teplova VV, Beloborodova NV (2010) Participation of phenolic acids of microbial origin in the dysfunction of mitochondria in sepsis. Biological Membranes 27: 60-66.
- Merfort I, Heilmann J, Weiss M, Pietta P, Gardana C (1996) Radical scavenger activity of three flavonoid metabolites studied by inhibition of chemiluminescence in human PMNs. Planta Med 62: 289-292.
- Limasset B, Ojasoo T, le Doucen C, Doré JC (1999) Inhibition of chemiluminescence in human PMNs by monocyclic phenolic acids and flavonoids. Planta Med 65: 23-29.
- Brahmachari S, Jana A, Pahan K (2009) Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol 183: 5917-5927.
- Monagas M, Khan N, Andrés-Lacueva C, Urpí-Sardá M, Vázquez-Agell M, et al (2009) Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br J Nutr 102: 201-206.
- Kalbag SS, Palekar AG (1988) Sodium benzoate inhibits fatty acid oxidation in rat liver: effect on ammonia levels. Biochem Med Metab Biol 40: 133-142.
- Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, et al. (1996) Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol 62: 3094-3100.
- Lambert LA, Abshire K, Blankenhorn D, Slonczewski JL (1997) Proteins induced in Escherichia coli by benzoic acid. J Bacteriol 179: 7595-7599.
- Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8: 67-113.
- Stefková J, Poledne R, Hubácek JA (2004) ATP-Binding Cassette (ABC) transporters in human metabolism and diseases. Physiol Res 53: 235-243.
- Higgins CF (2001) ABC transporters: physiology, structure and mechanism--an overview. Res Microbiol 152: 205-210.
- Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, et al. (2008) Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10: 193-199.
- Meredith D, Christian HC (2008) The SLC16 monocaboxylate transporter family. Xenobiotica 38: 1072-1106.
- Poole RC, Halestrap AP (1993) Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol 264: 761-782.
- Kang KW, Jin MJ, Han HK (2006) IGF-I receptor gene activation enhanced the expression of monocarboxylic acid transporter 1 in hepatocarcinoma cells. Biochem Biophys Res Commun 342: 1352-1355.
- Juel C, Halestrap AP (1999) Lactate transport in skeletal muscle - role and regulation of the monocarboxylate transporter. J Physiol 517: 633-642.
- Halestrap AP, Meredith D (2004) The SLC16 gene family-from Monocarboxylate Transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447: 619-628.
- Aliverdieva DA (2008) Dicarboxylate yeast transporters: some structural features and substrate specificity. Vestnik of Dagestan Scientific Center 32: 21-28.
- Vellonen KS, Häkli M, Merezhinskaya N, Tervo T, Honkakoski P, et al. (2010) Monocarboxylate transport in human corneal epithelium and cell lines. Eur J Pharm Sci 39: 241-247.
- Morris ME, Felmlee MA (2008) Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse γ-hydroxybutyric acid. AAPS J 10: 311-321
- Majumdar S, Gunda S, Pal D, Mitra AK (2005) Functional activity of a monocarboxylate transporter, MCT1, in the human retinal pigmented epithelium cell line, ARPE-19. Mol Pharm 2: 109-117.
- Kimura O, Tsukagoshi K, Endo T (2009) Uptake of phenoxyacetic acid derivatives into Caco-2 cells by the monocarboxylic acid transporters. Toxicol Lett 189: 102-109.
- Vaidyanathan JB, Walle T (2003) Cellular uptake and efflux of the tea flavonoid (-) epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther 307: 745-752.
- Lengelera Y, Drevsa G, Shlegelya G (2009) Modern Micriobiology. Prokaryotes.
- Johnson BF, Stanier RY (1971) Regulation of the -ketoadipate pathway in Alcaligenes eutrophus. J Bacteriol 107: 476-485.
- Collier LS, Gaines GL 3rd, Neidle EL (1998) Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcriptional activator. J Bacteriol 180: 2493-2501.
- Harayama S, Rekik M, Bairoch A, Neidle EL, Ornston LN (1991) Potential DNA slippage structures acquired during evolutionary divergence of Acinetobacter calcoaceticus chromosomal benABC and Pseudomonas putida TOL pWW0 plasmid xylXYZ, genes encoding benzoate dioxygenases. J Bacteriol 173: 7540-7548
- Grund E, Knorr C, Eichenlaub R (1990) Catabolism of benzoate and monohydroxylated benzoates by Amycolatopsis and Streptomyces spp. Appl Environ Microbiol 56: 1459-1464.
- Rather LJ, Knapp B, Haehnel W, Fuchs G (2010) Coenzyme A-dependent aerobic metabolism of benzoate via epoxide formation. J Biol Chem 285: 20615-20624.
- Anderson AJ, Harvey AL (1988) Effects of the facilitatory compounds catechol, guanidine, noradrenaline and phencyclidine on presynaptic currents of mouse motor nerve terminals. Naunyn Schmiedebergs Arch Pharmacol 338: 133-137.
- U.S. Department of Health and Human Services (1993) Hazardous Substances Data Bank (HSDB) National Toxicology Information Program, National Library of Medicine, Bethesda, USA
- Catechol (ICSC: 0411) (2005) Prepared in the context of cooperation between the International Programme on Chemical Safety and the Commission of the European Communities © IPCS. CEC.
- Andersen FA (1997) Amended Final Report on the Safety Assessment of Pyrocatechol. International Journal of Toxicology 16: 11-58.
- Harwood CS, Gibson J (1997) Shedding light on anaerobic benzene ring degradation: a process unique to prokaryotes? J Bacteriol 179: 301-309.
- Gibson J, S Harwood C (2002) Metabolic diversity in aromatic compound utilization by anaerobic microbes. Annu Rev Microbiol 56: 345-369.
- Mohapatra PK (2006) Textbook of Environmental Biotechnology. IK International Publishing House Pvt Ltd, New Delhi, India.
- Dakin JD (1910) The fate of sodium benzoate in the human organism. J Biol Chem 7: 103-108
- Granik VG (2006) Metabolism of endogenous compounds. Vuzovskaya kniga, Moscow, Russia.
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, et al. (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106: 3698-3703.
- SIAM (2001) Benzoates: Benzoic acid, Sodium benzoate, Potassium benzoate, Benzyl alcohol. SIDS Initial Assessment Report for 13th SIAM, Bern, Switzerland.
- Temellini A, Mogavero S, Giulianotti PC, Pietrabissa A, Mosca F, et al. (1993) Conjugation of benzoic acid with glycine in human liver and kidney: a study on the interindividual variability. Xenobiotica 23: 1427-1433.
- Deguchi T, Takemoto M, Uehara N, Lindup WE, Suenaga A, et al. (2005) Renal clearance of endogenous hippurate correlates with expression levels of renal organic anion transporters in uremic rats. J Pharmacol Exp Ther 314: 932-938.
- Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, et al. (2011) Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 6: 18438.
- Hani EK, Chan VL (1995) Expression and characterization of Campylobacter jejuni benzoylglycine amidohydrolase (Hippuricase) gene in Escherichia coli. J Bacteriol 177: 2396-2402.
- Beloborodova N, Moroz V, Osipov A, Bedova A, Sarshor Y, et al. (2014) Tyrosine metabolism disorder and the potential capability of anaerobic microbiota to decrease the value of aromatic metabolites in critically ill patients. Critical Care 18: 60.
- World Health Organization (1996) Toxicological evaluation of ceratin food additives - Benzyl acetate, Benzyl alcohol, Benzaldehyde, and Benzoic acid and its Salts. Food Additive Series 37, Geneva, Switzerland.
- Clemens PC, Schünemann MH, Hoffmann GF, Kohlschütter A (1990) Plasma concentrations of phenyllactic acid in phenylketonuria. J Inherit Metab Dis 13: 227-228.
- Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, et al. (2007) Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med 356: 2282-2292.
- Green TP, Mirkin BL (1981) Sodium benzoate in the treatment of hyperammonemia in newborns. Pediatric Research 15: 630.
- Schiff D, Chan G, Stern L (1971) Fixed drug combinations and the displacement of bilirubin from albumin. Pediatrics 48: 139-141.
- Mu Y, Lin J, Liu R (2011) Interaction of sodium benzoate with trypsin by spectroscopic techniques. Spectrochim Acta A Mol Biomol Spectrosc 83: 130-135.
- Tremblay GC, Qureshi IA (1993) The biochemistry and toxicology of benzoic acid metabolism and its relationship to the elimination of waste nitrogen. Pharmacol Ther 60: 63-90.
- Praphanphoj V, Boyadjiev SA, Waber LJ, Brusilow SW, Geraghty MT (2000) Three cases of intravenous sodium benzoate and sodium phenylacetate toxicity occurring in the treatment of acute hyperammonaemia. J Inherit Metab Dis 23: 129-136.
- Health & Consumer Protection Directorate-General (2002) Scientific Committee on Consumer Products - Opinion on Benzoic Acid and Sodium Benzoate. European Commission Health & Consumer Protection Directorate-General, Brussels, Belgium.
- Garrabou G, Morén C, López S, Tobías E, Cardellach F, et al. (2012) The effects of sepsis on mitochondria. J Infect Dis 205: 392-400.
- Protti A, Singer M (2006) Bench-to-bedside review: potential strategies to protect or reverse mitochondrial dysfunction in sepsis-induced organ failure. Crit Care 10: 228.
Citation: Beloborodova NV, Osipov AA, Bedova A Yu, Khabib ON (2016) Low-Molecular Weight Bacterial Metabolites in Host-Microbial Interaction. J Infect Non Infect Dis 2: 011.
Copyright: © 2016 Beloborodova NV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

