
Journal of Nuclear Medicine Radiology & Radiation Therapy Category: Medical
Type: Review Article
The Varied Clinical Presentations, Prognostic Factors and Management of Spontaneous Spinal Epidural Hematoma: A Review of Literature
*Corresponding Author(s):
Rajagopal NDepartment Of Neurosurgery, Sri Sathya Sai Institute Of Higher Medical Sciences, Bangalore, India
Tel:080-28411500,
Email:niranjana.rajagopal@gmail.com
Received Date: Jul 10, 2019
Accepted Date: Aug 01, 2019
Published Date: Aug 07, 2019
Abstract
Introduction
Spontaneous spinal epidural hematoma [SSEH] is an acute neurological condition that manifests from an accumulation of blood in the epidural space, causing cord compression, which when left untreated can result in permanent neurological damage. Complete Brown-Sequard syndrome with complete hemisection of the spinal cord is a very rare presentation in SSEH. We present one such a case with relevant review of literature.
Materials and Methods
A 65 years old lady, a known Hypertensive on medications, presented with a right hemiparesis for 4 hours. On evaluation, she was found to have a complete Brown-Sequard syndrome. MRI spine showed a C3 to C6 epidural hematoma. She underwent emergent decompressive surgery.
A literature search was performed using PubMed to identify articles on SSEH. A thorough review of literature was done to determine the varied clinical presentation, risk factors, evaluation, management options and prognosis.
Results
The patient recovered completely at the end of 2 weeks and the postoperative MRI showed complete resolution of the hematoma.
Conclusions
SSEH has a wide spectrum of clinical manifestations that can mimic various other pathologies. Thus, knowledge about the disease entity along with its differentials and diagnostic pitfalls with a thorough clinical evaluation and imaging is essential for a correct diagnosis. Early surgical intervention and hematoma evacuation is the treatment of choice in patients with progressive neurological deficits. The duration and severity of the pre-operative deficit are found to be independent predictors of poor outcome. Though there is no consensus about the accepted time frame for intervention, decompression within 12 hours and maximally up to 36 hours of symptom onset is essential to achieve a favourable outcome. The progressive interval, hematoma size, segmental distribution and rehabilitation are the other factors affecting clinical outcome.
Spontaneous spinal epidural hematoma [SSEH] is an acute neurological condition that manifests from an accumulation of blood in the epidural space, causing cord compression, which when left untreated can result in permanent neurological damage. Complete Brown-Sequard syndrome with complete hemisection of the spinal cord is a very rare presentation in SSEH. We present one such a case with relevant review of literature.
Materials and Methods
A 65 years old lady, a known Hypertensive on medications, presented with a right hemiparesis for 4 hours. On evaluation, she was found to have a complete Brown-Sequard syndrome. MRI spine showed a C3 to C6 epidural hematoma. She underwent emergent decompressive surgery.
A literature search was performed using PubMed to identify articles on SSEH. A thorough review of literature was done to determine the varied clinical presentation, risk factors, evaluation, management options and prognosis.
Results
The patient recovered completely at the end of 2 weeks and the postoperative MRI showed complete resolution of the hematoma.
Conclusions
SSEH has a wide spectrum of clinical manifestations that can mimic various other pathologies. Thus, knowledge about the disease entity along with its differentials and diagnostic pitfalls with a thorough clinical evaluation and imaging is essential for a correct diagnosis. Early surgical intervention and hematoma evacuation is the treatment of choice in patients with progressive neurological deficits. The duration and severity of the pre-operative deficit are found to be independent predictors of poor outcome. Though there is no consensus about the accepted time frame for intervention, decompression within 12 hours and maximally up to 36 hours of symptom onset is essential to achieve a favourable outcome. The progressive interval, hematoma size, segmental distribution and rehabilitation are the other factors affecting clinical outcome.
Keywords
Spontaneous spinal epidural hematoma; Differential diagnosis; Prognostic factors;Decompressive laminectomy;Conservative management
INTRODUCTION
Spontaneous spinal epidural hematomas [SSEH] is a potentially disabling disease accounting for less than 1% of spinal epidural space occupying lesions [1-4]. It is defined as an acute myelopathy leading to severe neurological deficits caused by the accumulation of blood in the vertebral epidural space in the absence of an underlying haematologic or haemostatic disorder, vascular malformation, trauma, tumour, systemic or local disease, drug use or any history of medications that interfere with the coagulation cascade [1,3,5,6]. It is a rare condition with an incidence of 0.1/ 100,000 people/year [1,5,7,8]. SSEH can produce a wide variety of clinical manifestations, which when not managed promptly, may result in devastating morbidity. Relevant imaging and timely intervention are required to prevent permanent neurological deficits in patients with this pathology. Owing to its emergent nature, no prospective trials have been possible, thus the persisting controversy about the etiopathogenesis, diagnosis, and timing of treatment regarding this condition.
PATIENTS AND METHODS
A literature search was performed using PubMed to identify articles pertaining to SSEH. A thorough review of literature was done to determine the varied clinical presentation, risk factors, evaluation, management options and prognosis along with an illustrative case report with a Brown-Sequard syndrome due to SSEH which is a rare initial presentation of the pathology.
Relevant articles with regard to clinical presentation, etiopathogenesis, management and prognostic factors were selected and reviewed. There are various hypotheses regarding the etiopathogenesis but there are no proven results till date thus, finding preventive measures for this pathological entity is an insurmountable task. A case example highlights the rarity of the event and potential pitfalls in diagnosis.
Relevant articles with regard to clinical presentation, etiopathogenesis, management and prognostic factors were selected and reviewed. There are various hypotheses regarding the etiopathogenesis but there are no proven results till date thus, finding preventive measures for this pathological entity is an insurmountable task. A case example highlights the rarity of the event and potential pitfalls in diagnosis.
ILLUSTRATIVE CASE
A 65 years old lady, who was a known case of Hypertension, on medications, presented to the emergency department with complaints of right hemiparesis for 4 hours. There was no history of trauma, smoking or drug use. The patient did not use anticoagulants or antiplatelets before admission. She had no neck pain or bladder dysfunction. The initial laboratory tests of blood, such as blood routine test, liver function test, blood creatinine, electrolytes, activated partial thromboplastin time, and international normalized ratio, were within normal limits. The blood pressure was normal. On examination, GCS was 15, with left hemiparesis of 3/5 MRC in the upper limb and 2/5 MRC in the lower limb, with ipsilateral loss of vibration sense, fine touch, proprioception, from C3 below and contralateral loss of pain, temperature and crude touch from C6 below. She was evaluated immediately with an MRI and MRA which showed an epidural hematoma, with a larger left paracentral component, from C3 to C6 with maximum cord compression and oedema at C4 level. The hematoma was isointense to the spinal cord on T1-weighted images and hyperintense on T2-weighted images. MRA did not reveal any abnormal vessels within the hematoma or elsewhere (Figure 1). DSA showed no evidence of any vascular malformations of the spine and brain. MRI brain was normal.
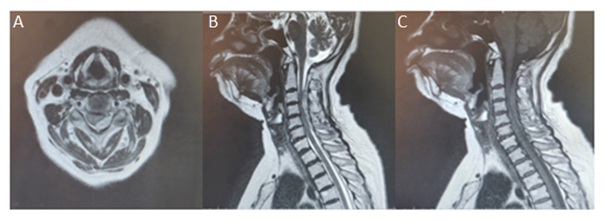
Figure 1: A) MRI - T2 axial image; B) T2 sagittal image; C) T1 sagittal image-shows C3 to C6 extradural spinal hematoma with a left paracentral component causing cord compression with edema. The hematoma is isointense on T1 and Hyperintense on T2 images.
She then underwent a C3 to C5 laminectomy and hematoma evacuation soon after the imaging. Thick, hematoma causing cord compression was noted in the epidural space, it was evacuated and cord was decompressed (Figure 2). There was no tumour/ vascular malformation found intraoperatively. The cord was lax at the end of surgery. The histopathological report showed organized clot with no evidence of vascular malformation or neoplasm.
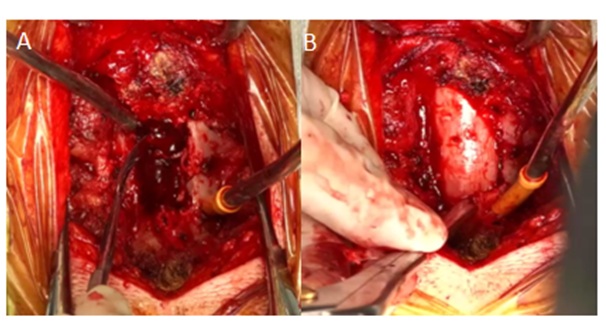
Figure 2: A) intraoperative view of the epidural hematoma. B) Shows complete evacuation of hematoma.
Postoperatively she gradually improved. A detailed rehabilitation program was initiated postoperatively. At discharge after 2 weeks, she was asymptomatic with complete recovery of her sensory and motor deficits. Postoperative MRI showed complete resolution of the hematoma (Figure 3).
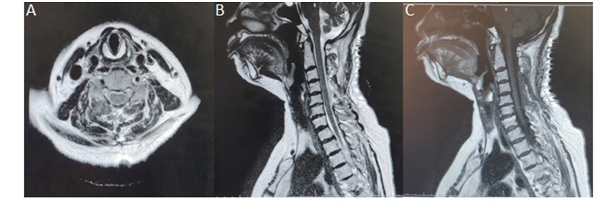 Figure 3: A) POST OP MRI - T2 axial image; B) T2 sagittal image; C) T1 sagittal image -shows complete evacuation of the hematoma with reduction in edema.
Figure 3: A) POST OP MRI - T2 axial image; B) T2 sagittal image; C) T1 sagittal image -shows complete evacuation of the hematoma with reduction in edema.DISCUSSION
Epidemiology and clinical presentation
SSEH is an acute condition with no clear aetiology, which when left untreated can have permanent neurological damage. It can occur at any age though it is more frequent between the fourth to sixth decades with men slightly more affected than women[5,7,9-11]. It is very rare in children and about 30 cases have been reported so far in literature[12]. It is the commonest cause of vascular non-traumatic spinal cord injury[3]. There is no significant correlation with race or gender [13]. The wide variation of clinical presentation makes it challenging to diagnose. It was first described by Blauby in 1808[3] followed by Jackson in 1869[14]. The first surgically-treated case was reported by Bain in 1897 [15]. Patients with SSEH may present with manifestations ranging from a simple neck or back pain with/without radiculopathy, paraparesis, and tetraparesis to hemiparesis depending on the site and severity of the compression. The time until the classical symptoms appear can vary from a few minutes to several days [6,16-19]. The absence or the atypical symptomatology is what causes the delay in diagnosis and intervention.
Due to its wide array of presentation, from mimicking a disc prolapse to that of a transient ischemic attack or a hemiparesis, it is impossible to consider SSEH as an initial diagnosis [5,13,17,18,20]. Thus, the differential diagnosis should include transverse myelitis, Guillain-Barré syndrome (GBS), epidural subarachnoid bleeding, spinal tumours, vertebral collapse, and acute spinal cord ischemia in the presence of a neurological deficit. Pulmonary emboli, spontaneous pneumothorax, and acute myocardial infarction should be the differentials considered in the absence of any deficit [21,22]. Infectious spinal diseases such as osteomyelitis, acute epiduritis, or epidural abscess, closely resemble the classical presentation of SSEH because of the pain lasting hours to days and the following flaccid paralysis. Infections will present with fever and have leukocytosis with elevated C-reactive proteins which will help in the differentiation. Painless sensorimotor paralysis is the main diagnostic point to differentiate SSEH from myelomalacia, hematomyelia, transverse myelitis and anterior spinal artery syndrome. A dissecting aneurysm of the aorta may closely mimic the clinical presentation of SSEH especially when it ruptures into the vertebral canal or the false lumen, occludes the Adamkiewicz artery, resulting in infarction of the thoracolumbar spinal cord. However, cardiovascular symptoms and signs can easily differentiate it from SSEH. MRI with gadolinium administration can differentiate all these lesions from a SSEH and help finalize the diagnosis [23].
SSEH is one of the differential diagnoses of ischemic stroke in patients without supraspinal signs [8,16]. There are reports of patients presenting with hemiplegia, being misdiagnosed as cerebral infarction and treated with anticoagulation/ anti-platelet therapy[24]and cases where the presentation was chest pain that was misdiagnosed as acute coronary syndromes and treated with dual antiplatelet therapy[25,26]. Thus, the clinical symptoms worsened due to increased bleeding. There is a case report by Aristedis et al. [27]in which SSEH symptoms mimicked that of a subarachnoid haemorrhage. There was a diagnostic delay and hence poor outcome owing to the delayed surgical intervention. Brown–Séquard syndrome and TIA are rare as clinical presentation of SSEH but have a favourable prognosis [4,7,28,29]. The relevance of these reports is for clinicians to understand the varied presentation of the pathology and confirm the presence of neck/ back pain or radiculopathy because it has clinical significance in the differential diagnosis of SSEH. A high index of suspicion and diagnostic imaging are critical as SSEHs can produce lasting neurologic deficits ranging from persistent paresis to even death.
Our case had an unusual presentation of spontaneous cervical epidural hematoma with Brown-Sequard syndrome findings of significant motor and sensory involvement of only one half of the body. Complete Brown-Sequard syndrome with complete hemisection of the spinal cord is very rare in SSEH. It is usually seen in extra medullary tumours or following trauma. The neurologic interruption is caused mainly by unilateral involvement of lateral corticospinal tract, dorsal column and ventral spinothalamic tract of the spinal cord. Kreppel(30) reviewed 613 cases from 1826 to 1996 and found only 17 patients with Brown-Sequard syndrome and Bakker [7] in his meta-analysis with 617 cases found only 46 of these cases. This presentation is another stroke mimic which can delay diagnosis and thus ending up with a morbid outcome. In our case, there was no delay in diagnosis as the spine imaging was done immediately as the CT brain was normal and clinically there was a definite sensory loss as exhibited with the absence of supraspinal signs.
Due to its wide array of presentation, from mimicking a disc prolapse to that of a transient ischemic attack or a hemiparesis, it is impossible to consider SSEH as an initial diagnosis [5,13,17,18,20]. Thus, the differential diagnosis should include transverse myelitis, Guillain-Barré syndrome (GBS), epidural subarachnoid bleeding, spinal tumours, vertebral collapse, and acute spinal cord ischemia in the presence of a neurological deficit. Pulmonary emboli, spontaneous pneumothorax, and acute myocardial infarction should be the differentials considered in the absence of any deficit [21,22]. Infectious spinal diseases such as osteomyelitis, acute epiduritis, or epidural abscess, closely resemble the classical presentation of SSEH because of the pain lasting hours to days and the following flaccid paralysis. Infections will present with fever and have leukocytosis with elevated C-reactive proteins which will help in the differentiation. Painless sensorimotor paralysis is the main diagnostic point to differentiate SSEH from myelomalacia, hematomyelia, transverse myelitis and anterior spinal artery syndrome. A dissecting aneurysm of the aorta may closely mimic the clinical presentation of SSEH especially when it ruptures into the vertebral canal or the false lumen, occludes the Adamkiewicz artery, resulting in infarction of the thoracolumbar spinal cord. However, cardiovascular symptoms and signs can easily differentiate it from SSEH. MRI with gadolinium administration can differentiate all these lesions from a SSEH and help finalize the diagnosis [23].
SSEH is one of the differential diagnoses of ischemic stroke in patients without supraspinal signs [8,16]. There are reports of patients presenting with hemiplegia, being misdiagnosed as cerebral infarction and treated with anticoagulation/ anti-platelet therapy[24]and cases where the presentation was chest pain that was misdiagnosed as acute coronary syndromes and treated with dual antiplatelet therapy[25,26]. Thus, the clinical symptoms worsened due to increased bleeding. There is a case report by Aristedis et al. [27]in which SSEH symptoms mimicked that of a subarachnoid haemorrhage. There was a diagnostic delay and hence poor outcome owing to the delayed surgical intervention. Brown–Séquard syndrome and TIA are rare as clinical presentation of SSEH but have a favourable prognosis [4,7,28,29]. The relevance of these reports is for clinicians to understand the varied presentation of the pathology and confirm the presence of neck/ back pain or radiculopathy because it has clinical significance in the differential diagnosis of SSEH. A high index of suspicion and diagnostic imaging are critical as SSEHs can produce lasting neurologic deficits ranging from persistent paresis to even death.
Our case had an unusual presentation of spontaneous cervical epidural hematoma with Brown-Sequard syndrome findings of significant motor and sensory involvement of only one half of the body. Complete Brown-Sequard syndrome with complete hemisection of the spinal cord is very rare in SSEH. It is usually seen in extra medullary tumours or following trauma. The neurologic interruption is caused mainly by unilateral involvement of lateral corticospinal tract, dorsal column and ventral spinothalamic tract of the spinal cord. Kreppel(30) reviewed 613 cases from 1826 to 1996 and found only 17 patients with Brown-Sequard syndrome and Bakker [7] in his meta-analysis with 617 cases found only 46 of these cases. This presentation is another stroke mimic which can delay diagnosis and thus ending up with a morbid outcome. In our case, there was no delay in diagnosis as the spine imaging was done immediately as the CT brain was normal and clinically there was a definite sensory loss as exhibited with the absence of supraspinal signs.
Etiopathogenesis
The exact aetiology of SSEH remains unclear in approximately 40 to 50% of the patients. Increasing age, vascular malformations, coagulopathy, epidural catheter, therapeutic thrombolysis, surgical trauma, cocaine abuse, factor XI deficiency, anticoagulant therapy, pregnancy, conditions causing increased intra-thoracic or intra-abdominal pressure such as cough and Valsalvamanoeuvre have been reported to cause SSEH [1,4,5,11,21,31]. Hypertension has been observed from 3% to 21.4% of patients with SSEH, but whether it is a risk factor of SSEH or not, is still an ongoing debate [7,32,33]. A meta-analysis by Bakker et al. [7]could not suggest the relationship between hypertension and the development of SSEH. According to Bakker [7], 22.8% of patients with SSEH were using oral anticoagulants which is higher than the general population, whereas, the use of platelet inhibitors was only 10.1% of all patients which was comparable with the general population. He thus concluded that the use of anti-coagulants seemed to be a risk factor for developing SSEH, while the use of platelet inhibitors was not.
SSEH can occur anywhere along the length of the cord but is commonly found at the posterior cervicothoracic (C5–T2) and thoracolumbar (T10–L2) segments with the peaks at C6 and T12 levels. The location also seems to be a prognostic factor for the clinical outcome because patients with a thoracic hematoma are more susceptible to compression-induced ischemia due to a narrower spinal canal compared to the cervical and lumbar canals [3,4,34,35]. Dorsal hematomas are much more common than the ventral ones because the ventral dura is firmly attached by Hoffmann ligaments to the posterior longitudinal ligament as well as the dorsal epidural venous plexus being larger than the ventral ones. It has also been hypothesized that the dorsal plexus may have areas called “locus minoris resistentiae” that are highly susceptible to rupture with minor changes in the intravenous pressure [11,13,30].
Both the venous and arterial theories are a probable hypothesis for SSEH, with most cases probably resulting from a venous bleed. The posterior epidural venous network is the most likely source of bleeding [6,7,16,17,30,36,37]. The vertebral venous plexus is a low-pressure valveless system, which is in continuity with the abdominal and thoracic venous system. Routine activities such as voiding, sneezing, bending, coughing or coitus causes fluctuations in the intrathoracic and abdominal pressures. These pressure variations lead to blood flow reversal in the valve-less epidural plexus resulting in their rupture as they are highly vulnerable to any intrinsic pressure changes. A few authors also believe that hematomas occur at segments with more mobility like the cervicothoracic or thoracolumbar areas, as these areas are suspected to produce more tension on the epidural veins [6,32,36,38]. Though the arterial source for a SSEH is infrequent, it has definitely been hypothesized by many as a source of bleeding given the rapidity of the formation of the hematoma as the pressure of the epidural venous plexus is lower than the intrathecal pressure. So venous bleeding should not be able to compress the dural sac. Therefore, it seems logical that epidural arteries could be the cause due to the compression of the cord that can occur and the posterolateral location of most cervical epidural hematomas [4,37]. In comparison with an arterial source, when the hematoma is of a venous origin, there is no sudden pain or any other subjective symptoms initially and there is a relatively insidious onset of deficits, probably due to the slower expansion velocity of the haemorrhage [3,35].
Spinal cord compression leads to venous congestion, due to the impairment of the venous drainage of the spinal cord. This causes oedema and axonal swelling. With progressive oedema, blood flow to the cord is compromised leading to ischemia. Severe compression may also lead to the compression of major spinal arteries causing infarctions of the cord. Further damage ensues due to the propagation of a secondary cascade of biochemical and cellular processes following the primary insult [7,39]. Thus, surgical decompression is warranted even if the presentation is an ASIA A deficit or in a delayed manner [39].
SSEH can occur anywhere along the length of the cord but is commonly found at the posterior cervicothoracic (C5–T2) and thoracolumbar (T10–L2) segments with the peaks at C6 and T12 levels. The location also seems to be a prognostic factor for the clinical outcome because patients with a thoracic hematoma are more susceptible to compression-induced ischemia due to a narrower spinal canal compared to the cervical and lumbar canals [3,4,34,35]. Dorsal hematomas are much more common than the ventral ones because the ventral dura is firmly attached by Hoffmann ligaments to the posterior longitudinal ligament as well as the dorsal epidural venous plexus being larger than the ventral ones. It has also been hypothesized that the dorsal plexus may have areas called “locus minoris resistentiae” that are highly susceptible to rupture with minor changes in the intravenous pressure [11,13,30].
Both the venous and arterial theories are a probable hypothesis for SSEH, with most cases probably resulting from a venous bleed. The posterior epidural venous network is the most likely source of bleeding [6,7,16,17,30,36,37]. The vertebral venous plexus is a low-pressure valveless system, which is in continuity with the abdominal and thoracic venous system. Routine activities such as voiding, sneezing, bending, coughing or coitus causes fluctuations in the intrathoracic and abdominal pressures. These pressure variations lead to blood flow reversal in the valve-less epidural plexus resulting in their rupture as they are highly vulnerable to any intrinsic pressure changes. A few authors also believe that hematomas occur at segments with more mobility like the cervicothoracic or thoracolumbar areas, as these areas are suspected to produce more tension on the epidural veins [6,32,36,38]. Though the arterial source for a SSEH is infrequent, it has definitely been hypothesized by many as a source of bleeding given the rapidity of the formation of the hematoma as the pressure of the epidural venous plexus is lower than the intrathecal pressure. So venous bleeding should not be able to compress the dural sac. Therefore, it seems logical that epidural arteries could be the cause due to the compression of the cord that can occur and the posterolateral location of most cervical epidural hematomas [4,37]. In comparison with an arterial source, when the hematoma is of a venous origin, there is no sudden pain or any other subjective symptoms initially and there is a relatively insidious onset of deficits, probably due to the slower expansion velocity of the haemorrhage [3,35].
Spinal cord compression leads to venous congestion, due to the impairment of the venous drainage of the spinal cord. This causes oedema and axonal swelling. With progressive oedema, blood flow to the cord is compromised leading to ischemia. Severe compression may also lead to the compression of major spinal arteries causing infarctions of the cord. Further damage ensues due to the propagation of a secondary cascade of biochemical and cellular processes following the primary insult [7,39]. Thus, surgical decompression is warranted even if the presentation is an ASIA A deficit or in a delayed manner [39].
Radiology
MRI is the preferred modality of investigation in a suspected case of SSEH. It provides accurate information on location, size, and severity of the spinal cord compression. It is usually iso/ hypointense to the spinal cord in T1weighted images and hyperintense/heterogeneous in T2 weighted images within the first 24 hours and hyperintense in both T1 and T2 sequences by 48 hours. Chronic hematomas may be hypointense on both T1 and T2-weighted images. Occasionally we might see contrast enhancement within the hematoma which indicates persistent bleeding. Contrast imaging is not required to diagnose SSEH, but it could help us in the differential diagnosis of other lesions such as tumours, or vascular anomalies with epidural bleeding. Cord oedema is another sensitive indicator of prognosis as it plays a crucial role in neural dysfunction. Cord oedema usually presents as hyperintense / heterogenous in T2 weighted sequences. Oedema is found more commonly in the lower cervical and upper thoracic areas according to various reports. It is probably due to the reduced blood supply in these locations compared with the rest of the cord [3,4,8,10,13,17,40].
Spinal computed tomography [CT]/ computed myelography can be done when MRI is unavailable or contraindicated. CT remains the fastest, most accessible, and least expensive means for evaluation of patients with spinal pathology. CT scan may enable differentiation of hyper acute hematoma from adjacent fat and osseous structures[41]. A spinal angiogram can be used to diagnose as well as treat vascular malformations. However, it should not be performed as a routine investigation in an emergency setting because SSEH is known for its rapid deterioration and high disability rate [4].
Spinal computed tomography [CT]/ computed myelography can be done when MRI is unavailable or contraindicated. CT remains the fastest, most accessible, and least expensive means for evaluation of patients with spinal pathology. CT scan may enable differentiation of hyper acute hematoma from adjacent fat and osseous structures[41]. A spinal angiogram can be used to diagnose as well as treat vascular malformations. However, it should not be performed as a routine investigation in an emergency setting because SSEH is known for its rapid deterioration and high disability rate [4].
Management
There is no consensus on the best management paradigm for SSEH. The obscure aetiology, low incidence and vast array of presentation from being asymptomatic to tetraparesis is too wide a spectrum to generate a specific protocol. This variability has led to a generalized division of therapeutic options into 2 categories: surgical and conservative. This broad management principal is very sub-optimal considering the disease-related mortality of about 5.7%. Further, the literature search has revealed over 50% of patients continue to have some level of sensorimotor deficits. This morbidity is extremely high and obligates the need for improvement in the therapeutic options. We have analysed the available case series and their outcome to propose a management strategy.
Surgical treatment: Early surgical decompression and hematoma evacuation is the treatment of choice in patients with progressive neurological deficits[7,8,17]. The surgery comprises of a hemilaminectomy or a laminectomy followed by hematoma evacuation[4,33]. Endoscopic decompression using flexible devices can be considered in patients to minimize the extent of bony resection. They can also be considered as an operative adjunct as well as a surgical alternative in those whom extensive laminectomies are contraindicated[11,42,43].
Prognosis depends on the type and extent of pre-operative neurological deficits, the progressive interval, hematoma size, segmental distribution, and time of intervention from the onset of symptoms. Patients with minimal preoperative deficits are more likely to achieve complete recovery than those with major deficits. Postoperative rehabilitation is another important factor determining the clinical outcome[3,4,7,23,32,33].
Though there is an ongoing debate about establishing an acceptable time frame between ictus and surgery, there is no consensus about it till date. Bakker et al. [7]in his meta-analysis, has reported that the duration and severity of the pre-operative deficit were independent predictors of poor outcome.Lawton et al. [44]reported that surgery should not be delayed beyond 12 hours as patients who underwent surgery within this time frame had more satisfactory outcomes. Shin et al. [45]however, found that the neurological outcome was better in patients who underwent surgery within 24 hours. A few authors have opined that surgery should be performed within 48 hours after onset in patients with incomplete neurological dysfunction or within 36 hours in patients with complete dysfunction [23,46]. Analysis from the available studies and case reports reveals that patients should undergo decompression within 12 hours and maximally up to 36 hours of symptom onset to achieve a favorable neurological outcome or at least within 48 hours, because any further delay may cause irreversible damage to the cord [4,17,23,47]. A shorter progression interval is another prognostic indicator which often led to a worse prognosis. Patients with a progression interval <12 hours developed a more serious neurological deficit than those with a progression interval >12 hours [18,30,45]. Wang et al.[4] in his retrospective analysis found that outcomes were better in patients with incomplete neurological deficits than in those with complete neurological deficits. He also found a statistically significant association between the initial ASIA grade and outcome. According to Liao et al. [36], complete recovery was seen in 88.9% with incomplete deficits and only in 37.5% with complete deficits at the end of 1 year. A literature search revealed that 30% of patients who presented with an ASIA score of A did not improve with surgery whereas; every SSEH patient who presented at an ASIA score of C or D improved with surgery. Thus proving that the severity of preoperative deficit is the strongest predictor for a patient’s outcome [6,7,19,23,45].
Rehabilitation is another important factor to prevent disability, thus determining the clinical outcome in these patients. Since there is no instability factor in the case of SSEH due to the absence of trauma, early rehabilitation particularly is suitable in this subset of patients to improve outcomes[3].
Our patient underwent C3 to C6 laminectomy and decompression within 5 hours of the onset of hemiparesis. Post operatively, a gradual recovery in the neurological status and improvement of sensation was observed within 24 hours. A complete motor and sensory recovery was observed at the end of second week, at the time of discharge.
Conservative management: A few authors have reported that conservative management can be considered as a treatment option in patients with no neurological deficits or patients showing spontaneous recovery [4,16,35]. Groen et al. [35] in his study focusing on conservative management, reported 84% of patients treated non-operatively recovered completely. Raasck et al. in his retrospective analysis of literature found that 73% of patients managed conservatively made a full recovery (ASIA score E) as opposed to the 48% who were managed surgically. However, both the reports emphasize that patients selected for conservative management had, on average, a smaller neural deficit than those who were managed surgically, and within this comparison was an inherent bias. There are various theories regarding the spontaneous resolution of SSEH. Many authors believe that the hematoma reduction is due to the tracking of blood along the epidural space thereby decompressing the cord and reducing the mass effect [35]. This view was accepted because the duration of hematoma was longer in the conservative group. Few others suggested that blood could seep through the intervertebral foramen and cause spinal cord decompression [48].
If a patient is managed non-operatively, then the patient needs to be monitored with serial examinations while on strict bed rest. A sudden increase in the size of the hematoma and the neural deficits are the possible factors to be considered prior to decision making [49]. Thus the decision of conservative management should never be considered if the hospital in question has no imaging or surgical spine services. Cases initially treated conservatively have been reported to deteriorate even after a marked period of recovery, ultimately requiring surgery [47,49]. All these factors have to be considered prior to the decision and the patients should be counselled accordingly. Since there are no large series available to confirm the safety of conservative management and given the nature of the pathology, the consensus is for emergent or at least urgent surgical intervention on the appearance of a deficit in an otherwise asymptomatic patient.
Since SSEH has no randomized control trials to date, some guidelines from the management of similar conditions such as acute spinal cord injury (SCI) is taken into consideration. Hence a mean arterial pressure (MAP) of 85–90 mmHg for 7 days after acute SCI should be considered [50]. Since SSEH has predominantly a venous origin, augmentation of blood pressure with vasoactive agents may not increase the risk of a rebleed. Joshua et al. [51]found a positive correlation between higher MAP and neurologic improvement in patients who are ASIA grade A–C. By continuing to asses conservatively managed cases, perhaps we can identify and substantiate more parameters that indicate the likelihood of spontaneous recovery or degree of benefit from decompressive surgery.
ASIA score is a reliable indicator to assess and compare the preoperative and postoperative deficits. It also aids in prognosticating and determining the time and type of intervention required. Hence a detailed preoperative and post-operative assessment is essential in the management of these cases, as this will guide us to change the management based on the ongoing clinical status of the patient [4,6,52].
To summarize the management strategy, an emergent surgical intervention is mandatory when the ASIA score is A or B. If the score is C or D, urgent care is recommended but time is not as strong a factor and if the score is E, the patient can be managed conservatively [4,6,52]. Conservative management can also be considered in patients presenting with minimal neural deficits, or in those where spontaneous recovery has started before or during surgical preparations. Observation should include repeat MRIs and repeated clinical assessment with the consideration that, surgical intervention may still be necessary even after a period of marked recovery.
Surgical treatment: Early surgical decompression and hematoma evacuation is the treatment of choice in patients with progressive neurological deficits[7,8,17]. The surgery comprises of a hemilaminectomy or a laminectomy followed by hematoma evacuation[4,33]. Endoscopic decompression using flexible devices can be considered in patients to minimize the extent of bony resection. They can also be considered as an operative adjunct as well as a surgical alternative in those whom extensive laminectomies are contraindicated[11,42,43].
Prognosis depends on the type and extent of pre-operative neurological deficits, the progressive interval, hematoma size, segmental distribution, and time of intervention from the onset of symptoms. Patients with minimal preoperative deficits are more likely to achieve complete recovery than those with major deficits. Postoperative rehabilitation is another important factor determining the clinical outcome[3,4,7,23,32,33].
Though there is an ongoing debate about establishing an acceptable time frame between ictus and surgery, there is no consensus about it till date. Bakker et al. [7]in his meta-analysis, has reported that the duration and severity of the pre-operative deficit were independent predictors of poor outcome.Lawton et al. [44]reported that surgery should not be delayed beyond 12 hours as patients who underwent surgery within this time frame had more satisfactory outcomes. Shin et al. [45]however, found that the neurological outcome was better in patients who underwent surgery within 24 hours. A few authors have opined that surgery should be performed within 48 hours after onset in patients with incomplete neurological dysfunction or within 36 hours in patients with complete dysfunction [23,46]. Analysis from the available studies and case reports reveals that patients should undergo decompression within 12 hours and maximally up to 36 hours of symptom onset to achieve a favorable neurological outcome or at least within 48 hours, because any further delay may cause irreversible damage to the cord [4,17,23,47]. A shorter progression interval is another prognostic indicator which often led to a worse prognosis. Patients with a progression interval <12 hours developed a more serious neurological deficit than those with a progression interval >12 hours [18,30,45]. Wang et al.[4] in his retrospective analysis found that outcomes were better in patients with incomplete neurological deficits than in those with complete neurological deficits. He also found a statistically significant association between the initial ASIA grade and outcome. According to Liao et al. [36], complete recovery was seen in 88.9% with incomplete deficits and only in 37.5% with complete deficits at the end of 1 year. A literature search revealed that 30% of patients who presented with an ASIA score of A did not improve with surgery whereas; every SSEH patient who presented at an ASIA score of C or D improved with surgery. Thus proving that the severity of preoperative deficit is the strongest predictor for a patient’s outcome [6,7,19,23,45].
Rehabilitation is another important factor to prevent disability, thus determining the clinical outcome in these patients. Since there is no instability factor in the case of SSEH due to the absence of trauma, early rehabilitation particularly is suitable in this subset of patients to improve outcomes[3].
Our patient underwent C3 to C6 laminectomy and decompression within 5 hours of the onset of hemiparesis. Post operatively, a gradual recovery in the neurological status and improvement of sensation was observed within 24 hours. A complete motor and sensory recovery was observed at the end of second week, at the time of discharge.
Conservative management: A few authors have reported that conservative management can be considered as a treatment option in patients with no neurological deficits or patients showing spontaneous recovery [4,16,35]. Groen et al. [35] in his study focusing on conservative management, reported 84% of patients treated non-operatively recovered completely. Raasck et al. in his retrospective analysis of literature found that 73% of patients managed conservatively made a full recovery (ASIA score E) as opposed to the 48% who were managed surgically. However, both the reports emphasize that patients selected for conservative management had, on average, a smaller neural deficit than those who were managed surgically, and within this comparison was an inherent bias. There are various theories regarding the spontaneous resolution of SSEH. Many authors believe that the hematoma reduction is due to the tracking of blood along the epidural space thereby decompressing the cord and reducing the mass effect [35]. This view was accepted because the duration of hematoma was longer in the conservative group. Few others suggested that blood could seep through the intervertebral foramen and cause spinal cord decompression [48].
If a patient is managed non-operatively, then the patient needs to be monitored with serial examinations while on strict bed rest. A sudden increase in the size of the hematoma and the neural deficits are the possible factors to be considered prior to decision making [49]. Thus the decision of conservative management should never be considered if the hospital in question has no imaging or surgical spine services. Cases initially treated conservatively have been reported to deteriorate even after a marked period of recovery, ultimately requiring surgery [47,49]. All these factors have to be considered prior to the decision and the patients should be counselled accordingly. Since there are no large series available to confirm the safety of conservative management and given the nature of the pathology, the consensus is for emergent or at least urgent surgical intervention on the appearance of a deficit in an otherwise asymptomatic patient.
Since SSEH has no randomized control trials to date, some guidelines from the management of similar conditions such as acute spinal cord injury (SCI) is taken into consideration. Hence a mean arterial pressure (MAP) of 85–90 mmHg for 7 days after acute SCI should be considered [50]. Since SSEH has predominantly a venous origin, augmentation of blood pressure with vasoactive agents may not increase the risk of a rebleed. Joshua et al. [51]found a positive correlation between higher MAP and neurologic improvement in patients who are ASIA grade A–C. By continuing to asses conservatively managed cases, perhaps we can identify and substantiate more parameters that indicate the likelihood of spontaneous recovery or degree of benefit from decompressive surgery.
ASIA score is a reliable indicator to assess and compare the preoperative and postoperative deficits. It also aids in prognosticating and determining the time and type of intervention required. Hence a detailed preoperative and post-operative assessment is essential in the management of these cases, as this will guide us to change the management based on the ongoing clinical status of the patient [4,6,52].
To summarize the management strategy, an emergent surgical intervention is mandatory when the ASIA score is A or B. If the score is C or D, urgent care is recommended but time is not as strong a factor and if the score is E, the patient can be managed conservatively [4,6,52]. Conservative management can also be considered in patients presenting with minimal neural deficits, or in those where spontaneous recovery has started before or during surgical preparations. Observation should include repeat MRIs and repeated clinical assessment with the consideration that, surgical intervention may still be necessary even after a period of marked recovery.
CONCLUSION
SSEH has a very low incidence in the general adult population. Prompt diagnosis and surgical intervention are mandatory, as it can cause catastrophic consequences if there is delayed treatment. SSEH can mimic various other pathologies due to its wide spectrum of clinical presentations, thereby causing a delay in diagnosis. Thus, a thorough clinical evaluation and imaging are essential for a correct diagnosis. The treating physicians should be made aware of this rare but treatable disease entity and its diagnostic pitfalls. Early surgical decompression and hematoma evacuation is the treatment of choice in patients with progressive neurological deficits. The duration and severity of the pre-operative deficit are found to be independent predictors of poor outcome. There is no consensus about the accepted time frame for intervention. However, decompression within 12 hours and maximally up to 36 hours of symptom onset is essential to achieve a favorable neurological outcome.
The progressive interval, hematoma size, segmental distribution and rehabilitation are the other factors affecting clinical outcome.Conservative management can be considered in patients who are asymptomatic individuals, patients with minimal neural deficits or in those with spontaneous recovery. It should include constant clinical assessment and imaging. The rarity of this pathology makes it hard to study underlying risk factors. A sufficiently large, single-institution series which is required to obtain relevant statistical data has not yet been described. Thus, necessitating the use of a review of published cases to collect enough data to analyze the aetiology, treatment and outcome of the disease.
The progressive interval, hematoma size, segmental distribution and rehabilitation are the other factors affecting clinical outcome.Conservative management can be considered in patients who are asymptomatic individuals, patients with minimal neural deficits or in those with spontaneous recovery. It should include constant clinical assessment and imaging. The rarity of this pathology makes it hard to study underlying risk factors. A sufficiently large, single-institution series which is required to obtain relevant statistical data has not yet been described. Thus, necessitating the use of a review of published cases to collect enough data to analyze the aetiology, treatment and outcome of the disease.
ACKNOWLEDGEMENTS
Nil
CONFLICT OF INTEREST
We have no conflict of interest.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil
REFERENCES
- Bhat KJ, Kapoor S, Watali YZ, Sharma JR (2015) Spontaneous epidural hematoma of spine associated with clopidogrel: A case study and review of the literature. Asian J Neurosurg 10: 54.
- Al-Mutair A, Bednar DA (2010) Spinal epidural hematoma. J Am Acad Orthop Surg 18: 494-502.
- Xian H, Xu L-W, Li C-H, Hao J-M, Wan W-X, et al. (2017) Spontaneous spinal epidural hematomas One case report and rehabilitation outcome. Medicine 96: e8473.
- Wang M, Zhou P, Jiang S (2017) Clinical Features, Management, and Prognostic Factors of Spontaneous Epidural Spinal Hematoma: Analysis of 24 Cases. World Neurosurg. 102: 360-369.
- Figueroa J, Devine JG (2017) Spontaneous spinal epidural hematoma: literature review. J Spine Surg 3: 58-63.
- Raasck K, Habis AA, Aoude A, Simões L, Barros F, et al. (2017) Spontaneous spinal epidural hematoma management: a case series and literature review. Spinal Cord Ser Cases 3: 16043.
- Bakker NA, Veeger NJGM, Vergeer RA, Groen RJM (2015) Prognosis after spinal cord and cauda compression in spontaneous spinal epidural hematomas. Neurology 84: 1894-1903.
- Romaniuc A, Maier S, Buruian M, Liptak L, B?la?a A (2018) Spontaneous spinal epidural haematoma mimicking acute ischaemic stroke: case report. ActaNeurologicaBelg 1-3.
- Li C, He R, Li X, Zhong Y, Ling L, et al. (2017) Spontaneous spinal epidural hematoma mimicking transient ischemic attack: A case report. Medicine 96: e9007.
- Domenicucci M, Mancarella C, Santoro G, Dugoni DE, Ramieri A, et al. (2017) Spinal epidural hematomas: personal experience and literature review of more than 1000 cases. J Neurosurg Spine 27: 198-208.
- Dziedzic T, Kunert P, Krych P, Marchel A (2015) Management and neurological outcome of spontaneous spinal epidural hematoma. J Clin Neurosci 22: 726-729.
- Suck Baek B, Woo Hur J, Kwon KY, Lee HK (2008) Spontaneous Spinal Epidural Hematoma. J Korean NeurosurgSoc 44: 40-42.
- Gopalkrishnan CV, Dhakoji A, Nair S (2012) Spontaneous cervical epidural hematoma of idiopathic etiology: Case report and review of literature. J Spinal Cord Med 35: 113-117.
- Jackson R (1869) Case of spinal apoplexy. Lancet 94: 5-6.
- Bain W (1897) A Case of Haematorrachis. Br Med J 2: 455.
- Akimoto T, Yamada T, Shinoda S (2014) Spontaneous Spinal Epidural Hematoma as a Potentially Important Stroke Mimic. J Cent NervSyst Dis 6: 15-20.
- Zuo B, Zhang Y, Zhang J, Song J, Jiang S, et al. (2018) Spontaneous Spinal Epidural Hematoma: A Case Report. Case Rep Orthop Res 1: 26-33.
- Matsumura A, Namikawa T, Hashimoto R, Okamoto T, Yanagida I, et al. (2008) Clinical management for spontaneous spinal epidural hematoma: diagnosis and treatment. Spine J 534-537.
- Fedor M, Kim ES, Ding K, Muizelaar JP, Kim KD (2011) Spontaneous Spinal Epidural Hematoma: A Retrospective Study on Prognostic Factors and Review of the Literature. Korean J Spine 8: 272–282.
- Kyu Kim J, Hong Kim T, Keun Park S, Soon Hwang Y, Shik Shin H, et al. (2013) Acute Spontaneous Cervical Epidural Hematoma Mimicking Cerebral Stroke: A Case Report and Literature Review Corresponding. Korean J Spine 10: 170-173.
- Wang P, Xin X, Lan H, Chen C, Liu B (2011) Spontaneous cervical epidural hematoma during pregnancy: case report and literature review. Eur Spine J 20: S176-S179.
- Ahn DK, Jung WS, Lee J Il(2015) Hemophilia A in a Senior Patient: A Case Report of Spinal Epidural Hematoma as First Presentation. Asian Spine J 9: 452.
- Liao CC, Hsieh PC, Lin TK, Lin CL, Lo YL, et al. (2009) Surgical treatment of spontaneous spinal epidural hematoma: a 5-year experience. J Neurosurg Spine 11: 480-486.
- Ueba Y, Hojo M, Munemitsu T, Suzuki K, Kawasaki T, et al. (2014) Two cases of spontaneous cervical epidural hematoma mimicking cerebral infarction. No ShinkeiGeka 42: 143-148.
- Mohammed N, Shahid M, Haque M, Qureshi M, Hoey ETD (2015) Spontaneous spinal epidural haematoma mimicking acute coronary syndrome. Quant Imaging Med Surg 5: 925-927.
- Estaitieh N, Alam S, Sawaya R (2014) Atypical presentations of spontaneous spinal epidural hematomas. Clin NeurolNeurosurg 122: 135-136.
- Aristedis R, Dimitrios B (2017) Spinal epidural hematoma mimicking subarachnoid hemorrhage: A case study. SurgNeurolInt 8: 182.
- Hee Yoon B, Seok Park K, Sam Jung S, Sun Park M, Kim S-M, et al. (2012) Spontaneous Cervical Epidural Hematoma Causing Brown-Sequard Syndrome. Korean J Spine 9: 297-299.
- Cai HX, Liu C, Zhang JF, Wan SL, Uchida K, et al. (2011) Spontaneous epidural hematoma of thoracic spine presenting as Brown-Sequard syndrome: report of a case with review of the literature. J Spinal Cord Med 34: 432-436.
- Kreppel D, Antoniadis G, Seeling W (2003) Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 26: 1-49.
- Kim K-T, Cho D-C, Ahn S-W, Kang S-H (2012) Epidural Hematoma Related with Low-Dose Aspirin?: Complete Recovery without Surgical Treatment. J Korean NeurosurgSoc 51: 308-311.
- Groen RJ, van Alphen HA (1996) Operative treatment of spontaneous spinal epidural hematomas: a study of the factors determining postoperative outcome. Neurosurgery 39: 494-508.
- Zhong W, Chen H, You C, Li J, Liu Y, et al. (2011) Spontaneous spinal epidural hematoma. J Clin Neurosci 18: 1490-1494.
- Foo D (1997) Operative treatment of spontaneous spinal epidural hematomas: a study of the factors determining postoperative outcome. Neurosurgery 41: 1218-1220.
- Groen RJMM, Goffin J (2004) Non-operative treatment of spontaneous spinal epidural hematomas: A review of the literature and a comparison with operative cases. ActaNeurochir (Wien) 146: 103-110.
- Liao CC, Lee ST, Hsu WC, Chen LR, Lui TN, et al. (2004) Experience in the surgical management of spontaneous spinal epidural hematoma. J Neurosurg 100: 38-45.
- Beati’y RM, Winston KR (1984) Spontaneous cervical epidural hematoma:A consideration of etiology. J Neurosurg 61:143-148.
- Koksal V, Yavasi O (2017) Controversies in the differential diagnosis of Brown-Sequard syndrome due to cervical spinal disease from stroke: A case series. Turk J Emerg Med 17: 115-120.
- Rajz G, Cohen JE, Harnof S, Knoller N, Goren O, et al. (2015) Spontaneous spinal epidural hematoma: The importance of preoperative neurological status and rapid intervention. J Clin Neurosci 22: 123-128.
- Holtås S, Heiling M, Lönntoft M (1996) Spontaneous Spinal Epidural Hematoma: Findings at MR Imaging and Clinical Correlation. Radiology 199: 409-413.
- Post MJ, Becerra JL, Madsen PW, Puckett W, Quencer RM, et al. (1994) Acute spinal subdural hematoma: MR and CT findings with pathologic correlates. AJNR Am J Neuroradiol 15: 1895-1905.
- Steel TR, Kellogg JX, Kuether TA, Favre J, Frank EH (1998) Endoscopic treatment of spinal epidural hematoma. J Clin Neurosci 5: 460-463.
- Kessel G, Böcher-Schwarz H-G, Ringel K, Perneczky A (1997) The Role of Endoscopy in the Treatment of Acute Traumatic Anterior Epidural Hematoma of the Cervical Spine: case Report. Neurosurgery 41: 688-690.
- Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VKH, et al. (1995) Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 83: 1-7.
- Shin JJ, Kuh SU, Cho YE (2006) Surgical management of spontaneous spinal epidural hematoma. Eur Spine J 15: 998-1004.
- Liu Z, Jiao Q, Xu J, Wang X, Li S, et al. (2008) Spontaneous spinal epidural hematoma: analysis of 23 cases. SurgNeurol 69: 253-260.
- Yamao Y, Takagi Y, Kawauchi T, Arakawa Y, Takayama M, et al. (2015) Surgical management of recurrent spontaneous spinal epidural hematoma with 3 episodes. Spine (Phila Pa 1976) 40: 996-998.
- Inamasu J, Hori S, Aoki K, Aikawa N, Maruiwa H, et al. (2000) Spontaneous spinal epidural hematoma. Am J Emerg Med 18: 837-839.
- Duyll J, Sparrow OC, Millar J, Barker CS (2000) Can spontaneous spinal epidural haematoma be managed safely without operation? A report of four cases. J NeurolNeurosurg Psychiatry 69: 816-819.
- Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, et al. (2013) Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries: 2013 update. Neurosurgery 60: 82-91.
- Catapano JS, Hawryluk GWJ, Whetstone W, Saigal R, Ferguson A, et al. (2016) Higher Mean Arterial Pressure Values Correlate with Neurologic Improvement in Patients with Initially Complete Spinal Cord Injuries. World Neurosurg 96: 72-79.
- Maynard FM, Bracken MB, Creasey G, Ditunno JF, Donovan WH, et al. (1997) International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 35: 266-274.
Citation: Rajagopal N, Balaji A, Kawase T, Yamada Y, Kato Y (2019) The Varied Clinical Presentations, Prognostic Factors and Management of Spontaneous Spinal Epidural Hematoma: A Review of Literature. J Nucl Med Radiol Radiat Ther 4: 014.
Copyright: © 2019 Rajagopal N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Journal Highlights
© 2024, Copyrights Herald Scholarly Open Access. All Rights Reserved!
