
Effects of UVA+UVB Solar Radiation Levels and Cross-Linking on Animal Model with Bowman’s Layer: Chicken
*Corresponding Author(s):
Marîa Carmen Martínez-GarcíaDepartamento De Biologia Celular, Histología Y Farmacología. Facultad De Medicina. Grupo De Investigación Reconocido: Técnicas Ópticas Para El Diagnóstico, Universidad De Valladolid, Calle: Ramón Y Cajal 47005 Valladolid, Spain
Tel:+34 983184781,
Email:mariacarmen.martinez.garcia@uva.es
Abstract
Purpose: Evaluate the corneal damage and wound healing in an animal model with Bowman’s layer after UVA+UVB radiation and epi-off Cross-Linking (CXL).
Methods: Adult Brown Classic chickens were randomly divided into three groups. The UVA+UVB group (20 eyes) was exposed to three doses of radiation (4.60 J/cm2). The CXL group (12 eyes), was de-epithelialized and irradiated with UVA light (5.4 J/cm2) for 30 minutes. The contralateral eyes were used as a control (12 eyes). The animals have clinically follow-up, the epithelial closure was measured by fluorescein stain and corneal thickness by pachymetry. Keratocytes loss, subsequent cell repopulation, and healing events were analyzed after euthanasia at 24 hours, 15 and 30 days post-radiation on hematoxylin-eosin-stained sections. Cell death was detected by TUNEL assay, proliferation by BrdU (bromodeoxyuridine) immunofluorescence, and myofibroblasts differentiation by (αSMA) immunohistochemistry.
Results: Re-epithelialization was at 5 ± 2.3 days in CXL and at day 2 ± 0.9 days in UVA+UVB. Pachymetry was higher after UVA+UVB than CXL (p<0.05). Cell death affected the whole thickness of stroma in the UVA+UVB group and anterior and middle stroma in CXL. At day 1, the UVA+UVB group showed a more significant reduction in the number of stromal cells. After 30 days, both groups showed a free band of cells below the epithelium.
Conclusion: The damage and the healing response in chicken corneas after UVA+UVB and CXL demonstrated that Bowman’s layer has not been successful in protecting the cornea from radiation. Perhaps, other factors that are involved in corneal defense.
Keywords
Bowman’s layer; Chicken; Cornea; Crosslinking; Ultraviolet radiation
ABBREVIATIONS
UV: Ultraviolet
UVA: Ultraviolet A
UVB: Ultraviolet B
CXL: Cross-Linking
H-E: Haematoxilin-Eosin
ROS: Reactive Oxygen Species
TUNEL: Terminal Uridine Nick End-Labeling
Brdu: Bromodeoxyuridine
αSMA: Alpha Smooth Muscle Actin
INTRODUCTION
Eyes are regularly and continuously exposed to sunlight. Solar radiation is composed of ultraviolet (UV) radiation: UVA (320-400 nm), UVB (280-320 nm), UVC (100-280 nm) and visible light (400-700 nm). On the Earth's surface, UV radiation consists mainly of UVA (97%) and UVB (3%) wavelengths [1,2]. UV exposure induces oxidative damage, which derives from the formation of reactive oxygen species that cause cell death [2-4]. The absorption of solar radiation by different corneal layers is related to the wavelength. The cornea, aqueous humor and lens absorb UVA radiation, whereas the cornea primarily absorbs UVB radiation. The epithelium and Bowman's layer absorb 2 to 2.75 times more UV radiation up to 300 nm of wavelength (UVB) than the stroma [5,6]. Although UVB is the smaller part of the UV environmental radiation, it is the most harmful [5,6].
UV solar exposure is related to some ocular diseases, such as photokeratitis, pterygium, cataracts, climatic droplet keratopathy and climatic proteoglycan stromal keratopathy [7,8]. Indeed, there are many published studies regarding UVA and UVB radiation injuries in the corneas of animal models that try to explain this damage [1, 2, 9-11], although none of them were done in an animal with Bowman’s layer.
The CXL procedure is a medical strategy to increase the biomechanical stability of corneal stromal tissue [12-14]. In this treatment, UVA radiation is used with a photosensitizer, riboflavin that produces reactive oxygen species and radicals that lead to extracellular matrix protein cross-linking. It is used in patients with corneal ectasias (e.g., keratoconus), Pellucid Marginal Degeneration (PMD), and ectasias secondary to LASIK to increase corneal stiffness and halt the progression of these pathologies [15]. This safe and minimally invasive treatment is widely used in patients with keratoconus, and secondary ectasia was introduced by Wollensak et al. in 2003 since then has been carried out with similar parameters and protocol. Clinical results of CXL have shown it to be an effective means of halting keratoconus progression with scare reports of complications in patients with follow-up over ten years [12,15-18]. To minimize pain and discomfort in the early postoperative period due to epithelial removal, new variations in standard CXL have been proposals as transepithelial CXL and transepithelial iontophoresis-assisted CXL [16-18]. In the same way, the animal models used have never had Bowman’s layer.
This study aims to evaluate the effects of two types of UV radiation on the corneas of an animal model with Bowman’s layer that have similar histological proportions to human corneas. One is similar to conditions on the Earth's surface, and the other is a clinical treatment called CXL. Moreover, we evaluated the response and regeneration after radiation damage.
MATERIALS AND METHODS
Animals
The Animal Ethics Committee at the University of Valladolid approved the animal studies described in this research. Animals were cared for according to the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Female adult Lohmann Brown Classic chickens, weighing 2.5 kg, were supplied by a center listed in the official register as a provider of lab animals (Ibertec - Ibérica de Tecnología Avícola, S. A. U., Boecillo - Spain).
Treatment procedure
Twenty-two chickens were randomly divided into three groups. The first group, ten chickens (20 eyes), was exposed to three doses of UVA+UVB radiation. The second group, twelve chickens (12 eyes), was treated with the UVA - Riboflavin CXL procedure. The contralateral eye of the chickens in the CXL group (12 eyes) was used as a control (control group).
The chickens were anesthetized with an intramuscular injection of ketamine hydrochloride (Imalgene 500, Merial Laboratory S. A., Barcelona Spain; 30 mg/kg) and xylazine (Rompum, Bayer AG, Leverkusen, Germany; 5 mg/kg) in the thigh, followed by topical application of 0.5% tetracaine hydrochloride and 1 mg oxybuprocaine (Colircusi Anestésico Doble, Alconcusí SA, Barcelona, Spain) before exposure to UVA+UVB doses and the CXL procedure as well as at every clinical follow-up. After the treatment, chickens were treated with butorphanol tartrate solution (Torbugesic S.A. 2 mg/ml; Fort Dodge Animal Health, Iowa, USA) 0.1 ml/kg by intramuscular injection every 24 h to avoid pain.
UVA and UVB radiation
For radiation, a Super-Quiet Xenon Lamp L2273 (150 nm to 2000 nm, 150 W) (Hamamatsu Photonics K. K., Iwata City, Japan) was used. This lamp emits white light with 6000 K color temperature similar to the sun and deep UV and IR (185 nm to > 4000 nm). To get the right UVA and UVB radiation range without UVC radiation, we designed an apparatus composed of a UV xenon lamp (A), a screen with one black side to keep out the high heat of the lamp (B), and a WG305 filter (280-420 nm) that allowed only UVA+UVB radiation to directly reach the animal’s eyes (C). Two quartz lenses with 50 nm diameter and focal distance of 150 nm were employed to focus high power energy on the central light zone (D) of the animal’s cornea; these lenses amplified the UV xenon lamp power by 11,000 times. Another screen placed 32 cm from the xenon lamp was used to avoid scattering light on the animal’s eyes (E) (Figure 1). This experimental apparatus allowed us to reduce the exposure time and provided a light corneal zone less than 6 mm in diameter.
 Figure 1: Experimental apparatus to UVA+UVB radiation. A - Super-Quiet Xenon Lamp L2273; B - A screen with one black side, which keep out the high lamp’s heat; C - WG305 filter (280 - 420 nm) that allow only the pass of UVA+UVB; D - Two quartz lenses with 50 nm; E - The screen avoids light scattering on the animal.
Figure 1: Experimental apparatus to UVA+UVB radiation. A - Super-Quiet Xenon Lamp L2273; B - A screen with one black side, which keep out the high lamp’s heat; C - WG305 filter (280 - 420 nm) that allow only the pass of UVA+UVB; D - Two quartz lenses with 50 nm; E - The screen avoids light scattering on the animal.
Both of the animal’s eyes were exposed to radiation. Each eye was irradiated three times, every other day (4.60 J/cm2; days 1, 3, and 5), for a total dose of 13.80 J/cm2. The central cornea of the animals was exposed to a daily dose equal to 1 hour and 20 minutes of sunlight exposure (4.60 J/cm2). This dose corresponds to 1 hour and 30 minutes (4.10 J/cm2) of UVA exposure and 5 hours (0.499 J/cm2) of UVB exposure [2,19].
UVA - riboflavin CXL
The left eye of the animal was treated following the Dresden method [20]. Briefly, after de-epithelialization of a 5 mm diameter area, drops of 0.1% riboflavin solution in 20% dextran (La Botica Argensola, Madrid, Spain) were applied on the cornea every 5 minutes for 30 minutes. Then, the central cornea was exposed to UVA light with a wavelength of 370 ± 5 nm and an irradiance of 3 mW/cm2 for a total time of 30 minutes; this corresponds to a total dose of 3.4 J or a total radiant exposure of 5.4 J/cm2 to the cornea. During the radiation time, the cornea was also soaked with riboflavin/dextran solution every 5 minutes.
Clinical course
The anterior segment of the eyes of the animals was evaluated with a surgical microscope (Leica M220 F12, Leica Microsystems, Nussloch, Germany) before and after treatments and at different time points (1, 15 and 30 days post-radiation) in both groups. In the UVA+UVB group, the anterior segment was also evaluated at 2 and 4 days after each dose of radiation. In the clinical follow-up, the epithelial wound was stained by sodium fluorescein (Fluotest, Alcon Cusí S. A., Barcelona, Spain) every day until epithelial closure. Corneal opacity was graded from 0 to 4 according to the Fantes Grading Scale [21]. Conjunctival hyperemia was quantified from 0 to 4 according to the Efron Grading Scale [22]. Edema was quantified by corneal pachymetry and carried out with an ultrasonic pachymeter (Corneo-Gage Plus; Sonogage, Inc., Cleveland, OH, USA) before radiation, then at 1, 7, 15 and 30 days after treatment.
Tissue processing and light microscopy
Animals were euthanized 1, 15 and 30 days after treatments by intracardiac injection of sodium pentobarbital (Dolethal, Vetoquinol Especialidades Veterinárias, S. A., Madrid, Spain) under general anesthesia. Eyes were enucleated for histological studies. They were then fixed with 4% buffered paraformaldehyde and embedded in paraffin. Sections 7-µm thick was stained with Hematoxylin-Eosin (H-E). Sections were examined under an Olympus BX41 microscope (Olympus Life Science, Hamburg, Germany), and photomicrographs were obtained with an Olympus DP20 Digital Camera. Quantitative measurements of the photographs were then taken using Cell A software (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
Cell counting
In each cornea, three measurements of corneal thickness were taken at 100x magnification. All the H-E stained cells were then counted using the Touch Count function from Cell A software (Olympus Soft Imaging Solutions GmbH) in five columns of 90,000 µm2 (from epithelium to endothelium) in the center of the cornea (wound) (Figure 2 C), left and right of the center of the cornea (edges) (Figure 2 E) and finally in the zone next to both the limbi (Figure 2 L). Each column was divided into anterior, medial, and posterior zones, each approximately 30,000 µm2 (105.92 x 287.25 µm). Three measurements of the distance from the epithelium to the first stromal cell were also taken in the central cornea in five different zones. All H-E stained sections were prepared identically to facilitate comparison.
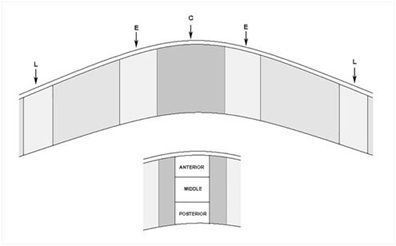 Figure 2: Cells were counted in the wound (C), edges (E) and limbus (L) in an area that corresponds to 90,000 μm2. This area was subdivided in anterior (a) middle (m) and posterior (p) stroma - 30,000 μm2.
Figure 2: Cells were counted in the wound (C), edges (E) and limbus (L) in an area that corresponds to 90,000 μm2. This area was subdivided in anterior (a) middle (m) and posterior (p) stroma - 30,000 μm2.
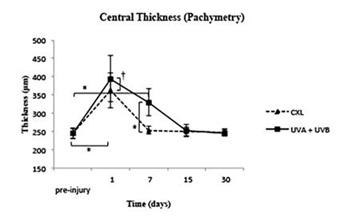 Figure 3: Measurements of pachymetry from pre-injury to 30 days.
Figure 3: Measurements of pachymetry from pre-injury to 30 days.
† p≤0.05 ; *p ≤ 0.001.
Cell death: TUNEL assay
Terminal Uridine Nick End-Labeling (TUNEL) assays were performed to detect DNA fragmentation associated with apoptosis in deparaffinized sections according to the manufacturer’s instructions (TUNEL Ref: G3250, Promega Corp., Madison, WI, USA). Nuclei were counterstained with 4’, 6-diamino-2 phenylindole (DAPI, D9542, Sigma-Aldrich, Munich, Germany). TUNEL assay sections were examined at 100x magnification, and the number of cells labeled was counted.
Myofibroblast differentiation
Immunofluorescence identified myofibroblasts with anti-α-SMA (alpha smooth muscle actin) monoclonal antibody (mouse clone 1A4, Dako Cytomation). The secondary antibody was a Texas red goat anti-mouse IgG (Molecular Probes). Nuclei were stained with DAPI (Molecular Probes). The walls of the limbal blood vessels served as positive controls, and omission of the primary antibody provided negative controls.
Immunofluorescence sections were examined, and cells were counted under an Axiophot fluorescence-incorporated microscope (Zeiss Axiophot HB0-50, Carl Zeiss, Germany). Photomicrographs were taken using an AxioCam HRC digital camera and Axiovision release 4.8 software (Carl Zeiss).
Statistical methods
Measured variables were analyzed by calculating the mean, Standard Deviation (SD), coefficient of variation, maximum and minimum values, asymmetry, and coefficient of kurtosis. After defining these parameters, variances were compared with the Levene test. If the variances were equal, an Analysis of Variance (ANOVA) table was used to test the equality of the means in the three groups. If the variances were different, then the Kruskal-Wallis test was used to compare the equality of the medians. A multiple range test was used to determine which means were significantly different from others.
RESULTS
Clinical evaluation
During the first week, the eyes of both treated groups presented abundant watering and hyperemic conjunctiva of grade 2-3 [22].
Fluorescein testing was positive in the UVA+UVB group 24 hours post-treatment and every day until 2 ± 0.9 days after the last dose of radiation. The de-epithelialization had an irregular border and varied sizes, which were in general shorter than the optical zone. In the CXL group, the epi-off zone (5 mm) was fluorescein testing positive from the beginning until 5 ± 2.3 days after treatment. The time of closure was significantly later in the CXL treated corneas as compared with the UVA+UVB treated corneas (p≤0.05). All treated eyes showed central corneal edema of grade 1-3 [22] during the first 15 days, which subsided spontaneously and disappeared at one month in all eyes. A very light corneal haze was present only in the CXL group from day 15 in the central cornea until day 30. Its severity decreased from grade 1 to 0.4 ± 0.3 from day 15 until the end of the study [21].
Pachymetry. At day 1, the corneal edema was maximum in both groups, and the pachymetry showed statistically significant differences between the UVA+UVB and CXL groups as well as both treatments with the pre-surgery values (245.2 ± 14.45 μm). At day 7, the corneas in the CXL group reached the pre-injury values, while those in the UVA+UVB group needed 15 days to reach pre-injury values.
Histological assessment
Hematoxylin-Eosin sections. One day after receiving the last dose of radiation, UVA+UVB corneas showed irregular ulcers in the optical zone. The epithelium did not cover the center of the wound, and there was a reduction from the top to the inner epithelial layers in the edges. At the same time (day 1), in CXL corneas, the epithelium did not cover the stroma, because it was manually removed in the optical zone.
At day 15, the epithelium thickness was similar to the control in both groups. At day 30, the epithelium thickness measurement showed slight hypertrophy but without a significant difference when compared to the control (Figure 4).
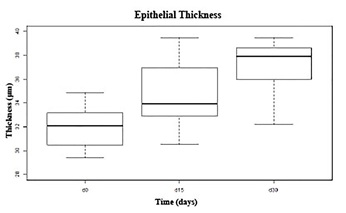 Figure 4: Corneal epithelial thickness measurements before and after treatments. The graphic shows the confidence gap of epithelial thickness during the wound healing in both groups. The progress of epithelial healing in both groups, until day 30, was similar with maximum confidence gap of 3 μm.
Figure 4: Corneal epithelial thickness measurements before and after treatments. The graphic shows the confidence gap of epithelial thickness during the wound healing in both groups. The progress of epithelial healing in both groups, until day 30, was similar with maximum confidence gap of 3 μm.
At day 1, in the UVA+UVB group, the anterior stroma was devoid of keratocytes, and the stromal cells had a condensed and eosinophilic cytoplasm with pyknotic nuclei (Figure 5A). In the corneas that underwent CXL treatment, there was a reduction of keratocytes in the anterior stroma, and the stromal cells also had a condensed and eosinophilic cytoplasm with pyknotic nuclei (Figure 5B). Corneas showed prominent loss of stromal cells as compared with the control corneas. The mean number of stromal cells was significantly smaller at the wound and edges in both treated groups as compared with the control group, although in the edges the decrease of stroma cells was much less in the edges (Figures 6A, 6B and 6C).
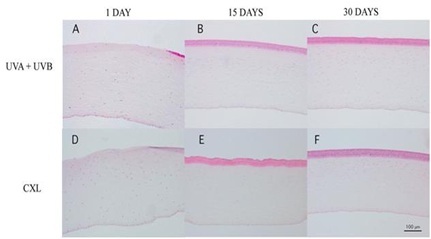 Figure 5: Corneal sections stained with H-E from UVA+UVB treatment (A, B, and C) and CXL (D, E, and F). In both treatments, there were no epithelium at day 1 (A and D) and appeared a band of stromal free cells at day 15 which remain at day 30.
Figure 5: Corneal sections stained with H-E from UVA+UVB treatment (A, B, and C) and CXL (D, E, and F). In both treatments, there were no epithelium at day 1 (A and D) and appeared a band of stromal free cells at day 15 which remain at day 30.
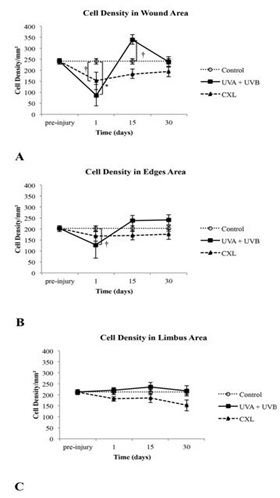 Figure 6: Total cell number in the stroma of control, UVA+UVB and CXL corneas in (A) the wounded central corneal, in (B) the edges of wounded central corneal and in (C) the untreated limbal area as a function of time after treatment.
Figure 6: Total cell number in the stroma of control, UVA+UVB and CXL corneas in (A) the wounded central corneal, in (B) the edges of wounded central corneal and in (C) the untreated limbal area as a function of time after treatment.
† p ≤ 0.05 ; *p ≤ 0.001.
At day 15, the corneal stroma in both groups was repopulated with new cells in an irregular distribution. Both treatments induced an evident stromal strip devoid of keratocytes near the anterior surface (Figures 5B and 5E). The thickness of this strip was pronounced in the central cornea, and there was no difference between the groups. The cell population increased a lot after 15 days; most of all in the UVA+UVB radiated corneas whose population became larger than the control. However, the CXL treated corneas did not reach the control values at this experimental time point.
At day 30, the anterior stroma still showed a keratocyte-free band in UVA+UVB and CXL corneas (Figure 6). In contrast, a great number of cells were situated immediately below the band. At day 30, with both treatments, the number of stromal cells was similar to control. The endothelium and limbic zone were well preserved in both groups during the study and seemed not to be affected by treatments.
Because the depth of damage was relevant, we quantified the number of cells per square millimeter in the anterior, middle and posterior stroma (30,000 μm2 each) at day 1 as well as at day 15 and day 30. At day 1, UVA+UVB radiation and CXL produced a significant decrease of cells in the anterior, middle and posterior stroma in the wound zone, being greater in UVA+UVB group. Similar decreases were found in the edges of the wound in the UVA+UVB group, but the edges were mainly affected in the middle stoma in the CXL group (Figures 7A, 7B and 7C).
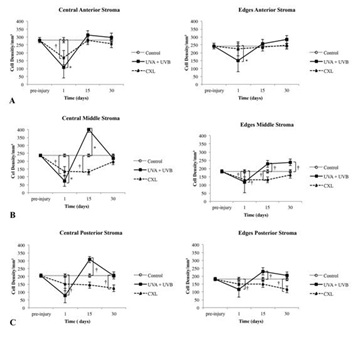 Figure 7: Number of cells in the central, where the wound was localized, and the edges of the wound at different depths in the stroma and at different times pre- and post-treatment. (A) Anterior one-third of the stroma, (B) middle one-third, (C) and posterior one-third.
Figure 7: Number of cells in the central, where the wound was localized, and the edges of the wound at different depths in the stroma and at different times pre- and post-treatment. (A) Anterior one-third of the stroma, (B) middle one-third, (C) and posterior one-third.
†p ≤ 0.05; *p ≤ 0.001.
At day 15, in the UVA + UVB radiated corneas, the population of cells increased a lot in the middle and posterior stroma, and the number of cells was significantly higher than that of the control in the wound zone and edges. The number of stromal cells from CXL treated corneas was recovered in the anterior stroma and nearly recovered in the posterior stroma in the wound zone (and in a similar way in the edges). Nevertheless, the middle stroma in both areas (wound and edges) still had less stromal cells than the control (Figure 7).
At day 30, the recovery of the number of cells was similar in the anterior, middle and posterior stroma of the wound and edges in both groups. However, the UVA+UVB group had a higher population of cells in the middle stroma at the edges as compared with the CXL group. The CXL group was not recovered in the posterior stroma of the wound and edges (Figure 7).
Cell death
One day after radiation, TUNEL-positive cells were detected in UVA+UVB corneas in the anterior, middle and posterior stroma. They were also detected in CXL corneas in the anterior stroma (Figure 8). These areas coincided with the optical zone irradiated and the area scraped to remove the epithelium, respectively.
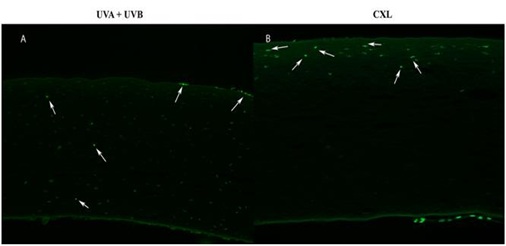 Figure 8: Positive cells TUNEL at day 1 in (A) UVA+UVB in the anterior, middle and posterior stroma and (B) CXL in the anterior stroma.
Figure 8: Positive cells TUNEL at day 1 in (A) UVA+UVB in the anterior, middle and posterior stroma and (B) CXL in the anterior stroma.
There were no TUNEL-positive cells detected in the stroma of both groups at 15 and 30 days after treatment.
Myofibroblast differentiation
No α-SMA-positive cells (myofibroblasts) were observed at any time in the corneas from both treated groups. The artery walls in the limbi were used as positive controls for α-SMA immunohistochemistry.
DISCUSSION
Our study demonstrated that UVA+UVB radiation similar to 1 hour 20 minutes of the direct sun produces epithelial damage that leaves the stroma of the cornea unprotected, even in an animal model with Bowman’s layer, inducing a loss of keratocytes deep in the stroma. On the other hand, chicken corneas subjected to CXL treatment, and hence, to de-epithelialization, did not show such deep damage. In both cases, the recovery was nearly complete with regard to the number of cells after 30 days, being slower in the CLX group as compared to the UVA+UVB group.
We used two medical approaches. One with a high exposition to UV solar radiation that produces several pathologies described as ophthalmohelioses, which include photokeratitis, ulcers, cataracts, pterygium, and age-related macular degeneration. The other, CXL treatment, is used to stabilize infectious corneal ulcers [23], halt keratectasia after LASIK [24], and treat keratoconus [25]. Both procedures have a common photochemical reaction producing Reactive Oxygen Species (ROS) and free radicals, which are responsible for the majority of the damage observed in these corneas [4,26]. The effects of these ROS are cytotoxicity and new bonds generation between collagen fibers.
This study was conducted on chickens as a domestic and easy to handle representation of birds. Birds are exposed to sunlight and have similar corneal histology layers, which includes Bowman’s layer; in addition, these layers have similar proportions to the human cornea. For these reasons, we hypothesized that the reaction to UV radiation would be similar to that of the human eye. Furthermore, chickens represented a good animal model and have been included in many other studies about wound healing [27-30]. There are no studies currently available about the effects of UV radiation in this animal model.
The results of our study showed that the UVA+UVB and CXL groups shared common clinical signs, such as photokeratitis (as red-eye), corneal edema, ulcers, and corneal opacity. These signs were also described in CXL studies made in rabbits [31]. In studies carried out in human patients after CXL treatment, the most common clinical sign found was corneal stromal haze [14,16,32-34]. Although the UVA+UVB group had time to recover (24 hours from one dose to another), the clinical signs worsened at the end of radiation periods, suggesting that the doses were cumulative.
Ulcers in the UVA+UVB radiated corneas healed faster than the de-epithelialized zone in the CXL group. It might be due to the fact that the ulcers in this group were smaller than in the CXL group, with irregular edges that easily stick to the stroma, which undoubtedly provide a faster closure of the wound. The central corneal thickness gradually increased during the study, showing significant edema. This edema was more significant in the UVA+UVB treated corneas as compared to the CXL treated corneas, even though epithelial recovery was later. We believe that less edema in the CXL group corneas was most likely because of the protective effect of dextran, which creates a high osmotic pressure gradient that drives water from the cornea [17,35]. At day 15, the corneal edema receded, and the central corneal thickness was quite similar to the previous day of UV radiation in both groups.
It is well known that UV radiation, as explained before due to ROS radicals, produce DNA damage and finally epithelial and keratocyte apoptosis [2,36-38]. Several studies have demonstrated that 75% of UVA radiation is transmitted through the cornea and absorbed by the lens. UVB light is absorbed by the cornea and, mainly, by the Bowman’s layer (being the absorption in the Bowman’s layer is eight times greater than in the stroma) [6,7,19,39].
Some of these studies were carried out in mice exposed to chronic (3 times a week for up to 50 weeks) UVR (280-400 nm). Corneal lesions were microscopically characterized by marked hypocellularity of the stroma. Acute exposition (one single dose-1.5 times chronic doses) showed rare apoptotic corneal stromal cells [11].
On the other hand, rabbits exposed only to 280 nm (UVB) displayed a loss of epithelial cells in the treated area, but no damage to keratocytes was detected. When this cornea was only manually de-epithelialized, it showed a loss of keratocytes in the anterior quarter to a half of the corneal stroma, with the remaining stroma populated by keratocytes. Meanwhile, UVB plus manual scraping of the corneal epithelium produced serious and involved nearly all the deepness of the stroma. Keratocytes had disappeared throughout the entire thickness of the stroma and in the optical zone, even in endothelial cells [7].
Other authors, also in rabbits, described that UVA radiation alone produced a superficial death of Keratocytes [40]. Nevertheless, Wollensak did not find significant epithelial changes with UVA radiation alone, and there were no apoptotic keratocytes in the underlying stroma [26].
The sum of UVA plus UVB radiation, in our results, was demonstrated to be very harmful, implicating the whole thickness of the cornea. The cell density showed a better number of cells that were described [7] in rabbits after de-epithelialization and UVB radiation, perhaps because the epithelium was maintained.
As shown in our results, CXL treatment produces apoptotic cells in the anterior, middle, and posterior stroma, although cell death is lesser than with UVA and UVB radiation.
Careful studies that determine by cell count and apoptotic labeling the depth and number of dead cells in the stroma showed that, in rabbits, only the anterior two-thirds of the stroma were involved [31,40]. As well studies cared out in human with 12-24 months follow-up have found keratocytes loss in the anterior and intermediate corneal stroma in patients treated with standard CXL and transepithelial CXL [14,16]. Only a previous study from Wollensak showed the whole stroma thickness was implicated [26].
Keratocyte apoptosis is an initiator of the corneal wound healing response and the beginning of the complex cascade events [41,42]. As a consequence of this cascade, at day 15, a regeneration of the stromal cells was produced; being higher in UVA+UVB treated corneas than in those of the CXL treated corneas. This repopulation started deep in the stroma in the UVA+UVB group, being observed above all in the posterior and middle stroma, where, following our results, the number of cells was higher than the control. At day 30, cell proliferation seems to reduce, and the number of cells tends to reach the control values. Nevertheless, in CXL treated corneas, cell proliferation was low.
In keratoconus humans patients treated with CXL, keratocytes repopulation was observed form third month after treatment. The complete repopulation was observed after 6-12 months postoperative in transepithelial CXL, being a slower tendency in the CXL epi-off [14,16].
In spite of the differences between UVA+UVB radiation and CXL procedure, the histological findings were very similar at the end of the experiment. Although the complex wound healing cascade started quickly after treatments, we could not determine when the healing ended.
In conclusion, Bowman’s layer has not been successful in protecting, at least in chicken corneas, from UVA and UVB radiation. It is possible that other elements in the defense against ROS are involved. This study emphasizes the importance of UVA+UVB radiation sunlight exposure and how it can affect the cornea.
ACKNOWLEDGEMENT
The authors thank to Roberto Cantalapiedra and Cristina Herrero-Pérez for their technical assistance and the statistical group of University of Valladolid. Thanks to Proof-Reading-Service.com Ltd for has been professionally proofread this manuscript.
CONFLICT OF INTEREST
The authors have no financial and no conflict of interest to disclose.
REFERENCES
- Lucas RM (2011) An epidemiological perspective of ultraviolet exposure--public health concerns. Eye Contact Lens 37: 168-175.
- Ibrahim OM, Kojima T, Wakamatsu TH, Dogru M, Matsumoto Y, et al. (2012) Corneal and retinal effects of ultraviolet-B exposure in a soft contact lens mouse model. Invest Ophthalmol Vis Sci 53: 2403-2413.
- Lodovici M, Caldini S, Morbidelli L, Akpan V, Ziche M, et al. (2009) Protective effect of 4-coumaric acid from UVB ray damage in the rabbit eye. Toxicology 255: 1-5.
- Cejková J, Stípek S, Crkovská J, Ardan T, Pláteník J, et al. (2004) UV Rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res 53: 1-10.
- Zigman S (1995) Environmental near-UV radiation and cataracts. Optom Vis Sci 72: 899-901.
- Kolozsvari L, Nogradi A, Hopp B, Bor Z (2002) UV absorbance of the human cornea in the 240- to 400-nm range. Invest Ophthalmol Vis Sci 43: 2165-2168.
- Podskochy A (2004) Protective role of corneal epithelium against ultraviolet radiation damage. Acta Ophthalmol Scand 82: 714-717.
- Hayes S, Cafaro TA, Boguslawska PJ, Kamma-Lorger CS, Boote C, et al. (2011) The effect of vitamin C deficiency and chronic ultraviolet-B exposure on corneal ultrastructure: a preliminary investigation. Mol Vis 17: 3107-3115.
- Hwang HS, Kim MS (2013) Ultraviolet-visible light spectral transmittance of rabbit corneas after riboflavin/ultraviolet-A (365 nm) corneal collagen cross-linking. Mol Vis 19: 2113-2123.
- Kozak I, Klisenbauer D, Juhas T (2003) UV-B induced production of MMP-2 and MMP-9 in human corneal cells. Physiol Res 52: 229-234.
- Newkirk KM, Chandler HL, Parent AE, Young DC, Colitz CM, et al. (2007) Ultraviolet radiation-induced corneal degeneration in 129 mice. Toxicologic pathology 35: 819-826.
- Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T (2007) Safety of UVA-riboflavin cross-linking of the cornea. Cornea 26: 385-389.
- Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, et al. (2006) Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg 32: 279-283.
- Mazzotta C, Hafezi F, Kymionis G, Caragiuli S, Jacob S, et al. (2015) In Vivo Confocal Microscopy after Corneal Collagen Crosslinking. Ocul Surf 13: 298-314.
- Cezón J (2009) Técnicas de modelado corneal desde la Orqueratología hasta el Cross-linking. Mac Line, S. L, Sociedad Española de Cirurgía Ocular Implnto-Refractiva
- Bikbova G, Bikbov M (2016) Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol 94: 600-606.
- Hayes S, Morgan SR, O'Brart DP, O'Brart N, Meek KM (2016) A study of stromal riboflavin absorption in ex vivo porcine corneas using new and existing delivery protocols for corneal cross-linking. Acta Ophthalmol 94: 109-117.
- Raiskup F, Theuring A, Pillunat LE, Spoerl E (2015) Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: Ten-year results. J Cataract Refract Surg 41: 41-46.
- Cejka C, Rosina J, Sirc J, Michalek J, Brunova B, et al. (2013) The reversibility of UV-B induced alterations in optical properties of the rabbit cornea depends on dose of UV irradiation. Photochem Photobiol 89: 474-482.
- Wollensak G, Spoerl E, Seiler T (2003) Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135: 620-627.
- Fantes FE, Hanna KD, Waring GO, Pouliquen Y, Thompson KP, et al. (1990) Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol 108: 665-675.
- Efron N, Morgan PB, Katsara SS (2001) Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt 21: 17-29.
- Micelli Ferrari T, Leozappa M, Lorusso M, Epifani E, Micelli Ferrari L (2009) Escherichia coli keratitis treated with ultraviolet A/riboflavin corneal cross-linking: a case report. Eur J Ophthalmol 19: 295-297.
- Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T (2007) Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg 33: 2035-2040.
- Zhang X, Tao XC, Zhang J, Li ZW, Xu YY, et al. (2015) A review of collagen cross-linking in cornea and sclera. J Ophthalmol 2015:289467.
- Wollensak G, Iomdina E, Dittert DD, Herbst H (2007) Wound healing in the rabbit cornea after corneal collagen cross-linking with riboflavin and UVA. Cornea 26: 600-605.
- de la Cera EG, Rodriguez G, de Castro A, Merayo J, Marcos S (2007) Emmetropization and optical aberrations in a myopic corneal refractive surgery chick model. Vision Res 47: 2465-2472.
- Martinez-Garcia MC, Merayo-Lloves J, Blanco-Mezquita T, Mar-Sardana S (2006) Wound healing following refractive surgery in hens. Exp Eye Res 83: 728-735.
- Merayo-Lloves J, Blanco-Mezquita T, Ibares-Frias L, Fabiani L, Alvarez-Barcia A, et al. (2010) Induction of controlled wound healing with PMMA segments in the deep stroma in corneas of hens. Eur J Ophthalmol 20: 62-70.
- Ibares-Frias L, Gallego P, Cantalapiedra-Rodriguez R, Valsero MC, Mar S, et al. (2015) Tissue reaction after intrastromal corneal ring implantation in an experimental animal model. Graefes Arch Clin Exp Ophthalmol 253: 1071-1083.
- Lorenzo-Martin E, Gallego-Muñoz P, Ibares-Frias L, Marcos S, Perez-Merino P, et al. (2018) Rose Bengal and Green Light Versus Riboflavin-UVA Cross-Linking: Corneal Wound Repair Response. Invest Ophthalmol Vis Sci 59: 4821-4830.
- Arbelaez MC, Sekito MB, Vidal C, Choudhury SR (2009) Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: One-year results. Oman J Ophthalmol 2: 33-38.
- Gkika M, Labiris G, Kozobolis V (2011) Corneal collagen cross-linking using riboflavin and ultraviolet-A irradiation: a review of clinical and experimental studies. International ophthalmology 31: 309-319.
- Ting DSJ, Rana-Rahman R, Chen Y, Bell D, Danjoux JP, et al. (2019) Effectiveness and safety of accelerated (9 mW/cm(2)) corneal collagen cross-linking for progressive keratoconus: A 24-month follow-up. Eye (Lond) 33: 812-818.
- Hamaoui M, Tahi H, Chapon P, Duchesne B, Fantes F, et al. (2001) Corneal preparation of eye bank eyes for experimental surgery. Cornea 20: 317-320.
- Fris M, Tessem MB, Cejkova J, Midelfart A (2006) The effect of single and repeated UVB radiation on rabbit cornea. Graefes Arch Clin Exp Ophthalmol 244: 1680-1687.
- Muresan S, Filip A, Muresan A, Simon V, Moldovan R, et al. (2013) Histological findings in the Wistar rat cornea following UVB irradiation. Rom J Morphol Embryol 54: 247-252.
- Tessem MB, Midelfart A, Cejkova J, Bathen TF (2006) Effect of UVA and UVB irradiation on the metabolic profile of rabbit cornea and lens analysed by HR-MAS 1H NMR spectroscopy. Ophthalmic Res 38: 105-114.
- Walsh JE, Bergmanson JP, Koehler LV, Doughty MJ, Fleming DP, et al. (2008) Fibre optic spectrophotometry for the in vitro evaluation of ultraviolet radiation (UVR) spectral transmittance of rabbit corneas. Physiol Meas 29: 375-388.
- Salomao MQ, Chaurasia SS, Sinha-Roy A, Ambrosio R Jr, Esposito A, et al. (2011) Corneal wound healing after ultraviolet-A/riboflavin collagen cross-linking: A rabbit study. J Refract Surg 27: 401-407.
- Bilgihan K, Bilgihan A, Adiguzel U, Sezer C, Yis O, et al. (2002) Keratocyte apoptosis and corneal antioxidant enzyme activities after refractive corneal surgery. Eye (Lond) 16: 63-68.
- Podskochy A, Gan L, Fagerholm P (2000) Apoptosis in UV-exposed rabbit corneas. Cornea 19: 99-103.
Citation: Gonçalves GC, Ibares-Frías L, Mar-Saldaña S, Martínez-García MC (2019) Effects of UVA+ UVB Solar Radiation Levels and Cross-Linking on Animal Model with Bowman’s Layer: Chicken. J Ophthalmic Clin Res 6: 059.
Copyright: © 2019 Gisele C Gonçalves, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

