ABSTRACT
Legg-Calvé-Perthes disease (or Perthes disease) is a disorder of the hip, which is caused by a vascular disorder ischemia of the femoral head and passes through four stages. It affects mostly infants and children of caucasian population. The cause of the ischemia is not yet known and therefore cannot be prevented norreversed. Limping, restriction of the movement and pain can lead to the diagnosis. X-rays as well as Magnetic Resonance Imaging (MRI) confirms it. The classification of Herring (A-C) is helpful to make a clinical prognosis and further treatment. The main aim of therapy is to improve joint the mobility and the joint’s congruence (“containment”), build up muscle strength and reduce pain. Physiotherapy is an undisputed method, lasts over years involving an extensive movement therapy of the affected hip, the torso and the non-affected limb. Short-term drug-related analgesia, botulinum injection into the adductors and a weight relief can accompany therapy. An indication for operative treatment is given by subluxation within stadium B or B/C of Herring. Containment (hip centering/ joint’s congruence) can be achieved by femoral varization osteotomy or pelvic osteotomy. Only one quarter of all patients is treated with surgery and physiotherapy remains the most relevant therapy on Perthes disease.
KEYWORDS
Conservative treatment; Femoral varization osteotomy; Legg-Calvé-Perthes disease; Necrosis of the femoral head; Physiotherapy; Triple osteotomy
Introduction
Legg-Calvé-Perthes Disease (LCPD) is a pediatric disease which affects the hips and is caused by a bleeding disorder within the femoral head,which leads to a necrosis of the femoral head (also known as a “melting of the head”), which then passes through the various stages of disease. The disorder is one of the few within the hip thatcannot be prevented nor reversed. From the orthopedic point of view, physiotherapy plays an important role in the overall treatment of the disease.
An unclear cause one reason behind the lack of control and influence over the disease is due to the inability to find its cause (s). However, research has found that various factors play a role in its development. Next to genetic factors [1,2] and a possible coagulation disorder [3,4], LCPD is caused by a local circulatory disorder [5] on the basis of a skeletal disorder, with delayed growth occurring between the ages of 4-6 years [6-9].
Incidence
The incidence of the disease in the Caucasian population is 8.8 per 100,000 children and adolescents (0-15 years of age) per year [10,11]. In the Asian population it is 1.0 per 100,000 per year and in the black population it is 0.4. Boys are four times more affected than girls.
Clinics and diagnostics young patients can be identified through their limping or through the pain within their hip joints. Sometimes these symptoms are directly associated with limited hip joint mobility. Often the hip pain occurs approximately 6-8 weeks before the radiology’s findings. As a result of a possible collection of liquid within the joint, LCPD can be confounded by a coxitis fugax.
Changes in the scintigram or MRI can already be found at the initial stage. However, given the current state of research, early diagnosis does not alter the subsequent treatment. In any case, the initial stage of LCPD would not be treated, unless a restricted mobility of the hip is present. If this persists for more than two weeks, physiotherapy is prescribed, independent of whether it is a LCPD or a coxits fugax. Six weeks later LCPD can be easily diagnosed, if found to be present on a conventional X-ray image (method of choice) [12]. It is therefore possible to spare the child the MRI examination, which is expensive and invasive due to the anesthesia at this age [13].
Currently it is being tested in various centers, whether an early MRI-diagnosis and early surgical indication have clinical advantages instead of X-rays. A special perfusion-MRI therein specifies the extent of the necrosis of the femoral head (MRI-classifications) [14].
Classifications
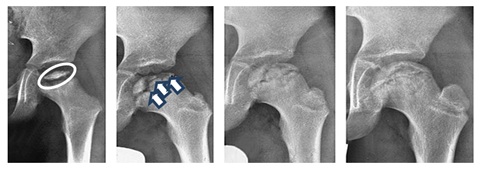
The disease progresses into 4 chronological stages (Figure 1):
• Condensation stage (Ø 7 months)
• Fragmentation stage (Ø 8.5 months)
• Reossification stage (Ø 18 months)
• End stage [15]
The older the child at the beginning of the disease, the longer the individual phases last. All classifications are based on a morphological classification according to an X-ray image [16-18]. However, classifications are only useful if they had also contributed to the prognosis and thus to a decision as to therapy. The Herring classification has proven to be the best method in the last years. In addition to the radiological risk factors, table 1 also shows the clinical factors and their relevance to a poor prognosis. Many long-term studies have verified this [19-22].
Methods
As physiotherapy plays an important role, we made a narrative review about the Perthes disease and its physiotherapy and publish our own treatment concept in this publication. The literature research was taken in the literature on PubMed with the above mentioned keywords. The last current literature was researched on 31.12.2017.
Therapy
The main aim of therapy for LCPD is to improve mobility and the joint’s congruence and relieve the hip joint pain [23,24]. Especially the hip joint mobility, the abduction and the inner rotation of the leg as well as the mobility of the lumbar spine could be improved by physiotherapy. In the long term, a hip arthrosis requiring a prosthetic replacement should be avoided.
According to the duration of the disease, an accompanying therapy has to take place over years with different frequencies. In acute painful phases, short-term drug-related analgesia, botulinum injection into the adductors and a discharge can treat as an adjuvant therapy. With the reduction of pain, better mobility can be quickly achieved as an improvement of the hip congruence, as well as the important adaptation of the head in the conversion to its joint partner, the acetabulum. All long-lasting relief methods (bed rest, relief abduction orthoses and crutches) are psychologically quite stressful. Since therapeutic effect has never been established, we never apply them [13].
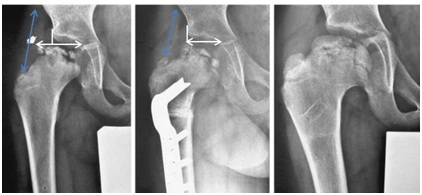
Operative treatment
The maintenance of mobility is a prerequisite to possible operative treatment in order to improve the joint congruence, respectively centering the hip joint. An indication for operative treatment is given if the femoral head is subluxed within Stadium B or B/C of Herring, but with at least an abduction of >10°. Often, children older than 6 years are already in the early stage of fragmentation. Unfortunately no international consensus on a standardized surgical indication and the kind of appropriate surgery exists [25]. The hip centering can be achieved either by centering the femoral head in the acetabulum via an intertrochanteric, femoral varization osteotomy (Figure 2) or centering the acetabulum over the femoral head via a pelvic osteotomy (triple/Salter osteotomy) (Figure 3). Due to many years of experience, good outcomes and results in the literature [26,27], we prefer the pelvic osteotomy of the femoral osteotomy, since there is no shortening of the leg and thus, no change in the lever arm of the abductors. Subsequently there is a lower risk of adduction contracture and a verticalization of the epiphysis, as it is the case with femoral varization osteotomy. However, the surgery (pelvic osteotomy) is technically more difficult. The lever arm of the abductors is also an important factor for the mobility and the stability of the pelvis after successful operative treatment. An unfavorable biomechanically difference occurs between the head center and the trochanter major (insertion of the abductors), if the head becomes flatter at the end stage. The growth of the trochanter major can be stopped by a small percutaneous surgery (epiphysiodesis of the trochanter by 2 screws). The shortening of the lever arm of the abductor is thereby countered. Nonetheless, only a quarter of all children with LCPD are treated with major surgical procedures in our clinic. The main focus of our clinic is to provide physiotherapy towards achieving an increase of mobility within the hip joint.
Conservative Treatment for LCPD: Physiotherapy for patients with LCPD can last several years and places high demands on patients and their families. A major challenge for the physiotherapist is the compliance of the patient. Patients with LCPD usually has a more or less reduced mobility of the hip joint. Initially the restriction is of functional origin, later of structural origin [28] and affects above all the abduction, extension and internal rotation of the hip [28]. In addition the patients suffer a lack of power in their lower extremities and the trunk. Also a leg length discrepancy of the patient’s legs exists. The main points of the treatments are discussed below.
Objective and indication of physiotherapy the outcome of therapy is related to hip joint mobility. For that reason the restoration of the mobility is most important. Physiotherapy is indicated from initial diagnosis.
Medical prescription includes a report and a current X-ray image. Additional relevant diagnostic data needs to be put in writing (e.g., Attention Deficit Disorder, treated with medication, see table 2 for examples). If hip mobility worsens lasting more than 2-3 sessions, the treating physician should be contacted [29].
Physiotherapeutic assessment an exact documentation including the joint status must be made (see table 3 for components).
Possible differential diagnosis should be known by the physiotherapist (e.g., coxitisfugax, epiphyseal dysplasia, chondroblastoma). A status check should be repeated twice a year. This also applies to any extended therapy pauses. The Swiss quality circle "Children’s Orthopedic and its physiotherapy" has created an appropriate medical report.
Frequency of physiotherapy treatment involves an extensive movement therapy, which not only treats the affected hip but also the trunk and the non-affected limb. The frequency of treatment is adapted to each individual patient and must be reevaluated by the physiotherapist. If mobility decreases, the number of sessions should be increased to four times a week. Where hip mobility improves, therapy can be reduced to one or two times per month.
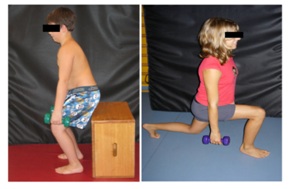
Joint mobility the Range Of Motion (ROM) should be improved passively by manual treatment. At the same time it should be improved by active and passive stretching of the affected muscle. Therefore the bone structures must allow abduction movement. An X-ray image is very important for the therapist. Ideally, after mobilization and stretching the new attained ROM can be exercised with strength-training e.g., squat-lunges or abduction-squats (Figure 4).
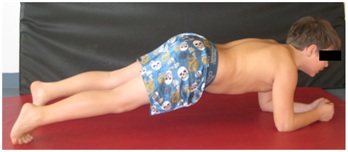
Balance and coordination training balance and coordination training as well as “core stability” (Figure 5) are important elements of every therapy session. To avoid more stress on the hip joint we don’t do any kind of jumps.
Medical advice on LCPD
Medical aid and non-weight-bearing is a highly controversial subject. We only advise use of crutches to provide relief if the patients complain of great pain. We basically reject the use of a wheelchair. We consider a bicycle or a balance bicycle for children. Restricted mobility reduces the quality of life, too much [30]. Furthermore, no evidence supports that the use of a wheelchairresults in a lesser deformation to the femoral head.
Home exercises must be written down. They are adapted to age and to the individual patient. They must be regularly reviewed.
Therapy consensus for Switzerland currently every physiotherapist uses their own exercise program and treatments, depending on experience. Questionnaires concerning the quality of life are still used to rarely. Such questionnaires, especially for “non-curable” diseases, are very important. Various guidelines exist for the treatment of patients with LCPD [31]. We should bring this information and our know-how together, and adapt it accordingly to our environment. Our goal should be to achieve consensus on a treatment in Switzerland to implement it in practice.
Conclusion
LCPD is an idiopathic necrosis of the femur head at the approximate age of 4-8years. Young patients with LCPD are noticed by unilateral limping, hip pain, and absence of trauma, fever and a history of previous infection. Initially the children receive symptomatic treatment with analgesia and physiotherapy. The parents must be informed about the disease pattern and the unavoidable, natural course of the disease.
Physiotherapy plays the most important role in the treatment of the LCPD. Although the cause of the disease cannot be prevented or cured, physiotherapy maintainship joint mobility which is highly important. It is indicated from the initial diagnosis and can last several years. It includes an extensive movement therapy for the affected hip but also the torso and the non-affected limb as well as balance, coordination training and home exercises. The frequency depends on the improvement or deterioration of the hip mobility and the pain. It is not advisable to have an orthotic support or to have a longer-lasting weight relief during the entire period of illness. In particular cases of the decentering of the hip, it is necessary to initiate an operative treatment.
Figures
Figure 1: Four stages of how the disease progresses (left to right): a) 3-year old boy, head of femur within condensation stage. b) Fragmentation stage at the age of 5 (see arrow for fragmentation line). c) Reparation stage at the age of 7. d) End stage at the age of 9, good relation between the height of the femoral head and the trochanter major (see double arrow).
Figure 2: Patient with Perthes disease (Herring type C). a) Typical risk signs on the femoral head with lateral calcification and subluxation (white double arrow). b) Postoperatively following an intertrochanteric varising osteotomy, the abduction lever is shortened (blue double arrow). c) Healing in the end stage, good congruence (=containment) of the femoral head but with leg length discrepancy of 2cm and shorten abductors.
Figure 3: Improvement of the congruence (containment) by triple osteotomy in a nearly 7-year-old girl. a) Preoperative with lateralization and bump development b) Postoperatively, c) 5 years postoperatively. Immediately postoperatively the containment is not ideal, but improves rapidly by further growth and continuous movement. This is a typical development after triple osteotomy in Perthes disease.
Figure 4: The newly gained degree of movement is exercised in its function: a) Squat b)Squat lung.
Figure 5: Training of the core stability.
Tables
Factors of Clinical and Radiological Prognosis | Relevance |
Condition of the lateral hip pillar 1 | +++ |
Lateral calcification 2 | ++ |
Subluxation (“containment”) 2 | ++ |
Age (>6 years) | +++ |
Agility | ++ |
Sex (female) | ++ |
Extent of necrosis 3 | + |
Metaphysical participation 2 | + |
Horizontalization of the growth gap 2 | - |
1. Risk factor according to Herring classification 2. Risk factor according to Catteral/“head at risk-sign” 3. Classification according to Catteral/Salter & Thompson |
Table 1: Prognostic factors in LCPD and their relevance to the prognosis for the end stage: Clinically the age (<6 years) at the first diagnosis of the disease, the poor mobility and the female sex, radiographically a preservation of the lateral head <50% (=Herring C), as well as the subluxation and the lateral calcification are factors for a bad prognosis of large (=++) or very large (=+++) relevance.
Prescription of long-term therapy with a clear diagnosis |
Example: LCPD right leg, stage 3 Length length discrepancy of 2cm Hyperlordosis of the lumbar spine Attention Deficit Disorder/Specific Learning Disability (SLD) |
Table 2: Example of a prescription.
Medical Assessment of LCPD |
Anamnesis/Medical report Pain (with the visual analogue scale) Passive and active ROM of the lower extremities as well as the lumbar spine (with a goniometer) Strength of the entire lower extremity (usually with a hand-held dynamometer) Statics, including indirect leg length testing (with small boards put underneath) Walking (walking analysis or video recording) Balance (standardized assessment) |
Table 3: Medical assessment of LCPD