
Full Recovery after Spinal Cord Injury and Cauda Equina Syndrome from Thoracolumbar Interlaminar Epidural Steroid Injection: Case Report and Literature Review
*Corresponding Author(s):
Rachel Yinfei XuMount Sinai Health System, New York, United States
Email:Rachelyxu88@gmail.com
Abstract
Background
Since the first Epidural Steroid Injection (ESI) in 1952, advancements in imaging and refinements in technique have greatly improved safety measures. Although potential benefits are significant and devastating complications are rare, interventionalists must be prepared to manage such outcomes.
Objective
1) To present an atypical case of Spinal Cord Injury (SCI) and superimposed Cauda Equina Syndrome (CES) after ESI, with full recovery through comprehensive care.
2) To review literature on timing of surgery.
3) To discuss management of procedural complications.
Study design
Case report and literature review.
Setting
Tertiary inpatient rehabilitation.
Results
Our patient is a 79-year-old female with a large T11-12 herniated disc who underwent fluoroscopy-guided T12-L1 ESI and sustained an acute SCI. MRI showed enlarged herniation causing cord compression. She had early symptomatic improvement that suggested transient injury, so urgent surgery was deferred for conservative management and acute inpatient rehabilitation. While under close observation, the team recognized an unexpected turn of neurological deterioration attributed to her pre-injection pathology. The patient subsequently underwent decompressive surgery. Comprehensive rehabilitative measures were continued and by 4-month outpatient follow-up, she had made a dramatic full recovery.
Discussion
Complication management decisions are complex and require interdisciplinary conversations, close clinical observation, and up-to-date knowledge of literature. We will explore the possible mechanisms of injury, review literature on indications for ESI and discuss in detail the controversial surgical timing in acute SCI, CES and central cord syndrome. An understanding of surgical indications is valuable for all treating teams as this will expedite recognition of neurologic deterioration and timely escalation of care. Regardless of management decisions, physician must help patients set realistic expectations for outcome and provide surgical, rehabilitative, psychological and social services as an integral part of care.
Conclusion
This case demonstrates that with comprehensive complication management, sub-optimal circumstances can be optimized to facilitate the best potential for recovery.
Keywords
BACKGROUND
CASE REPORT
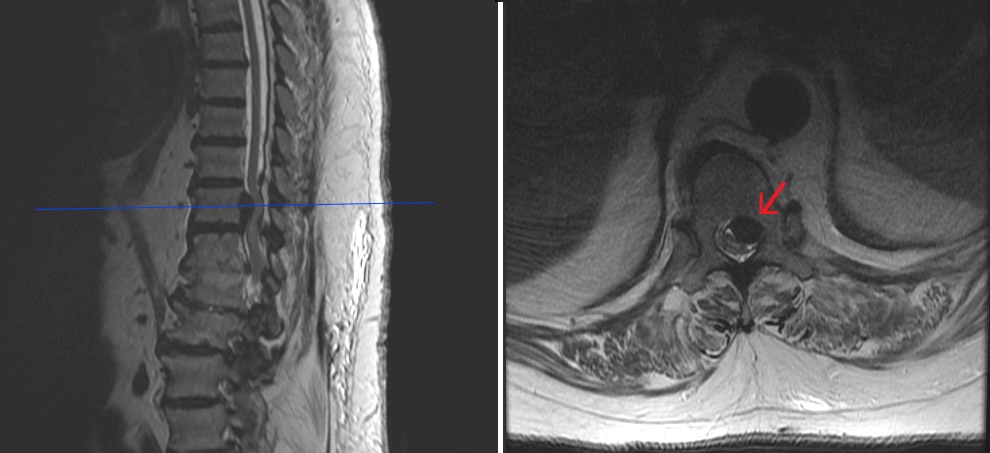
Figure 1: Prior to ESI showing large T11-12 extrusion with spinal cord impingement.
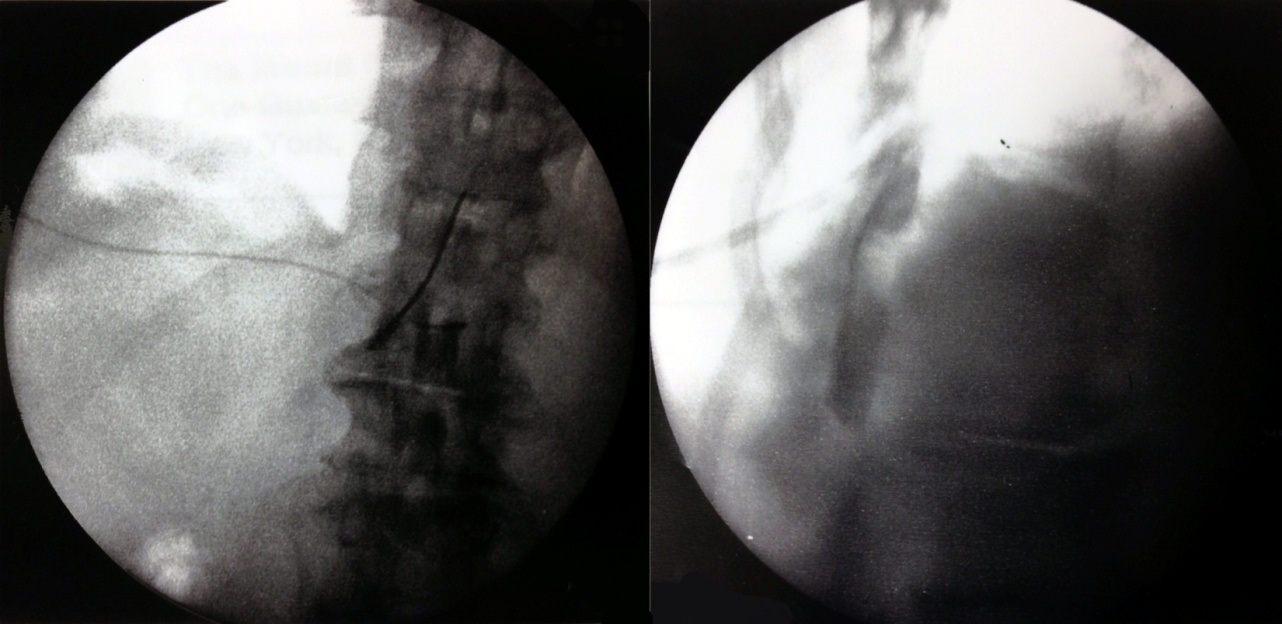
During needle advancement, patient experienced sudden, excruciating left leg pain so practitioner retrieved the needle and waited for acute pain to resolve. After repositioning the needle and ensuring no flow on syringe pullback, 1cc of iohexol was injected. Good laminar flow was visualized and 3cc of 10mg dexamethasone with 2cc of normal saline was administered (Figure 2). Immediately after the procedure, she developed left leg flaccid paralysis, paresthesia and severe pain. She was transferred to the ED where stat MRI showed enlarged T11-12 herniation causing cord shift, enhancement within herniation and increased signal intensity with in compressed cord at T11 (Figure 3). Within 3 hours, motor and sensation had already begun to return. There was no saddle anesthesia, bowel or bladder incontinence. Despite MRI findings, orthopedic-spine surgeon felt conservative management with observation and intravenous dexamethasone outweighed risks of emergent decompression. Symptoms remained stable and patient was transferred to acute inpatient SCI rehabilitation a week later. Pain physician who administered the injection continued extensive discussions with the patient and family members and was in close contact with the inpatient treating teams.
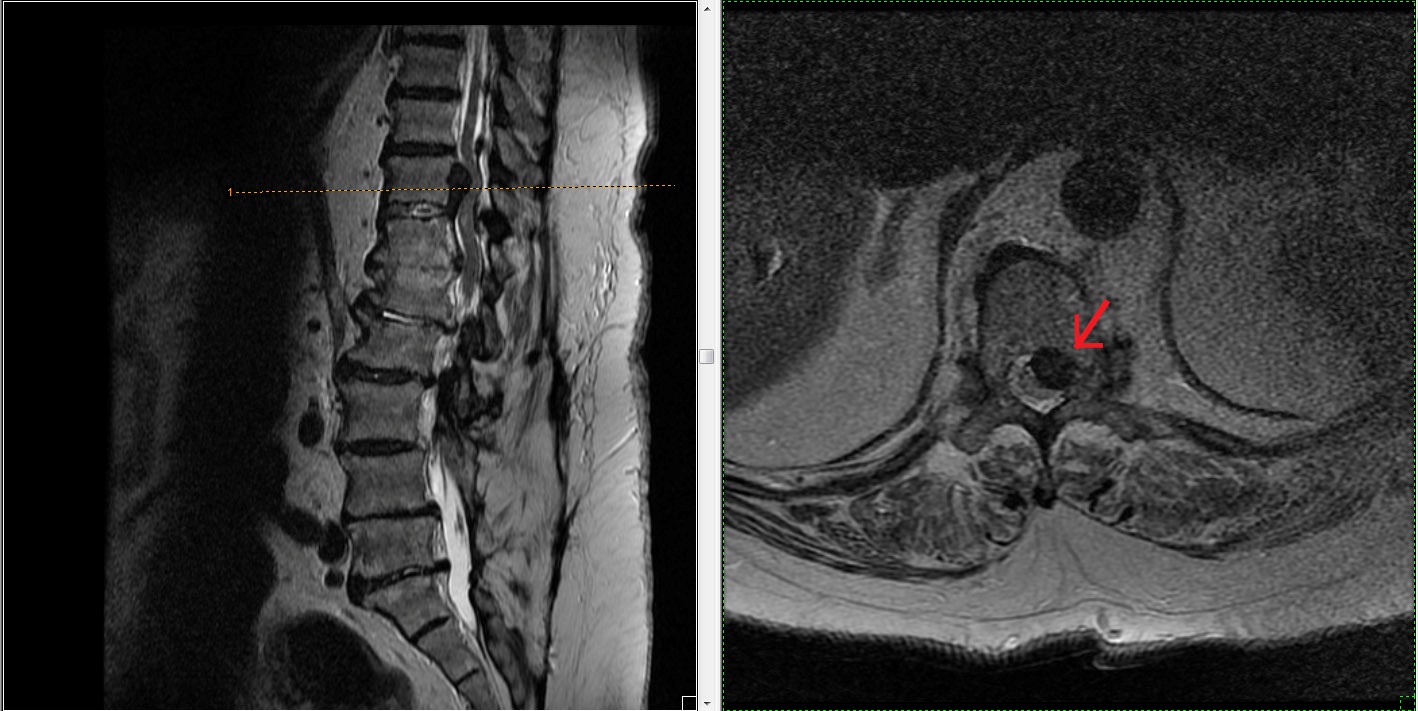
On the SCI unit, admission exam revealed incomplete L1 AIS C with lower extremity motors score of 17/25 including 3/5 strength in both proximal leg muscles, 1 to 2/5 distally, decreased leg and rectal tone with impaired sacral sensation. She was started on progressive therapy, which became intolerable as pain severely worsened over the following days despite medical management on opioids and pregabalin. She also developed new urinary retention and constipation requiring urinary catheter and daily bowel program. Because of these progressive CES symptoms, she underwent surgical decompression with T11-12 laminectomy and discectomy. Pain improved dramatically and therapy was quickly resumed, however left leg paresis continued to persist. Further workup with Electrodiagnostic Studies (EDS) showed diabetic polyneuropathy and right L2, 3 and left L2 through S1 polyradiculopathy, such as that seen in CES or multi-level spinal stenosis.
On discharge to subacute rehabilitation facility a month later, right leg strength improved but remained weak on the left. She was able to ambulate 50-75’ with rolling walker and assistance. However, she still required bowel program and urinary catheter. She never developed Upper Motor Neuron (UMN) signs such as limb spasticity. Clinical symptoms remained consistent with Lower Motor Neuron (LMN) injury. Rehabilitative services were continued in the community and by 4-month follow-up with long-term pain physician, her pain and function had returned to pre-injury baseline with full bowel and bladder recovery. FIMTM score improved from 46/126 on admission to 105. Our patient was in great spirits and genuinely grateful for her care.
DISCUSSION
Why was surgery delayed until 3 weeks after injury?
Management decisions are not laid out in an algorithmic flowchart but require interdisciplinary conversations, close clinical observation, and up-to-date knowledge of literature. This discussion will explore our patient’s possible mechanisms of injury as their recognition is the first step to prevention. Literature pertaining to the timing of surgical intervention for acute SCI, CES and Central Cord Syndrome (CCS) will be reviewed. It is ideal for all primary treating teams to have an understanding of the different surgical indications for the different entities above, as it will expedite escalation to appropriate care.
Epidural injection indications and complications
Major complications from thoracic and lumbar epidurals are rare. One study determined the overall complication rate in thoracic injections to be 4.1%, all of which were minor except one avoidable pneumothorax [11]. In 10,000 fluoroscopic-guided epidural injections, Manchikanti et al., confirmed that minor side effects were common but major complications were rare [2]. Potential complications include infection, estimated at 1-2% of lumbar injections [3], hematoma, intravascular injection, nerve damage, subdural injection, air emboli, disc entry and hypersensitivity, most of which are avoidable with standard precautions. Hematomas may compress nerves in an already crowded space and cause further neurological compromise. Intravascular injections are more common with TF injections. The risk of dural puncture can be minimized by using an oblique trajectory [12]. When recognized, dural punctures can occur without significant sequela other than spinal headache; otherwise, further advancement of needle leads to direct neurologic injury. Subsequent intrathecal injection of anesthesia, particularly with hyperbaric types such as bupivacaine, transiently blocks sodium channels in spinal nerves and may cause transient bowel and bladder impairment, respiratory depression, cardiac symptoms and complete spinal anesthesia [13,14].
For our patient the mechanism of injury had a wide differential. Hematoma or loculated contrast can mimic the appearance of enlarged T11-12 herniation, especially with the steep craniad angle of entry in a T12-L1 injection. This was less likely as epidural needle position was confirmed on fluoroscopy and vascularization within the herniation suggested chronicity over acutely-induced extrusion. Despite accurate location, injection of volume into a highly stenotic area risks further compression of already compressed structures. Injection of adjacent epidural spaces is considered in such cases. Finally, needle pressure on the hypertrophied ligamentum flavum may have caused buckling onto the neurological structures. Both direct and indirect trauma lead to cord edema correlating with increased signal intensity at T12-L1. In addition, several factors may have contributed to the worsening herniation seen on MRI (Figures 1 and 3) such as positional changes, increased intrathoracic pressure during exertion, inadvertent manipulation during ESI, or simply the natural progression of the disease.
Literature review of surgical timing
We chose conservative management with surgery deferred until a later date under optimal rather than emergent conditions. There is substantial risk in urgent surgical decompression of the inflamed cord as the combination of anesthesia and decompression can cause deadly spinal hypotension [13,14]. Alternatively, after acute instability is ruled out, CCS can be managed with close observation and intravenous steroids started within 8 hours and continued for 24-48 hours, shown to be beneficial in the NASCIS trial [15]. In the setting of neurological improvement, risks of surgery outweighed the benefits and it was not until CES symptoms presented that the benefits of surgery outweighed the risks. Though SCI was initially suspected, persisting symptoms of poor rectal tone, urinary retention and leg weakness suggested LMN picture of CES. Patient also had a history of diabetic neuropathy which complicates the clinical diagnosis, however CES was further supported by EDS showing polyradiculopathy. EDS may be of limited value in evaluating Upper Motor Neuron (UMN) injury however, studies have shown peripheral nervous system degeneration following SCI in the form of spontaneous activity and decreased sensory and motor amplitudes [16]. For our patient, diagnostic and clinical evidence suggested a combination of transient acute cord trauma related to ESI with superimposed progressive CES from enlarging thoracic herniation.
CES is a rare condition with incidence of 1 in 33,000 to 1 in 100,000 [17] and comprises 2-6% of lumbar disc operations [18-20]. Red flag symptoms include severe LBP, sciatica especially when bilateral, saddle/genital sensory deficits, and bladder and bowel dysfunction. Most common causes are large lower lumbar disc herniation [21] and less commonly epidural hematoma, infections, trauma, and spinal anesthesia. The syndrome is further distinguished into incomplete (CES-I) with impaired urinary sensation, urgency to void, and partial saddle anesthesia versus complete (CES-R) with painless urinary retention and perineal sensory loss. The bulbocavernosus reflex is useful in evaluating for sacral nerve root function. Similar to acute SCI, CES generally requires urgent surgical treatment with definitive time-frame complicated by variable presentations. Much of literature is cautious about making dogmatic statements in the setting of an ill-defined condition with high medico-legal profile. Literature cites from within 24 to 48 hours of onset [22,23] to bold statements that emergent surgery does not influence recovery for those with CES-R [1,24]. CES-I has better prognosis where early surgery within 48 hours is recommended for those with progressive symptoms.
Complication management
Throughout the hospital course, our patient continued to have persisting CES symptoms after surgical decompression. Regardless of management route 20% of all CES still end up with poor outcomes [1] and recovery of bladder and sexual dysfunction can continue for many years, likely a reflection of compensatory strategies [24]. Given the complex medical decision making and highly varied degrees of recovery, good communication with the patient and family members will improve rapport, informed decision-making, and overall patient-care. It is imperative that physicians discuss prognosis so that expectations remain realistic. Social and psychological support will help patients accept their condition and focus their efforts on rehabilitation rather than on taxing medico-legal endeavors. Such interdisciplinary efforts improved our patient’s quality of life and continue to maximize independence in the community after a full neurologic recovery (See Checklist).
Complication management checklist
- Minimize risk
- Sterile technique, image-guidance, digital subtraction imaging, lidocaine safety test, choice of injectate mixture
- Recognition of injury
- Close observation, thorough physical exam
- Timely escalation of care
- Transfer to ED, immediate surgical evaluation, verbal turnover
- Interdisciplinary discussion
- Physiatrists, surgeons, therapists, nurses, psychologists, social workers
- Literature support
- Tailor evidence-based medicine to individual cases
- Communication
- Explain injury to patient and family members
- Discuss options and set realistic expectations for prognosis
- Rehabilitative services
- Comprehensive therapy program, psychological and social services
- Continued community support after discharge
- Review case for future prevention
CONCLUSION
Regardless of the medical routes chosen, conversations with interdisciplinary teams, patient and family members will improve rapport and establish realistic expectations for prognosis, all of which in turn impact risk management issues. We believe this case demonstrates that by being advocates for our patients and through providing comprehensive care, sub-optimal circumstances can be optimized to facilitate the best potential for recovery.
REFERENCES
- Gardner A, Gardner E, Morley T (2011) Cauda equina syndrome: a review of the current clinical and medico-legal position. Eur Spine J 20: 690-697.
- Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, et al. (2012) A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician 15: 131-140.
- Goodman BS, Posecion LWF, Mallempati S, Bayazitoglu M (2008) Complications and pitfalls of lumbar interlaminar and transforaminal epidural injections. Curr Rev Musculoskelet Med 1: 212-222.
- Fehlings M, Sekhon L (2000) Cellular, ionic and biomolecular mechanisms of the injury process. Manag Spinal Cord Inj.
- Raslan AM, Nemecek AN (2012) Controversies in the surgical management of spinal cord injuries. Neurol Res Int 2012: 417834.
- Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte DW, et al. (2012) Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 7: 32037.
- Vanichkachorn JS, Vaccaro AR (2000) Thoracic disk disease: diagnosis and treatment. J Am Acad Orthop Surg 8: 159-169.
- Manchikanti L, Singh V, Cash KA, Pampati V, Falco FJ (2014) A randomized, double-blind, active-control trial of the effectiveness of lumbar interlaminar epidural injections in disc herniation. Pain Physician 17: 61-74.
- Bogduk N (2013) Applications of Thoracic Transforaminal Access. In: Practice Guidelines for Spinal Diagnostic and Treatment Procedures. International Spine Intervention Society, San Francisco, USA. Pg no: 346-349.
- Manchikanti L, Cash KA, McManus CD, Pampati V, Benyamin RM (2014) Thoracic interlaminar epidural injections in managing chronic thoracic pain: a randomized, double-blind, controlled trial with a 2-year follow-up. Pain Physician 17: 327-338.
- Wang A, Pilgram TK, Gilula LA (2011) Immediate complications and pain relief associated with 296 fluoroscopically guided thoracic foraminal nerve blocks. AJR Am J Roentgenol 197: 1410-1416.
- Cousins MJ, Bridenbaugh PO (1988) Epidural neural blockade. Neural Blockade in Clinical Anesthesia and Management of Pain. Pg no: 8253-8360.
- Nowak DD, Lee JK, Gelb DE, Poelstra KA, Ludwig SC (2009) Central Cord Syndrome. J Am Acad Orthop Surg 17: 756-765.
- Lenehan B, Fisher CG, Vaccaro A, Fehlings M, Aarabi B (2010) The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976) 35: 180-186.
- Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, et al. (1984) Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251: 45-52.
- Riley DA, Burns AS, Carrion-Jones M, Dillingham TR (2011) Electrophysiological dysfunction in the peripheral nervous system following spinal cord injury. PM R 3: 419-425.
- Anthony S (2000) Cauda equina syndrome. Medical Protection Society, UK Casebook 20: 9-13.
- Neininger OV (2008) EWHC 548 (QB), Casecheck, Dundee, UK.
- Gleave JR, Macfarlane R (2002) Cauda equina syndrome: what is the relationship between timing of surgery and outcome? Brit J Neurosurg 16: 325-328.
- Harrop JS, Hunt GE, Vaccaro AR (2004) Conus medullaris and cauda equine syndrome as a result of traumatic injuries management principles. Neurosurg Neurosurg Focus 16: 4.
- Kostuik JP (2004) Medico-legal consequences of cauda equina syndrome an overview. Neurosurg Focus 16: 8.
- Ahn UM, Ahn NU, Buchowski JM, Garrett ES, Sieber AN, et al. (2000) Cauda equina syndrome secondary to lumbar disc herniation: a meta-analysis of surgical outcomes. Spine (Phila Pa 1976) 25: 1515-1522.
- Kohles SS, Kohles DA, Karp AP, Erlich VM, Polissar NL (2004) Time-dependent surgical outcomes following cauda equina syndrome diagnosis: comments on a meta-analysis. Spine (Phila Pa 1976) 29: 1281-1287.
- Gleave JR, Macfarlane R (1990) Prognosis for recovery of bladder function following lumbar central disc prolapse. Br J Neurosurg 4: 205-209.
- Manchikanti L, Falco FJ, Singh V, Benyamin RM, Racz GB, et al. (2013) An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part I: introduction and general considerations. Pain Physician 16: 1-48.
- Manchikanti L, Pampati V, Falco FJ, Hirsch JA (2013) Assessment of the growth of epidural injections in the medicare population from 2000 to 2011. Pain Physician 16: 349-364.
Citation: Xu RY, Kahn SB, Baeza-Dager J, Qureshi SA, Kirschner J, et al. (2016) Full Recovery after Spinal Cord Injury and Cauda Equina Syndrome from Thoracolumbar Interlaminar Epidural Steroid Injection: Case Report and Literature Review. J Phys Med Rehabil Disabil 2: 013.
Copyright: © 2016 Rachel Yinfei Xu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

