
Safety Versus Toxicity of Inhaled Dextromethorphan in the Mice: Hematological, Biochemical and Histological approaches
*Corresponding Author(s):
Avi A WeinbroumDepartment Of Animal Laboratory, Tel Aviv Sourasky Medical Center And The Sackler School Of Medicine, Tel Aviv University, Tel Avic, Israel
Tel:+972 35440904,
Fax:+972 35440904
Email:draviw@tasmc.heatlh.gov.il
Abstract
Dextromethorphan (DM), an antitussive, was previously proven to effectively reduce cough when pre-medicating patients before bronchoscopy. The purpose of this study was to evaluate the safety vs. toxicity of inhaled DM in animals.
Methods
Female BALB/c mice were exposed to repeatedly administered aerosolized DM of 1 or 10mg/kg (safety phase) or 1, 60 or 360mg/kg (toxicological phase). Control animals were exposed to 0.9% NS-based aerosol. At the end of each series of treatments histological, hematological and toxicological analyses were performed on collected samples.
Results
Treatment-related microscopic lesions were observed only in the lungs, ranging from negligible to severe and diffused alveolar damage (DAD). These findings were detected 5 days after the end of 5-session trials of the 10mg/kg dose and 24 h after 15 inhalations of the toxic doses, respectively. The 1mg/kg dose was innocuous. No damage was detected after single sessions; nor were there biochemical or hematological pathologies detected in any of the groups.
Conclusion
A direct correlation may exist between DM dosing and the resulting severity of lung histopathological damage. Additional variables inducing it were multiplicity of inhalations and the time elapsing between the last session of inhalation and the pathological findings of DAD. The single dose of 1mg/kg DM, that is above the clinically accepted DM oral dose, proved safe in the tested animals.
Keywords
INTRODUCTION
Based on these results, we assumed that inhaled DM, if applied in patients immediately before bronchoscopy, especially when they are on NPO regimen, would equally benefit them. Following this rationale, we aimed at testing the effectiveness of inhaled DM, as could be obtained clinically in a fasting patient scheduled to undergo bronchoscopy.
Nevertheless, it has been previously demonstrated that 10 and 20mg/kg of DM, when administered in rats who underwent superior mesenteric artery clamping-unclamping, attenuated the increase in peak ventilatory pressure by 85%, reduced the PO2/FiO2 ratio by 45%, allowed for 4-12-fold increase in bronchoalveolar lavage-retrieved volume, and bettered the polymorphonuclear leukocytes/bronchoalveolar cells ratio [2]. Ischemic-reperfused-40mg/kg DM-treated animals demonstrated worse lung parameters. The lung tissue total xanthine oxidase activity, the reduced glutathione, and the wet-to-dry weight ratio, also remained within normal ranges in the two lower dose-treated groups, as compared to the higher one. The former regimens were also associated with longer post-experimental animal survival vs. the latter, all suggesting DM dose-dependent lung DAD.
This study thus employed non-operated mice inhaling low and high doses of DM, and whose organs and blood samples were investigated after 1, 5 or 15 consecutive sessions.
METHODS
Animals
Nebulization and Characterization of the Aerosol Particles
The sizes of the DM particles were characterized by Mass Median Aerodynamic Diameter (MMAD), their Geometric Standard Deviation (GSD), and the concentration of the aerosolized compound. These were determined using the Cis-100 Analyzer (Stanford Research Systems Inc., Sunnyvale, California, USA) and an analyzer’s video channel (Ankersmid, Yokneam, Israel). The Ankersmid video analyzer was connected to the Cis-100 Analyzer, thus allowing to procure the MMAD and GSD characteristics. To determine the concentration of DM in each of the various solutions, aliquots were collected from the Aeroneb Pro apparatus at the end of each session, and were measured by the Cis-100 analyzer.
Study Design
The second phase consisted of toxicological investigations following DM given at doses of 1, 60, 360mg/kg. Each (30-min) session was repeated 15 times to each animal, 5 times/week. The analyses in this phase were undertaken 24h past the last session each animal underwent. Noteworthy, each phase of the study comprised a control group that was exposed to aerosolize NS only.
The estimated total deposited amounts (D) of the inhaled drug in the solutions were calculated by the following formula [4]:
Animal handling and execution: The animals were weighed every other day. While exposed to the aerosol, all mice were placed unrestrained in a sealed plastic cage. Following each exposure, the animals were returned to their original cages and observed for signs of misbehavior, anxiety, or sedation [7,8].
At due time, the animals were weighed, then anesthetized intraperitoneally with ketamine plus xylazine (100mg/kg, 10mg/kg, respectively), and autopsied. Blood samples for clinical chemistry and general hematology were obtained via a direct cardiac puncture. Then, all internal organs were removed, weighed, and were subjected to pathological examinations. Analyses were performed by skilled individuals who were blinded to the protocol the materials were related to.
Hematology and blood biochemistry: Blood cells were counted by an automatic blood cell counter available at the institutional hematology laboratory (Becton Dickinson, Franklin Lakes, NJ, USA) and blood serum ingredients (urea nitrogen, creatinine, total bilirubin, alkaline phosphatase, aspartate transaminase, alanine transaminase, total protein, albumin, and globulin) were analyzed using an appropriate automated analyzer (Roche Diagnostics Corporation, Indianapolis, IN, USA).
Gross and microscopic pathology: The organs were initially fixed in 10% neutral buffered formalin for histopathological evaluation. After the fixation, the tissues were embedded into paraffin and sections of 5-µm thickness and were stained with hematoxylin-eosin. Whenever organ microscopic lesions were identified, they were graded for severity and evaluated by the pathologists. All histopathological studies were performed by the same protocol-blinded observers.
Statistical Analysis
RESULTS
Aerosol Characteristics
Overall Animal Behavior, Gross Pathology and Blood Analyses Data
No significant changes in the animals’ and organs’ weights were detected at the end of the study (Table 1). The hematological and biochemical analyses that followed both the safety and the toxicity phases of the study were similar among all the groups of animals. Table 2 and Table 3 reflect sampled data.
|
DM dose |
Body |
Lungs |
Heart |
Liver |
Spleen |
Kidney |
|
NaCl 0.9% |
21.9 (20-22.7) |
0.25 (0.231-0.278) |
0.141 (0.123-0.153) |
1.12 (1.132-1.229) |
0.111 (0.91-0.125) |
0.157 (0.130-0.170) |
|
60mg/kg |
21.1 (20-23.3) |
0.257 (0.230-0.280) |
0.137 (0.120-0.160) |
1.113 (1.100-1.380) |
0.117 (0.90-0.130) |
0.177 (0.150-0.200) |
|
360mg/kg |
23.7 (23.1-24.3) |
0.267 (0.260-0.290) |
0.16 (0.160-0.161) |
1.333 (1.250-1.410) |
0.127 (0.90-0.170) |
0.177 (0.140-0.190) |
|
DM dose |
WBC |
RBC |
Hb |
Hct |
MCV |
MCH |
MCHC |
RDW |
|
NaCl 0.9% |
4.9 (4.1-5.6) |
8.8 (7.3-9.2) |
12.3 (12.1-13.6) |
40.2 (37.7-46.1) |
51 (50.5-52.5) |
16.1 (15.7-16.9) |
32.3 (31.0-31.8) |
16.5 (16.1-17.7) |
|
60mg/kg |
4.5 (4.4-4.6) |
7.8 (7.3-8.2) |
12.9 (12.1-13.6) |
40.4 (37.7-46.1) |
52 (51.7-52.3) |
16.6 (16.0-17.2) |
31.9 (31.7-32.0) |
16.9 (16.7-17) |
|
360mg/kg |
5.1 (4.1-6.1) |
6.9 (6.9-7.4) |
11.3 (10.3-12.3) |
35.2 (32.5-37.9) |
50.1 (50.2-51.5) |
16.3 (15.8-16.7) |
32 (31.5-32.5) |
16.5 (16.2-16.9) |
|
DM dose |
Urea |
Creatinine |
Na |
K |
Cl |
GOT |
GPT |
|
NaCl 0.9% |
22.0 (20-26) |
0.40 (0.39-0.51) |
150 (149-151) |
3.7 (3.3-3.5) |
121 (118-122) |
66.5 (52-92) |
32 (24-51) |
|
60mg/kg |
21.3 (19-28) |
0.42 (0.34-0.49) |
151 (151-152) |
3.4 (3.3-3.5) |
123 (119-124) |
66.5 (51-82) |
34 (22-44) |
|
360mg/kg |
23 (20.1-26.3) |
0.45 (6.9-7.4) |
150.5 (150.3-152.3) |
3.87 (3.8-3.9) |
119 (117-119) |
57 (49-65) |
30.5 (27-34) |
Histopathology
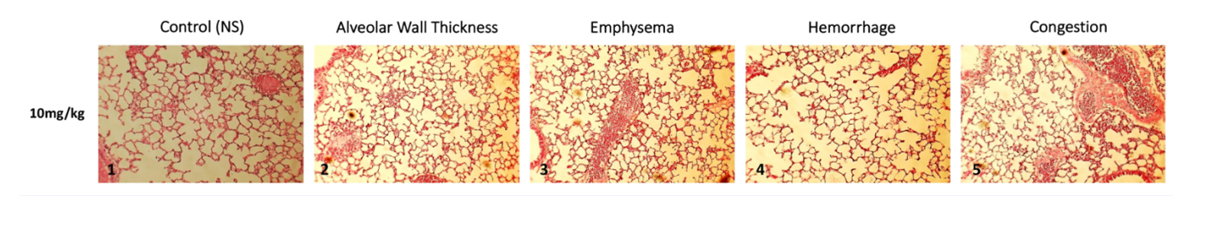 Figure 1: Lung histopathological findings following 5 consecutive DM 10 mg/kg inhalations.
Figure 1: Lung histopathological findings following 5 consecutive DM 10 mg/kg inhalations.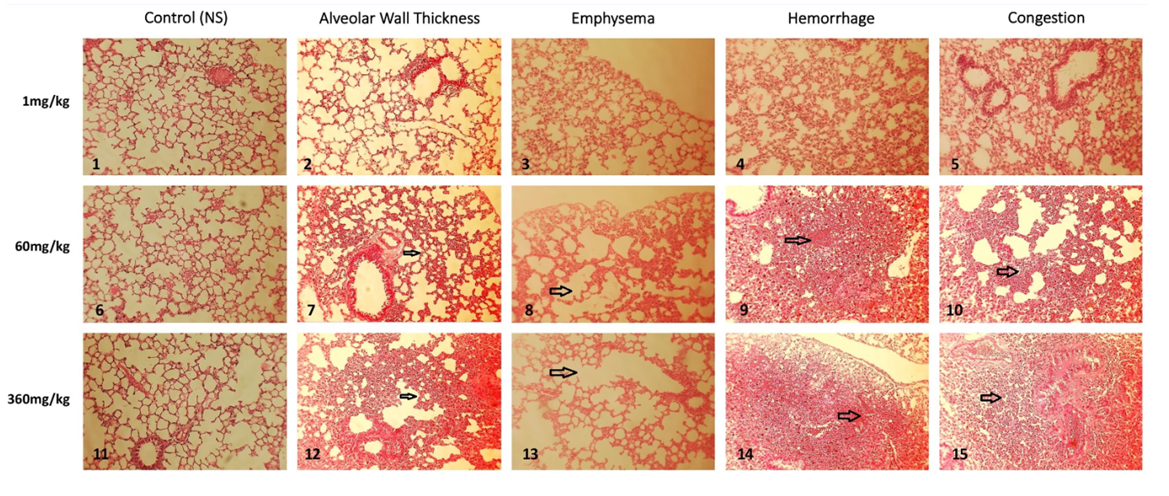 Figure 2: Lung histopathological findings following 15 consecutive inhalations.
Figure 2: Lung histopathological findings following 15 consecutive inhalations.Toxicology Study Phase: Lung damage that followed DM-60mg/kg treatments was characterized by mild thickening of the alveolar walls, appearance of peripheral emphysema, multiple limited intra-alveolar hemorrhagic spots, associated with occasional atelectasis areas, and mild-to-moderate congestion (Figure 2).
Following the use of DM at 360mg/kg for 15 consecutive times, prominent findings of alveolar damage became obvious (Figure 2). These included intense lymphocytes infiltration into alveolar walls, peripherally and centrally-located areas of emphysema, appearance of multiple intra-alveolar hemorrhagic areas, extended atelectasis, and congestion. Noteworthy, the left-sided column, comprising pictures No. 1, 6, and 11, represent the controls for each of the study groups.
DISCUSSION
This is the first study of its kind showing tangible correlations between lung damage and three DM variables that appeared to affect it: (1) the higher was the concentration of DM that the animal inhaled, the more severe appeared the damage. The 10mg/kg dose, but not the 1mg/kg, generated minimal histopathological changes; (2) nevertheless, the 10mg/kg-induced changes could be detected only after 5 consecutive sessions but not after a single one; (3) the above traces of damage apparently developed slowly, requiring at least 5 days before their appearance. From the clinical and laboratory aspects of the data, animals did not demonstrate any abnormal behavior, even when the inhaled doses were as high as 60 or 360mg/kg [10]. The detection of lung pathological damage as soon as 24h after the 15-session-exposures, would support the authors’ suggestion that both the concentration and the repetition of inhalation are relevant factors, since the 1mg/kg dose administered multiple times was innocuous as were the findings after 5-times inhalations of 10mg/kg.
Many drugs have been associated with pulmonary manifestations of interstitial inflammation and fibrosis, bronchospasm, pulmonary edema, and pleural effusion [11]. Among various medications, chemotherapeutic agents have been reported to exert toxicity-dependent interstitial lung disease (ILD) [12,13]. Drugs can virtually produce all histopathological patterns of interstitial pneumonia, including hypersensitivity pneumonitis, organizing pneumonia, diffused alveolar damage (DAD), eosinophilic pneumonia, pulmonary hemorrhage, and granulomatous pneumonitis [14,15]. The mechanisms by which they occur include both direct and indirect pulmonary toxicity, via inflammatory reactions of various lung components.
The herein presented pathological pattern of post-DM inhalation was consistent with acute interstitial pneumonia that is characterized by intra-alveoli and alveolar septal infiltration of small lymphocytes and varying numbers of plasma cells. The histological basis of acute interstitial pneumonia is the DAD, which is characterized by the appearance of hyaline membranes in the acute stage, and interstitial fibroblast proliferation in the late (organizing) stage. The latter histological feature illustrates the characteristic diffused interstitial thickening. Lung injury further results in sloughing of alveolar epithelial cells, causing the formation of protein-rich hyaline membranes. Neutrophils adhere to the activated capillary endothelium and then migrate into the alveolus and the septae [2]. Loss of alveolar capillary membrane barrier integrity induces the accumulation of a neutrophilic inflammatory exudate in the interstitium and air space.
In addition to hyaline membranes and proliferation of interstitial fibroblasts, several other histological findings are variably present in DAD. These include alveolar collapse/atelectasis, edema within the alveolar septa, thrombi within small pulmonary arteries, mild interstitial chronic inflammation and Diffused Arterial Hemorrhage (DAH) [16-18]. Most of the above pathological features were variably present in the animals that were repeatedly treated with moderate-high aerosol DM concentrations. Contrarily, the lower dosages, as would be the case in clinical practice, did not induce lung damage, even allowing inflammatory reactions evolve over a 5-day period.
It is yet unclear how DM directly induces a similar inflammatory reactions, unless an intermediary compound is involved, which requires time to recruit it and engineer its action, as demonstrated herein [19]. Overdose-related phenomena derive mostly from post-recreational systemically-used DM; they are related to mild-to-severe central stability and cognitive losses as the dose is acutely consumed [20,21].
Adverse effects associated with abnormally higher than the clinically-recommended antitussive doses, are also rare, but may induce unwanted effects as well, although of different receptor-induced symptomatology. The effects of mega dose (5-10 times the therapeutic dose) are ataxia, abnormal muscle movements, respiratory depression, and dissociative hallucination. Increase in heart rate, blood pressure and body temperature may lead to fatality.
While all the above symptoms were not identifiable in the investigated mice, neither signs of physical disturbances, nor damage to other organs in the body were noted, even after the mega dose (360mg/kg) repeatedly administered. Changes in blood components were neither identified. A limitation of this study may be the lack of long-term follow-up of the animals, although the finding that no damage was noted in any of the internal organs, and the absence of misbehavior of the animals, could minimize the limitation. However, this would be the next step in this dual-phase investigational study of DM.
Finally, a recent study evaluated the antitussive effectiveness of systemically-administered DM in healthy volunteers [22]. Dextromethorphan 30mg was found to attenuate cough sensitivity that was provoked by applying incremental capsaicin challenges, more effectively than four doses of butamirate. It is the opinion of the present authors that inhalational administration of DM would be as therapeutic as (if not superior to) the systemic administration before bronchoscopy, because of its direct effect in the airways. It also may obviate possible systemic bio-pharmacological untoward effects. This latter assertion awaits further confirmation by large-scale RCTs.
CONCLUSION
AUTHOR CONTRIBUTIONS
All authors have read and approved the final version of the manuscript. All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
COMPLIANCE WITH ETHICAL STANDARDS
• This study was not funded whatsoever.
• This research involved animals only.
• Informed consent: NR.
REFERENCES
- Schwarz Y, Greif J, Lurie O, Tarrasch R, Weinbroum AA (2007) Dextromethorphan premedication reduces midazolam requirement: objective and subjective parameters in peribronchoscopy. Respiration 74: 314-319.
- Ben-Abraham R, Guttman M, Flaishon R, Marouani N, Niv D, et al. (2006) Mesenteric artery clamping/unclamping-induced acute lung injury is attenuated by N-methyl-D-aspartate antagonist dextromethorphan. Lung 184: 309-317.
- Ben Abraham R, Marouani N, Weinbroum AA (2003) Dextromethorphan mitigates phantom pain in cancer amputees. Ann Surg Oncol 10: 268-274.
- Koshkina NV, Kleinerman ES (2005) Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer 116: 458-463.
- Phalen RF, Mannix RC, Drew RT (1984) Inhalation exposure methodology. Environ Health Perspect 56: 23-34.
- Koshkina NV, Giovanells B, Roberts LE, Gilbert GE, Knight V (2004) Cyclosporin A aerosol improves the anticancer effect of Paclitaxel aerosol in mice. J Aerosol Med 17: 7-14.
- Masneuf S, Lowery-Gionta E, Colacicco G, Pleil KE, Li C, et al (2014) Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology 85: 190-197.
- Marinho EA, Oliveira-Lima AJ, Santos R, Hollais AW, Baldaia MA, et al (2015) Effects of rimonabant on the development of single dose-induced behavioral sensitization to ethanol, morphine and cocaine in mice. Prog Neuropsychopharmacol Biol Psychiatry 58: 22-31.
- Imai Y, Miki T, Ishikawa T, Aoki T, Yamaguchi T (2012) Deposition of micrometer particles in pulmonary airways during inhalation and breath holding. J Biomech 45: 1809-1815.
- Ben Abraham R, Marouani N, Kollender Y, Meller I, Weinbroum AA (2002) Dextromethorphan for phantom pain attenuation in cancer amputees: a double-blind crossover trial involving three patients. Clin J Pain 18: 282-285.
- Muller NL, White DA, Jiang H, Gemma A, et al. (2004) Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 91: S24-S30.
- Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, et al. (2003) Severe acute interstitial pneumonia and gefitinib. Lancet 361: 137-139.
- Okamoto I, Fujii K, Matsumoto M, Terasaki Y, Kihara N, et al. (2003) Diffuse alveolar damage after ZD1839 therapy in a patient with non-small cell lung cancer. Lung Cancer 40: 339-342.
- Flieder D, Travis W (2004) Pathologic characteristics of drug-induced lung disease. Clin Chest Med 25: 37-45.
- Nemery B, Bast A, Behr J, Borm PJA, Bourke SJ, et al. (2001) Interstitial lung disease induced by exogenous agents: factors governing susceptibility. Eur Respir J 18: 30S-42S.
- Ieki R, Saitoh E, Shibuya M (2003) Acute lung injury as a possible adverse drug reaction related to gefitinib. Eur Respir J 22: 179-181.
- Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, et al. (2006) Predictive factors for interstitial lung disease, antitumor response, and survival in non–small-cell lung cancer patients treated with gefitinib. J Clin Oncol 24: 2549-2556.
- Higenbottam T, Kuwano K, Nemery B, Fujita Y (2004) Understanding the mechanisms of drug-associated interstitial lung disease. Br J Cancer 91: S31-S37.
- Yang HH, Hou CC, Lin MT, Chang VP (2012) Attenuating heat-induced acute lung inflammation and injury by dextromethorphan in rats. Am J Respir Cell Mol Biol 46: 407-413.
- Stanciu CN, Penders TM, Rouse EM (2016) Recreational use of dextromethorphan, "Robotripping"-A brief review. Am J Addict 25: 374-377.
- Zajac M, Andrzejczyk A, Kuich A, Tyra?ska-Fobke A, Waldman W, et al. (2013) Recreational usage of dextromethorphan--analysis based on internet users experiences. Przegl Lek70: 525-527.
- Faruqi S, Wright C, Thompson R, Morice AH (2014) A randomized placebo controlled trial to evaluate the effects of butamirate and dextromethorphan on capsaicin induced cough in healthy volunteers. Br J Clin Pharmacol 78: 1272-1280.
Citation: Schwarz Y, Star AN, Barshay A, Marmor S, Weinbroum AA (2019) Safety Versus Toxicity of Inhaled Dextromethorphan in the Mice: Hematological, Biochemical and Histological approaches. J Pulm Med Respir Res 5: 021.
Copyright: © 2019 Yehuda Schwarz, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

