
Enhancement of Atrophic Non-Union Fracture Healing Using Autologous Progenitor Cell-Rich Bone Marrow
*Corresponding Author(s):
Venkatesh PonemoneTotipotentrx Centre For Cellular Medicine Cesca Therapeutics Inc, Fortis Memorial Research Institute, Gurgaon, India
Tel:+91 124 4962251,
Email:ponemone@gmail.com
Abstract
Keywords
INTRODUCTION
Non-union has been described as failure of the fracture to heal within six to nine months in a patient where progressive repair has not been observed radiographically, within six months following fracture. Non-unions can be classified as hypertrophic, atrophic and pseudoarthrosis. Hypertrophic non-unions are rich in callus and have a rich blood supply in the ends of the bone fragments, which can be managed by proper reduction and stabilization of the bone. However, they result from insecure fixation (inadequate stability) or premature weight bearing in a reduced fracture whose fragments are viable. While in atrophic or oligotrophic non-unions there is minimal or no callus formation with absent or poor blood supply to the ends of the fragments making the non-union inert and incapable of biologic reaction and these are the types of non-unions that may benefit most from cell based therapies. Pseudoarthrosis generally known as ‘false joint’ is the third type of non-union fracture that has no chances on mending without interventions. In pseudoarthrosis, cartilage cap or fibrous tissue form at the end of bone fragments which interrupt in healing the fracture, due to which the body perceives bone fragments as separate bones and does not attempt to unite them [1].
Most mal-union and non-unions require open surgery to realign the fractured fragments into their normal anatomical position (open reduction) and stabilize the fracture by use of metal plates, rods, screws, and/or wires (internal fixation). Bone graft is placed at the surgical site to stimulate fracture healing. Treatment of non-union may be complemented with autograft (obtained from the same individual), synthetic bone graft or a graft from another individual (allograft, homogeneous graft). Some cases, whether treated surgically or with non-invasive techniques (closed reduction), benefit from the use of electrical, electromagnetic, or ultrasonic stimulation to promote fracture healing and bone growth. Infection requires surgical removal of any infected bone or tissue (debridement), followed by intensive antibiotic treatment. Newer approaches are using recombinant Bone Morphogenetic Protein (BMP) and bone marrow aspirates [5,6]. BMP use could lead to rising mutations in the body and it has been reported as not a very safe treatment method [7].
Fracture healing is a multidimensional process consisting of four well established remodeling stages; an initial inflammatory response, soft callus formation, initial bony union and bone remodeling. At the cellular level, inflammatory cells, vascular cells, osteochondral progenitors including stem cells and osteoclasts are fundamental in the repair process [8]. In the tissues surrounding non-unions, a decrease of progenitor cells is observed. This deficiency is often found in regions afflicted by infections, previous trauma, tissue defects and scars, as well as the compromised vascularity frequently associated with non-unions. This suggests that normal tissue repair may be limited by decreased population of progenitor cells locally. If the cells in the osteogenic layer of the periostium and endostium are too far apart and do not have sufficient osteogenic potential, bone consolidation can occur only by the osteoblasts present on the two fractured surfaces. In addition to necrosis, which can be a consequence of trauma at the fractured extremities, the osteoblasts are not numerous within the bone tissue [9].
Cell therapy with bone marrow cells has emerged as a promising new approach for bone regeneration. Several preclinical and clinical studies have shown that stem cells isolated from the bone marrow, can induce callus formation when injected in the non-union site of a fractured bone [10]. Bone marrow consists of progenitor cells that have the potential to promote angiogenesis, osteoinduction and osteogenesis thereby promoting bone healing/remodeling. We hypothesize that the presence of blood vessels, growth factors, and (proliferative) precursor cells are required to obtain successful fracture healing. Histologically it has been seen that in the tissues surrounding the non-union, there is a decrease in the number of progenitor cells. The concept of autologous cell therapy is based on the presence of beneficial growth factors and osteo-progenitors in the bone marrow and their property to differentiate into osteoblast cells [11].
The relationship between consolidation of fractures and activation of the bone marrow was apparently first observed by Ilizarov, who demonstrated that a 1% loss of blood volume induced an accelerated consolidation of osteotomy in rabbits [12]. This suggested a link between the haematopoietic activity of the iliac crest and osteogenesis localized around the focus of the osteotomy. This phenomenon has been confirmed by experiments in rats and mice by Bab [13] and in rabbits by Lippiello [14]. Animal studies done by Kadiyala et al (1997) on rat femora demonstrated that purified, culture-expanded syngeneic progenitor cells were capable of healing a clinically significant bone defect within 8 weeks [15]. The Kadiyala investigation further substantiates that compared to unprocessed marrow; concentrated mononuclear cells produce significantly more bone when placed in either an ectopic or an orthotopic site. Bruder et al., demonstrated bone formation at a segmental defect in adult athymic rats by implantation of human bone marrow-derived mesenchymal stem cells [16].
In 1990, Healey et al. [17] published good results in 7/8 cases of non-union after BM aspirate injection in humans. In 2005, Goel et al. presented results of Bone Marrow (BM) grafting in tibial non-unions [18]. Where 15/20 patients showed clinical and radio-graphical bone union after 14 weeks. Hernigou et al (2005) demonstrated significant results and union within 4-16 weeks (mean 12 weeks) in 53/60 patients who were treated using percutaneous injections of autologous bone marrow graft [8]. They also demonstrated a correlation between the volume of mineralized callus at four months and the number and concentration of fibroblast colony-forming units in the graft [8].
Surgical approach is still the most important tool in the management of non-union fractures. To date, autograft serves as the gold standard for bone grafts because they are histocompatible and non-immunogenic, and they offer all of the imperative properties required in a bone graft material. This is due to its high potential of osteogenesis (osteoprogenitor cells), osteoinduction and osteoconduction [19,20,21]. However, the classical autograft strategy has certain limitations that may result in significant donor site injury and co-morbidity, deformity, scarring and are associated with surgical risks such as bleeding, inflammation, infection, and chronic pain [22,23,24].
Current practice of treatment of fractured non-unions employs methods to promote osteogenesis including the use of autologous bone marrow cells, autogenous /synthetic grafts and corrective fixation [25]. To optimize these methods we propose the preparation of autologous bone marrow concentrate using a rapid point-of-care technology (in the operation theatre). Several scaffolding strategies have been developed to administer concentrated progenitor cells in a lesion site. Earlier studies have shown that autologous bone marrow cells when applied together with a synthetic graft were a potentially safe and effective method for treating atrophic non-union in humans [8]. The proposed protocol would reduce patient morbidity, infection rates, and vascular disruption through administration of the bone marrow concentrate. As a result of the clinical benefits, the patient could benefit from a reduction in medium and long-term healthcare costs. This was a non-randomized, open label, feasibility study to evaluate the safety and efficacy of autologous Bone Marrow Cell concentrate (aBMC) in patients with atrophic non-union fractures. The aBMC was produced at the point-of-care in the operation theatre for all the treated patients using our proprietary device the Res-Q 60 BMC system. All patients were consented to the procedure before commencing the study using an informed consent process. Our study indicates that administration of autologous bone marrow cell concentrate along with synthetic graft can be safe and effective in long-term healing of bone non-unions resulting in successful clinical outcomes in all patients.
MATERIALS AND METHODS
Patient selection
The inclusion criteria for the study was both male and female with atrophic non-union fracture diagnosed by X-ray or CT scan; a stable fracture with visible small gap (about 1-2 cm), and no signs of infection at the wound site or fracture site. Any patient with history of smoking but willing to quit was included in the study; corticosteroids were to be included only after cessation of smoking one month before cell therapy, (corticosteroids function by reducing the activity of immune system and decreasing inflammation). Also, patients must have a normal blood and marrow function as defined by: leukocytes ≥ 3000/µl, absolute neutrophil count ≥ 1500/ µl and platelets ≥ 100,000/ µl. Exclusion criteria for the study were patients with active systemic or local infection; any evidence of malignancy in the past five years; pregnancy or breastfeeding; patients who require corticosteroid or anti-inflammatory therapy after surgery; patients with genetic metabolic bone disease such as hypophosphatasia, or metabolic bone disorders such as primary or secondary hyperparathyroidism caused by chronic renal insufficiency or other disorders; patients that are positive by serology or PCR for HIV, hepatitis B or C infection; patients with uncontrolled Diabetes Mellitus (DM); patients under immunosuppressive therapy or taking anticoagulant agents; insufficient reduction of the fracture with displaced fragments; evidence of local sepsis by clinical signs; and multiple major fractures.
aBMC Preparation at point-of-care
The harvested bone marrow was thoroughly mixed, and filter transferred to the device for processing and concentrating using our intraoperative rapid, closed, point-of-care device, the Res-Q™ 60 BMC technology (Thermogenesis Corp. USA). This point-of-care system is an automated cell processing medical device that concentrates the bone marrow by a density gradient centrifugation method. The processing was carried out in the operating room in less than 20 minutes and required minimum operator intervention. Following processing at the patient’s bed side, 8 mL of aBMC was collected from the device that was rich in progenitor cells. Aliquot of 1 mL of aBMC (post-processed sample) was collected and later sent to the laboratory for analysis of cell counts and sterility evaluation, while the remaining 7 mL aBMC was preconditioned for 5-10 minutes with synthetic graft material i.e. tricalcium phosphate. During the ‘preconditioning’ of bone marrow concentrate, progenitor cells are bio-absorbed by the synthetic graft which makes a semi-solid matrix called ‘putty’. This ‘putty’ is administered between the ends of non-union bones for rapid healing of the fracture. The pre- and post- processed bone marrow samples were quantified using Coulter cell counter and sterility was evaluated using BacT/ALERT.
aBMC Delivery procedure
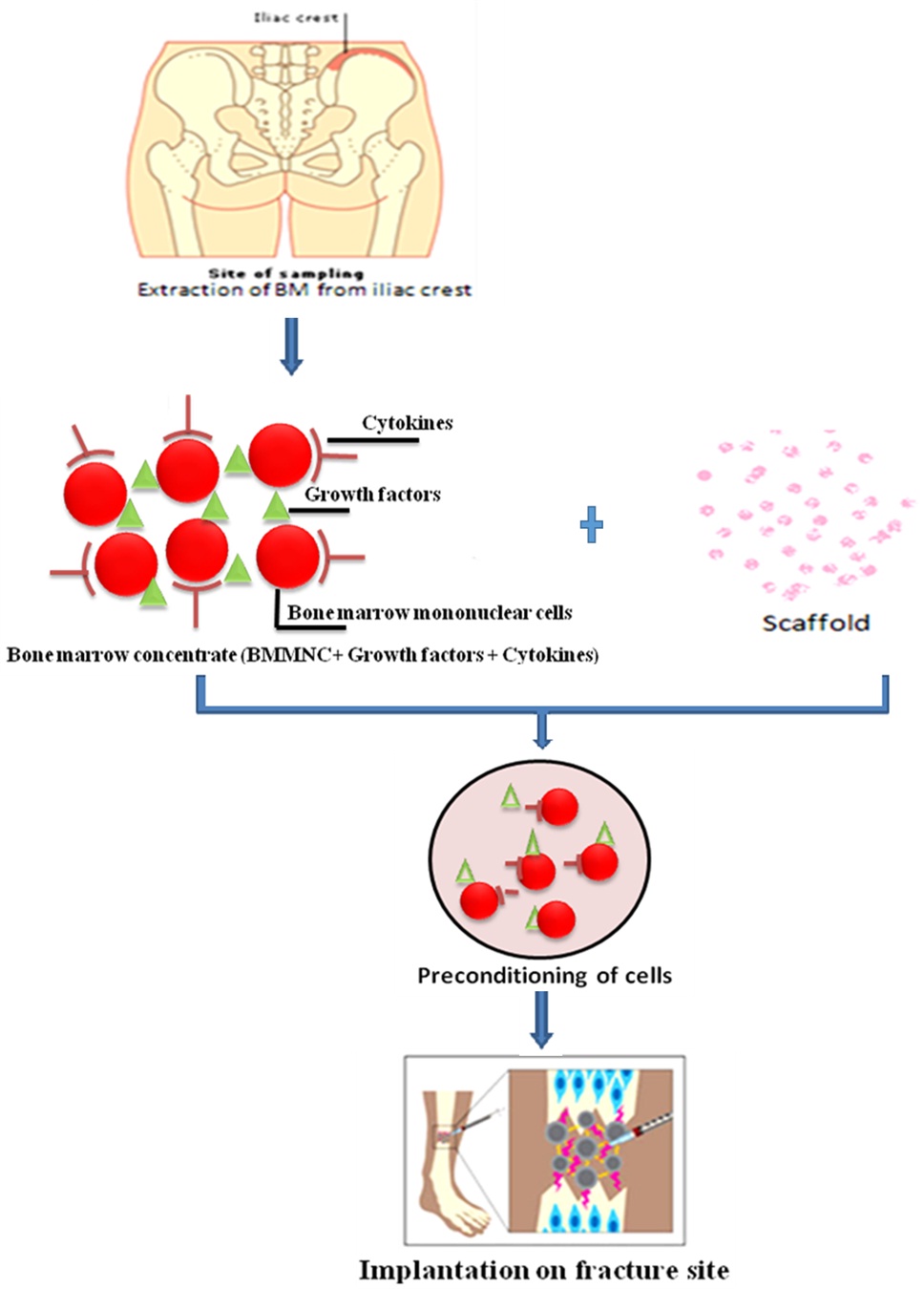
Post-operative management and follow-up
The primary objective of this clinical study was to demonstrate the efficacy of autologous bone marrow cell concentrate (aBMC) in the treatment of atrophic non-union fractures by determining the formation of callus at the fracture site. While, the secondary end points were healing time and quantitative reduction in pain measured using the Visual Analogue Scale (VAS).
Statistical analysis
RESULTS
| S. No. | Age (y) | Gender | Affected Bone | Diabetes (Y/N) | CVD (Y/N) | Infectious Disease (Y/N) | Smoking (Y/N) | Alcoholic | Time from Injury (months) | NU Status at 12 Months |
| 1 | 19 | Male | Tibia | N | N | N | N | N | 9 | United |
| 2 | 59 | Male | Tibia | Y | N | N | Y | Y | 10 | United |
| 3 | 31 | Male | Femur | N | N | N | N | Y | 6 | United |
| 4 | 62 | Male | Femur | Y | N | N | N | N | 8 | United |
| 5 | 40 | Male | Femur | N | N | N | N | Y | 6 | United |
| 6 | 51 | Male | Femur | Y | Y | N | Y | Y | 10 | Not United |
| 7 | 72 | Female | Femur | Y | Y | N | N | N | 12 | Not United |
| 8 | 39 | Male | Tibia | N | N | N | N | N | 8 | United |
| 9 | 30 | Male | Humerus | N | N | N | N | Y | 8 | United |
| 10 | 31 | Female | Tibia | N | N | N | N | N | 6 | United |
| 11 | 59 | Female | Tibia | Y | N | N | N | N | 7 | United |
| 12 | 41 | Male | Tibia | N | N | N | N | Y | 9 | United |
| 13 | 71 | Female | Femur | Y | N | N | N | N | 8 | United |
| 14 | 42 | Male | Wrist | Y | N | N | Y | Y | 8 | United |
| 15 | 64 | Female | Femur | Y | N | N | N | N | 7 | United |
| 16 | 41 | Male | Tibia | Y | N | N | Y | N | 9 | Not United |
| 17 | 24 | Male | Tibia | N | N | N | N | N | 9 | United |
Table 1: Demographic data of the patients in the study.
Bone marrow aspiration, processing and administration were accomplished in the operating room in a single sitting within 60 minutes using the Res-QTM 60 BMC system. Patients tolerated the procedure well, and there was no bleeding, infection, or procedure related complication, including local injection site swelling in all the subjects on the day of treatment after aBMC administration. The mean (±Standard Deviation) Total Nucleated Cell Count (TNCC) and Mononuclear Cell Count (MNCC) of all the patients were 5.54 x 108 ± 1.99 and 1.64 x 108 ± 0.86 respectively, in the bone marrow concentrate. The mean cell viability was found to be over 88% and the mean MNC dose administered to the patient was 1.34 x 108 cells.
Out of the 17 patients treated, signs of callus formation (confirmation of union of the bone) were observed in 14 patients with a union rate of 82%. The remaining 3 patients (18%) did not show any sign of bone union at the time of the last follow up (12 months). The average time for callus formation was observed to be 6 months following aBMC administration (Figure 2). Patient follow-up showed sign of clinical improvement at 3 month with respect to fracture healing, pain reduction and improved locomotive function of the limb. Radiographic images of the fractured bone, pre- and post- treatment are given in (Figure 2).
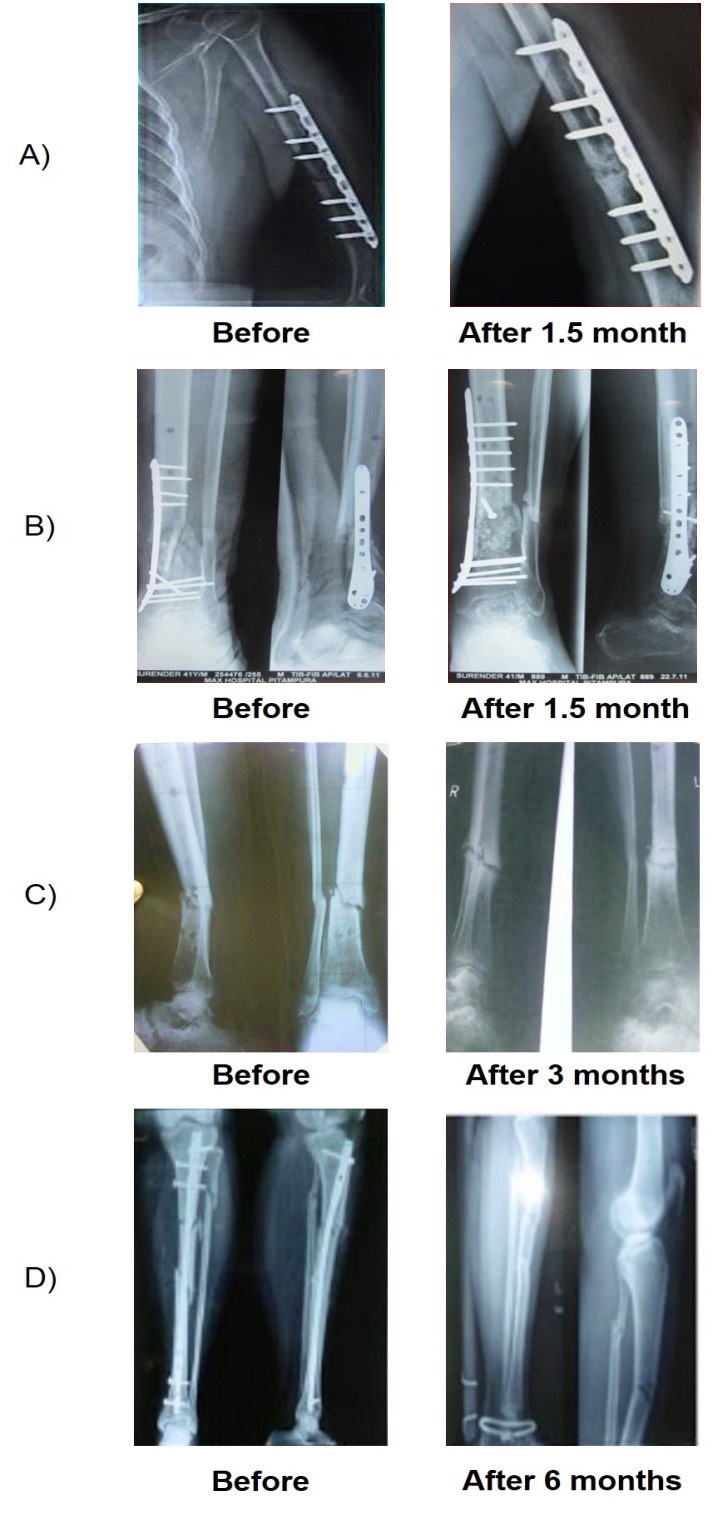
Figure 2: A) Figure showing the healing of a non-union fracture in the humerus bone
B) Bone healing is clearly evident in the tibia bone after 1.5 months
C) Figure showing callus formation in tibial bone after 3 months
D) Bone healing seen in non-union fracture in the tibia bone after 6 months.
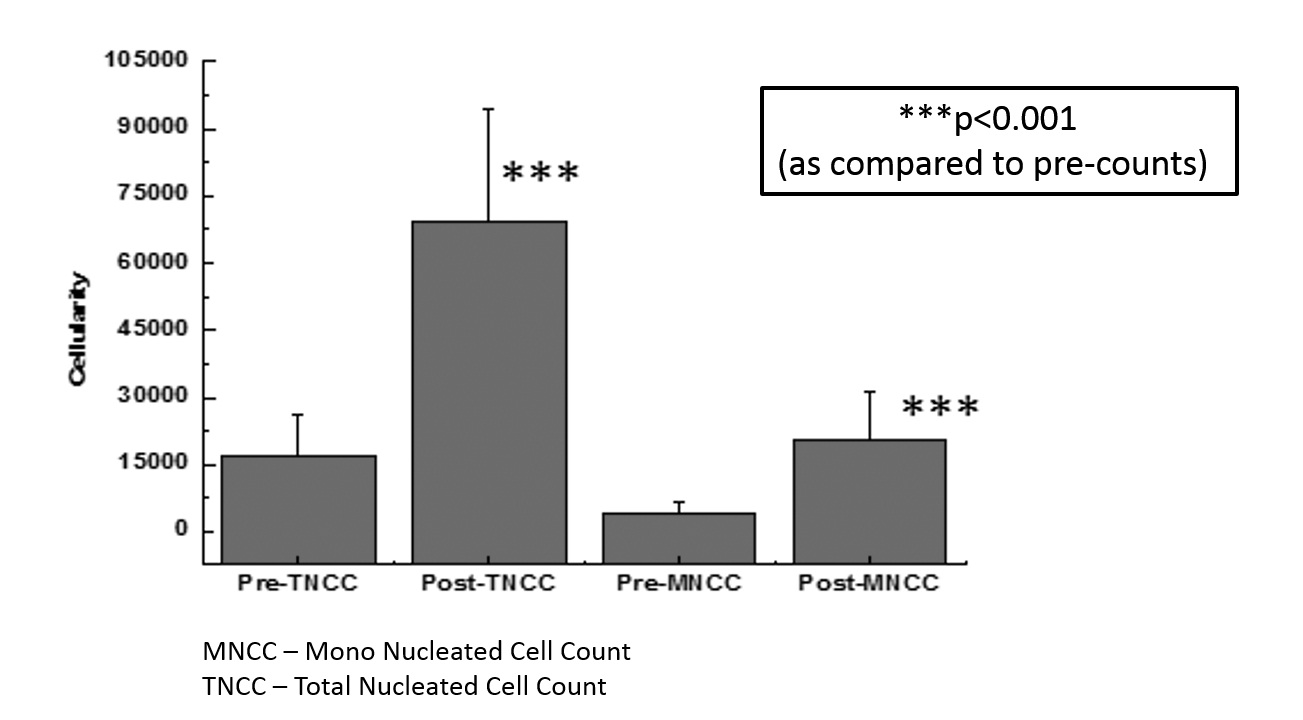
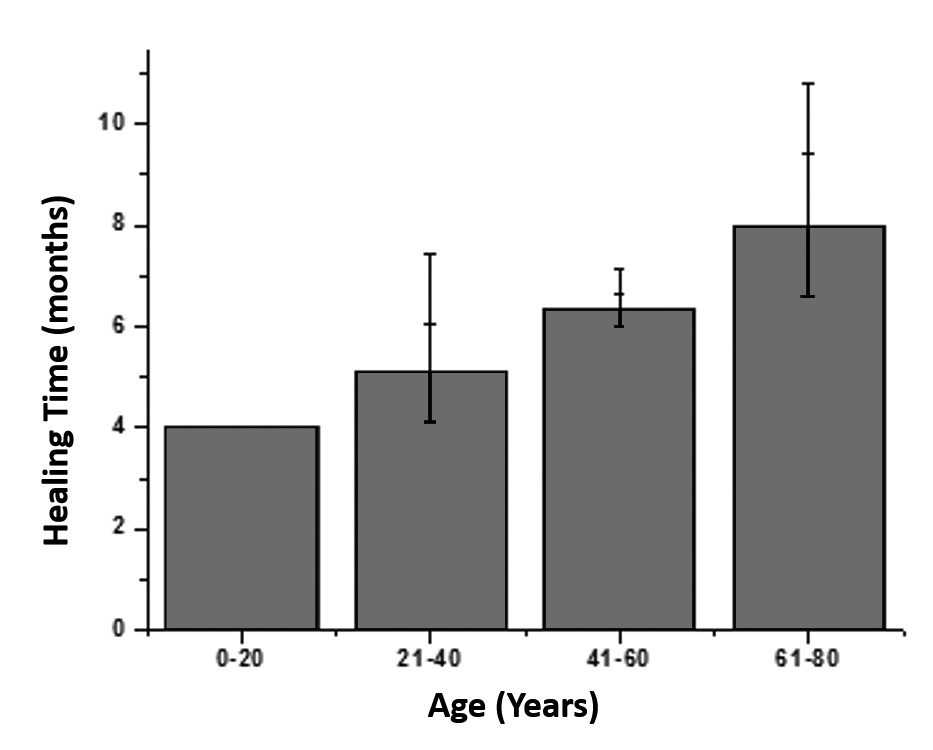 Figure 3B: Age vs Healing Time.
Figure 3B: Age vs Healing Time.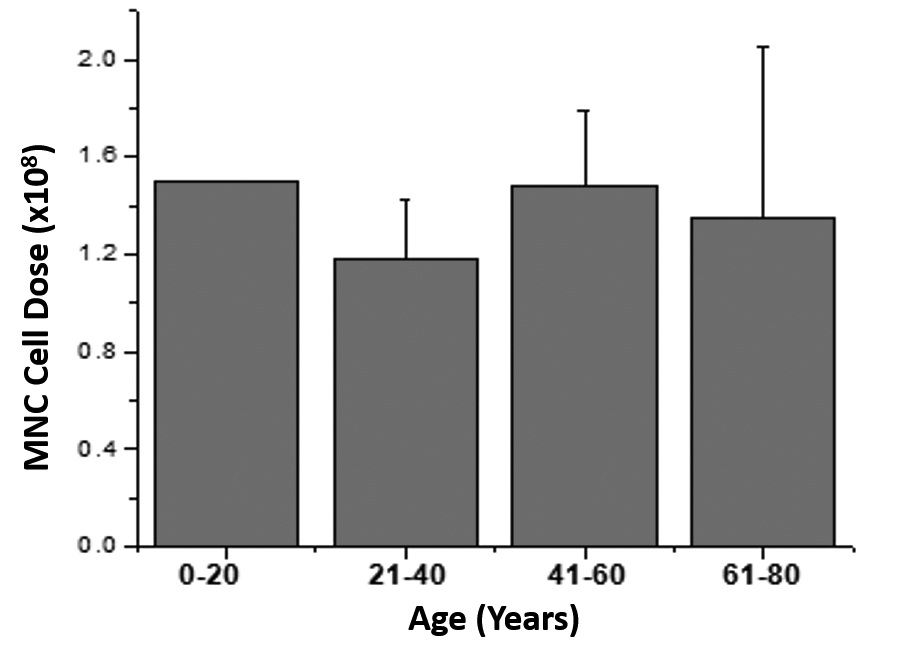
B) Graph representing the age versus healing time of all the patients
C) Graph showing the correlation between age and MNC Counts
DISCUSSION
Bone formation from autologous bone marrow combined with synthetic graft is believed to occur in two phases. During the first phase, which lasts approximately four weeks, the main contribution to bone formation is from the cells of the graft. However, during the second phase, cells from the host begin to contribute to the process. The endosteal lining cells and marrow stroma produce more than half of the new bone, whereas osteocytes make a small (10%) contribution. [27]. This feasibility, non-randomized, open label, single arm study, was a scientifically rigorous study designed to evaluate the safety and efficacy of autologous bone marrow cell concentrate conjugated with synthetic graft (tri-calcium phosphate). The present study shows that the application of autologous bone marrow cell concentrate mixed with synthetic graft in 14 out of 17 patients (82%) resulted in bone union at 12 month follow up period. The callus formation in radiographic imaging is indicative of bone healing and union in these patients, confirming the effectiveness of autologous bone marrow graft in the treatment of atrophic non-union fracture.
When a fracture occurs, the repair or healing of the bone takes place in three steps; inflammatory stage, reparative stage and a remodeling stage. In a damaged bone, the soft tissue envelope, including the periosteum and surrounding muscles, is torn, and numerous blood vessels crossing the fracture line are ruptured. There is an accumulation of hematoma within the medullary canal, between the fracture ends, and beneath any elevated periosteum. This blood rapidly coagulates to form a clot. The effect of this vascular damage is of paramount importance. Osteocytes are deprived of their nutrition and die as far back as the junction of collateral channels. Thus, the immediate ends of a fracture are dead; that is, they contain no living cells. The body begins to create cartilage around the fracture site to bridge the gap in the bone. This type of cartilage is called the soft callus, which is simply fibrous tissue. New blood vessels around the fracture site provide for nutrition. In the final stage of bone fracture healing, the body replaces old bone with new bone in a continual process called remodeling. Remodeling makes bones stronger and compact and blood circulation in the bone improves [28].
Historically, autologous bone grafts, usually the cancellous bone from the iliac crest is the gold standard and represents the most common therapeutic approach in orthopedic applications. The autologous graft is capable of providing all 3 elements of bone regeneration including osteogenic and osteoinductive as well as osteoconductive properties [21,25]. However, a bone autograft is limited in quantity and its harvesting represents an additional surgical intervention and donor site co-morbidity [6]. Therefore, investigations are needed to provide a safe and effective alternative approaches.
Bone marrow concentrate isolated contains progenitor cells such as Hematopoietic Stem Cells (HSCs), Mesenchymal Stem Cells (MSCs) and Endothelial Progenitor Cells (EPCs) along with abundant cytokines and growth factors. As bone marrow contains osteogenic progenitor cells, its implantation was proposed in order to potentially lead to efficient bone regeneration. In clinical practice, autologous bone marrow cells are harvested from the iliac crest and immediately transplanted into the site that is in need of skeletal repair. Low progenitor cell number is the major limiting factor of the direct bone marrow aspiration and injection into bone fracture site, and therefore concentration of bone marrow is important in order to provide abundant progenitor cells along with paracrine factors for bone healing.
This method of marrow-cell concentration and transfer or grafting is a relatively simple procedure that is inexpensive and can be performed intraoperatively at the bedside in a single sitting. Moreover, because it involves only the relocation of autologous tissue, it is not subjected to complex regulatory protocols. There have been some limitations observed with the traditional use of bone marrow derived cells for regenerative medicine. Earlier, after extraction of the bone marrow, the bone marrow sample had to be sent to a GMP facility for processing and isolation of progenitor cells. With the advent of technology, bedside processing of stem cells have become possible where the extraction, processing, as well as the injection of bone marrow cell concentrate can be done quickly. The advantage over traditional cell therapy processing is its rapid cell isolation at the bedside, single sitting procedure for the patient in comparison to the ex-vivo cultured autologous bone marrow cells, reduced cost, low to negligible infection rates and avoids the need for additional personnel. Another limitation of traditional cell therapy was the cost which has drastically come down owing to better technology for administering cell therapy. In our study, a rapid point-of-care device and technology, the Res-QTM 60 BMC system, was used for processing the autologous bone marrow cell concentrate and has several advantages over the traditional method, as it is a closed, sterile, automated system that processes cells with minimal manipulation. Our proprietary device reduced time, cost and labour-intensive procedure for harvesting, processing and injecting bone marrow cell concentrate at the fracture site and the complete procedure was completed within 60 minutes in the operation theatre. The bone marrow concentrate was allowed to conjugate with synthetic graft. This process is called ‘conditioning’ of the bone marrow which makes ‘putty’ for administration between the ends of fractured bone.
Furthermore, the infused cell dose plays a pivotal role in cellular therapy. Hernigou et al. [8] demonstrated in their study that the number of progenitor cells injected play an important role in determining the volume of callus formed and thereby, determine the healing of atrophic non-union fractures. Their group measured the concentration of progenitor cells harvested from bone marrow using the ‘Fibroblast- Colony Forming Unit’ (CFU-F) assay, and found a direct relationship with the amount of healing and indirect relation with the time of healing versus the concentration of CFU-F. In the same study, they also showed that absolute CFU counts less than 634 ± 187/ mL led to the failure of the procedure [8]. Another study by Desai et al. [29] demonstrated a mean total nucleated cell counts of 66.52 ± 19.47/ mL after concentration and absolute CFU count was 1270 ± 1009/mL, which they concluded as higher than other commercially available concentrate systems. In our study, our point-of-care device was capable of achieving mean Total Nucleated Cell Counts (TNCC) of 5.54 x 108 ± 1.99 (69.31 ± 25/ mL), while the Mono-Nuclear Cell Counts (MNCC) were 1.64 x 108 ± 0.86 (20.5 ± 10.7/ mL) in the final aBMC product that was administered into the patients. These cell counts were higher than the previously reported studies, and therefore our device could effectively be used for processing at the point-of-care in treating patients with atrophic non-union fractures using concentrated bone marrow cells. The limitation with our study was the absence of CFU assay for better quantification of the injected cells for potency. The sterility testing of the post-processed bone marrow samples using BacT/ALERT 14 day culture showed no bacterial or fungal growth in any of the patient samples. Since, our device is a completely closed system; the chances of infection are minimized and provide a safe and effective treatment procedure.
Researchers are now beginning to understand the differences among the progenitor cells harvested from various individuals. These differences depend on many variables, such as age, gender, and local and systemic diseases [30-32], and the variability in the osteogenic potential from patient to patient represents a limitation of this autologous bone marrow grafting technique [8]. Previous studies have observed that bone marrow cellularity declines with age, and there is also a decrease in the prevalence of connective-tissue progenitors with increasing age [33-34]. However, this was not evident in our small sample size, which is in concurrence with previously published studies by Hernigou et al. [8] Furthermore, our data shows that, bone healing time and age are directly related. However, due to the small sample size statistical significance could not be demonstrated and further large sample size studies are required to prove the exact co-relation. Increased age is associated with decreased bone marrow cellularity and connective tissue progenitors, which may explain our findings of increased bone healing time with increase in age [29].
The exact reason for failure of bone union post cell therapy intervention has not been yet demonstrated in any of the previous studies. Several studies have shown that bone-fracture consolidation is often delayed in heavy smokers and drinkers. Experiments in animals confirmed the fact that nicotine had an adverse effect on bone consolidation [35]. Studies on humans did not find a significant relationship between the prevalence of osteogenic progenitors and smoking, but nicotine increased the activity of osteoclasts, which could be causally related to deficient bone regeneration. Nicotine decreases microperfusion and tissue oxygenation which can lead to platelet aggregation and result in micro clotting [35]. Conversely, a direct analysis of patients with a history of chronic alcohol intoxication has shown that they have abnormally low levels of mesenchymal progenitors in the iliac crests. Among other toxic agents, chemotherapy causes differential effects on mesenchymal progenitors, which are resistant to agents usually used for bone-marrow transplantation in onco-haematological patients but sensitive to a panel of commonly used cytotoxic agents. Therefore, problems of consolidation may be linked to an overall reduction in the number of progenitor cells in the bone marrow [36]. Our analysis identified another risk factor for failure to heal, that is, late treatment of non-union. However, the exact relationship between late treatment for non-union and poor prognosis is less clear. We do accept the possibility that patients treated earlier may not have a true non-union and may be able to heal intrinsically; biological changes may occur over time at the site of non-union, thereby perpetuating the inability to heal. Therefore, the three (3) patients who did not show union of bone in our study, 12 months post cell therapy intervention, could be due to the above mentioned inferences.
The procedure is safe and feasible with no major complications or side effects like hematomas, infections or chronic pain at the aspiration or injection site. In our study, 82% of patients attained bone healing and showed callus formation radiographically at an average of 6 months; however, signs of healing were visible as early as 1.5 months post cell therapy. These results are similar to previously published studies, where Desai et al. [29] reported healing in 79.6% of patients at an average of 4.7 months and Hernigou et al. [8] reported higher healing rate of 88% with faster healing time of 12 weeks on an average. In their study, all patients underwent external fixation or conservative therapy for the initial fracture repair and in our study the primary intervention for all the patients was open reduction internal fixation. The longer healing time in our study could be due to many factors related to the fracture gap size, the underlying co-morbidities and patient age (older age vs younger patients). The limitation of our study was a small sample size and improper quantification of the fracture gap size. Therefore, further studies with larger patient population and proper quantification of gap size are required to better understand the effect of the above mentioned factors on healing time and callus formation. Nevertheless, no major complications were observed, and we have successfully demonstrated the safety and effectiveness of our device and technique in the treatment of non-union of long bones as a reliable alternative to traditional techniques.
The use of autologous bone marrow in non-union fracture sites with plates that had been put previously was discussed in another study conducted on patients of non-union fractures where the site of fracture was the distal tibia [37]. 11 patients were recruited for the study and 9 had an average healing time of 6 months and showed considerable healing with the use of autologous bone marrow cells. A follow up was done for 4.5 years and all patients reported a decline in the pain intensity and interference. Another very small pilot study by Centeno and group [38], where culture expanded mesenchymal stem cells were used to treat non-union in 6 patients (4 females, 2 males), and the treatment intervention was at an average of 8.75 months post-fracture, all but one patient showed improvement. The patient who lacked healing had a chronic fracture that lasted for more than 40 years. Overall the treatment showed faster healing of the fractures and culture expanded MSCs could be used as an alternative cell source for treating non-union fractures. Mesenchymal stem cells have shown their therapeutic capacity in several in vitro and in vivo studies for the regeneration of bone defects and non-unions. The supply of autologous mesenchymal stem cells is often limited. Nevertheless, their special immunological characteristics suggest that mesenchymal stem cells could be used in non-autologous applications. [39,40]. Also previous studies have shown that percutaneous injections of the autologous bone marrow has been effective in treating distal non-unions or delayed unions after internal fixation. [8,41-43].
Some studies have also reported the use of PRP (platelet rich plasma) for the treatment of non–unions fractures. [44-49] Platelet Rich Plasma is the conglomeration of cytokines and growth factors isolated from whole blood using density gradient separation. PRP upon activation releases growth factors such as primarily Transforming Growth Factor-β (TGFβ) and Platelet Derived Growth Factor (PDGF), vascular endothelial growth factor (VEGF), Fibroblast Growth Factor (FGF) and insulin-like growth factor and proteins such as fibrin, fibronectin, vitronectin and thrombospondin, which play a vital role at several stages of tissue healing [50]. With the effect of growth factors, PRP stimulates and activates the local stem cells in the circulation and bone marrow, which plays a major role in fracture healing. In the paper by Simman et al. [44] an animal study was conducted on 48 male rats. The animals femurs’ were fractured using a cat nail trimmer and bone fracture was analyzed in X-ray. PRP was injected on fracture sites and the healing was noted. In another study by Bielecki et al. [46], a single dose application of PRP was used for 32 cases of delayed and non-union. The results reported, showed union in the entire delayed union group and in 65% of the non-union group within 11 months after surgery. Therefore, they recommended the application of PRP in the treatment of delayed and non-union fractures.. Similarly, in the study by Calori et al. [47], revision surgery together with PRP was compared with the application of bone morphogenetic protein-7 (BMP-7). In 120 cases of atrophic non- union, union was achieved in 86.7% of the BMP group and 68.3% of the PRP group and clinical and radiological healing was reported earlier in the BMP group. While, Griffin and group [48] reviewed the use of PRP in clinical studies and reported that PRP use was safe but no clinical evidence was shown of benefit in acute or delayed fracture union. Also, in the study by Say et al. [49], union was determined in 30% of patients after three doses of PRP application. Results with the application of PRP together with allograft or autograft in surgical treatment have been varied. Some researchers have maintained that PRP has positive effects, while others have claimed that there is no benefit of PRP [51,52].
Bone marrow derived cells are able to elicit angiogenesis by the presence of endothelial progenitors in the cell fraction [53]. The bone marrow concentrate supply abundant number of progenitor cells and angiogenic cytokines that actively engage in vasculogenesis in tissue devoid of vessels and in neoangiogenesis from the pre-existing capillaries [54]. The local ischaemia activates the Hypoxia Inducible Factor (HIF)-1a signalling and mobilisation of circulating progenitors through the Stromal-cell-Derived Factor (SDF1) dependent pathway stimulates the angiogenesis and neovascularization for bone regeneration [55]. These mechanisms may explain the long-lasting effect of cell therapy in bone healing.
CONCLUSION
AUTHOR CONTRIBUTION
Venkatesh Ponemone: Conception and design, data analysis and interpretation; manuscript writing, final approval of manuscript.
Khushboo Choudhury, Saniya Gupta, Manish Suthar: Manuscript revisions, data collection, data analysis and interpretation
Kenneth Lee Harris, Dalip Sethi, Akshay Saxena, Manish Dalwani, Alok Sharma, Rakesh Mattoo, Nitiraj Oberoi: Data analysis and interpretation.
Harshavardhan Hegde: Conception and design, data analysis and interpretation; Manuscript revisions, final approval of manuscript.
CONFLICT OF INTEREST
REFERENCES
- Tseng SS, Lee MA, Reddi AH (2008) Nonunions and the potential of stem cells in fracture-healing. J Bone Joint Surg Am 90: 92-98.
- Healy WL, White GM, Mick CA, Brooker AF Jr, Weiland AJ (1987) Nonunion of the humeral shaft. Clin Orthop Relat Res 206-213.
- Murray IR, Foster CJ, Eros A, Robinson CM (2013) Risk factors for nonunion after nonoperative treatment of displaced midshaft fractures of the clavicle. J Bone Joint Surg Am 95: 1153-1158.
- Perumal V, Roberts CS (2007) Factors contributing to non-union of fractures. Orthopaedics and Trauma 21: 258-261.
- Bhargava R, Sankhla S, Gupta A, Changani R, Gagal K (2007) Percutaneous autologus bone marrow injection in the treatment of delayed or nonunion. Indian J Orthop 41: 67-71.
- Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, et al. (2015) Bone fracture healing: cell therapy in delayed unions and nonunions. Bone 70: 93-101.
- Ye M, Berry-Wynne KM, Asai-Coakwell M, Sundaresan P, Footz T, et al. (2010) Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum Mol Genet 19: 287-298.
- Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87: 1430-1437.
- Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H (2005) The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br 87: 896-902.
- Garg NK, Gaur S, Sharma S (1993) Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand 64: 671-672.
- O'Brien T, Barry FP (2009) Stem cell therapy and regenerative medicine. Mayo Clin Proc 84: 859-861.
- Ilizarov GA (1990) Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res : 8-26.
- Bab I, Gazit D, Massarawa A, Sela J (1985) Removal of tibial marrow induces increased formation of bone and cartilage in rat mandibular condyle. Calcif Tissue Int 37: 551-555.
- Lippiello L, Chavda D, Connolly J (1992) Colony-forming efficiency response of bone marrow stromal cells to acute blood loss. J Orthop Res 10: 145-148.
- Kadiyala S, Young RG, Thiede MA, Bruder SP (1997) Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant 6: 125-134.
- Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, et al. (1998) Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res 16: 155-162.
- Healey JH, Zimmerman PA, McDonnell JM, Lane JM (1990) . Clin Orthop Relat Res : 280-285.
- Goel A, Sangwan SS, Siwach RC, Ali AM (2005) Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury 36: 203-206.
- Bastos Filho R, Lermontov S, Borojevic R, Schott PC, Gameiro VS, et al. (2012) Cell therapy of pseudarthrosis. Acta Ortop Bras 20: 270-273.
- Jäger M, Herten M, Fochtmann U, Fischer J, Hernigou P, et al. (2011) Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res 29: 173-180.
- Hatzokos I, Stavridis SI, Iosifidou E, Karataglis D, Christodoulou A (2011) Autologous bone marrow grafting combined with demineralized bone matrix improves consolidation of docking site after distraction osteogenesis. J Bone Joint Surg Am 93:671-678.
- Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 20: 1055-1060.
- Ebraheim NA, Elgafy H, Xu R (2001) Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg 9: 210-218.
- St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, et al. (2003) Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop (Belle Mead NJ) 32: 18-23.
- Giannotti S, Bottai V, Ghilardi M, Dell'osso G, Fazzi R, et al. (2013) Treatment of pseudoarthrosis of the upper limb using expanded mesenchymal stem cells: a pilot study. Eur Rev Med Pharmacol Sci 17: 224-227.
- Giannoudis PV, Einhorn TA, Marsh D (2007) Fracture healing: the diamond concept. Injury 38: 3-6.
- Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84: 454-464.
- Hernigou P, Mathieu G, Poignard A, Manicom O, Beaujean F, et al. (2006) Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J Bone Joint Surg Am 88: 322-327.
- Desai P, Hasan SM, Zambrana L, Hegde V, Saleh A, et al. (2015) Bone Mesenchymal Stem Cells with Growth Factors Successfully Treat Nonunions and Delayed Unions. HSS J 11: 104-111.
- Muschler GF, Boehm C, Easley K (1997) Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am 79: 1699-1709.
- Hernigou P, Beaujean F (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res : 14-23.
- Muschler GF, Nitto H, Boehm CA, Easley KA (2001) Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 19: 117-125.
- Quarto R, Thomas D, Liang CT (1995) Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int 56: 123-129.
- D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA (1999) Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14: 1115-1122.
- Lee JJ, Patel R, Biermann JS, Dougherty PJ (2013) The musculoskeletal effects of cigarette smoking. J Bone Joint Surg Am 95: 850-859.
- Bidula J, Boehm C, Powell K, Barsoum W, Nakamoto C, et al. (2006) Osteogenic progenitors in bone marrow aspirates from smokers and nonsmokers. Clin Orthop Relat Res 442: 252-259.
- Braly HL, O'Connor DP, Brinker MR (2013) Percutaneous autologous bone marrow injection in the treatment of distal meta-diaphyseal tibial nonunions and delayed unions. J Orthop Trauma 27: 527-533.
- Centeno CJ, Schultz JR, Cheever M, Freeman M, Robinson B et al. (2011) A Case Series of Percutaneous Treatment of Non-union Fractures with Autologous, Culture Expanded, Bone Marrow Derived, Mesenchymal Stem Cells and Platelet Lysate. J Bioeng. Biomed Sci 2:007.
- Niemeyer P, Schönberger TS, Hahn J, Kasten P, Fellenberg J, et al. ( 2010) Xenogenic transplantation of human mesenchymal stem cells in a critical size defect of the sheep tibia for bone regeneration. Tissue Eng. Part A 16: 33-43.
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31: 890-896.
- Matsuda Y, Sakayama K, Okumura H, Kawatani Y, Mashima N, et al. (1998) Percutaneous autologous bone marrow transplantation for nonunion of the femur. Nihon Geka Hokan 67: 10-17.
- Siwach RC, Sangwan SS, Singh R, Goel A (2001) Role of percutaneous bone marrow grafting in delayed unions, non-unions and poor regenerates. Indian J Med Sci 55: 326-336.
- Wilkins RM, Chimenti BT, Rifkin RM (2003) Percutaneous treatment of long bone nonunions: the use of autologous bone marrow and allograft bone matrix. Orthopedics 26: 549-554.
- Simman R, Hoffmann A, Bohinc RJ, Peterson WC, Russ AJ (2008) Role of platelet-rich plasma in acceleration of bone fracture healing. Ann Plast Surg 61: 337-344.
- Galasso O, Mariconda M, Romano G, Capuano N, Romano L, et al. (2008) Expandable intramedullary nailing and platelet rich plasma to treat long bone non-unions. J Orthop Traumatol 9: 129-134.
- Bielecki T, Gazdzik TS, Szczepanski T (2008) Benefit of percutaneous injection of autologous platelet-leukocyte-rich gel in patients with delayed union and nonunion. Eur Surg Res 40: 289-296.
- Calori GM, Tagliabue L, Gala L, d'Imporzano M, Peretti G, et al. (2008) Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury 39: 1391-1402.
- Griffin XL, Smith CM, Costa ML (2009) The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury 40: 158-162.
- Say F, Türkeli E, Bülbül M (2014) Is platelet-rich plasma injection an effective choice in cases of non-union? Acta Chir Orthop Traumatol Cech 81: 340-345.
- Alsousou J, Thompson M, Hulley P, Noble A, Willett K (2009) The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br 91: 987-996.
- Wei LC, Lei GH, Sheng PY, Gao SG, Xu M, et al. (2012) Efficacy of platelet-rich plasma combined with allograft bone in the management of displaced intra-articular calcaneal fractures: a prospective cohort study. J Orthop Res 30: 1570-1576.
- Peerbooms JC, Colaris JW, Hakkert AA, Van Appeldorn M, Bruijn DJ, et al. (2012) No positive bone healing after using platelet rich plasma in a skeletal defect. An observational prospective cohort study. Int Orthop 36: 2113-2119.
- Roberts N, Jahangiri M, Xu Q (2005) Progenitor cells in vascular disease. J Cell Mol Med 9: 583-591.
- Conway EM, Collen D, Carmeliet P (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res 49: 507-521.
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, et al. (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858-864.
Citation: Ponemone V, Choudhury K, Gupta S, Suthar M, Sethi D, et al. (2016) Enhancement of Atrophic Non-Union Fracture Healing Using Autologous Progenitor Cell-Rich Bone Marrow. J Stem Cell Res Dev Ther 3: 07.
Copyright: © 2016 Venkatesh Ponemone, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

