School Of Pharmaceutical Sciences Shenzhen, Sun Yat-Sen University, Guangzhou 510275, China
School Of Pharmaceutical Sciences Shenzhen, Sun Yat-Sen University, Guangzhou 510275, China
School Of Pharmaceutical Sciences Shenzhen, Sun Yat-Sen University, Guangzhou 510275, China
Abstract
Mammalian skin has a natural capacity to replace dying cells and to heal wounds. This ability is based on stem cells that reside in skin epidermis and its appendages. Skin contains stem cells such as Interfollicular Epidermis Stem Cells (IFE-SCs), Hair Follicle Stem Cells (HF-SCs) and Sebaceous Gland Stem Cells (SG-SCs). IFE-SCs are mainly located in the basal layer of the epidermis, and with extensive potential of proliferation to supplement the aged and exfoliated keratinocytes. HF-SCs are mainly located in bulge, and have the function of fueling hair follicle cycling and hair growth. SG-SCs located in sebaceous gland maintain its normal metabolism. Skin-Derived Precursor Cells (SKPs) are an endogenous multipotent precursor cell present in human skin that can be isolated, expanded and differentiate into both neural and mesodermal cell types. This review focuses on the stem cells in skin, with introduction of their niche, molecular identity and function in normal homeostasis and wound repair, also include the perspective of their clinical application.
Keywords
Hair follicle; Interfollicular epidermis; Sebaceous gland; SKPs; Stem cells
ABBREVIATIONS
- SC- Stem Cell
- IFE-interfollicular Epidermis
- HF- Hair Follicle
- SG-Sebaceous Gland
- BMP-Bone Morphogenetic Protein
- YAP-Yes-Associated Protein
- LRCs-Label Retaining Cells
- SKPs-Skin-Derived Precursor Cells
- DP-Dermal Papilla Cells
- DS-Dermal Sheath
INTRODUCTION
Skin covers the body surface and is the largest organ of the human body. It protects all kinds of tissues and organs from physical, mechanical, chemical and pathogenic microorganisms. Skin has ability to replace dying cells, renew tissues and heal wounds. In these processes, the key role is the tissue-resident stem cells. When the skin barrier is out-of-balance or damaged, stem cells can self-renew and preserve the cell population, and differentiate into one or more specialized cells to maintain and repair the function of skin tissue.
The study of skin stem cells is conducive to wound management and repairment. Especially for severe skin defects caused by extensive skin burns, traumatic skin defects and skin ulcers, cell-replacement therapy can be carried out. In addition, given the high self-renewal and multi-directional differentiation potential of stem cells, it is possible to use skin stem cells as target cells for gene therapy of skin hereditary diseases.
STEM CELLS AND PROGENITOR CELLS IN INTERFOLLICULAR EPIDERMIS
The epidermis is the outermost layer of mammalian skin and comprises a multilayered Interfollicular Epidermis (IFE), with associated hair follicles, sebaceous glands, and eccrine sweat glands. The Interfollicular Epidermis (IFE) is one of the main cell update systems. In the IFE, only the basal layer can proliferate, and differentiate into the spinous, granular, and stratum corneum layers. The renewal and repair of human IFE is maintained by epidermal stem cells that locate in the basal layer; and these stem cells express Keratin-5 (K5) and Keratin-14 (K14), which are the first group of markers to characterize skin epithelial progenitor cells [1].
Wound healing is very important for the repair of damaged skin. The basal epidermal progenitor cells at the wound are mainly involved in IFE regeneration. The proliferation, migration and differentiation of stem cells are uncoupled during wound healing. When the IFE is injured, keratinocytes, whose WNT signaling is considered to play an important role, will migrate and proliferate locally. At the same time, progenitor cells divide faster, and stem cells become more active to renew progenitor cells [2,3].
During the IFE’s development, they produce higher WNT signals than other cells. However, if the underlying mesenchyme produces a high level of Bone Morphogenetic Protein (BMP) signal, the IFE will fail to form morphogen gradient and stratify to form IFE. Artificial stretching of keratinocytes promotes mitotic signaling pathways such as MAPK and PKC, and this effect can be weakened by blocking integrins. Epidermis progenitor cells can sense their body deformation and activate the appropriate signal pathway [2,4]. The expression of yes-associated protein (YAP) in epidermal stem cells and progenitor cells is very active after birth. Studies have shown that YAP plays an active molecular switch role in the IFE. At the same time, YAP may also be an important therapeutic target for some skin diseases such as skin cancer [2] (Figure 1 and Table 1).
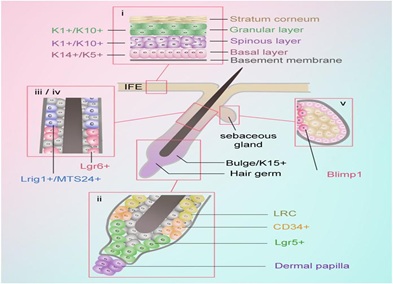 Figure 1:
Figure 1: Stem cells and progenitors in skin. i. K5+ and K14+ mainly exist in basal layer, while K10+are in Basal super stratum. K5, Keratin-5. K14, Keratin-14. K10, Keratin-10 ii. CD34+ exists in the bulge region and Lgr5+ exists in the lower outer root sheath of anagen hair follicles. Lgr, Leucine-rich repeat-containing GPCR. iii. Lgr6+ exists in the region above the follicle bulge. iv. Lrig1+/MTS24+ exist in the region between the sebaceous glands and infundibulum. Lrig1, Leucine-rich repeats and immunoglobulin-like domains protein 1. v. Blimp1+exists in the base of the sebaceous glands. Blimp1, B-lymphocyte-induced maturation protein 1.
|
Stem Cell location
|
Marker
|
Position
|
Regenerative Potentia
|
|
IFE
|
K5+
|
Basal layer
|
Have infinite proliferative capacity and can proliferate and differentiate into various functional cell groups in epidermis.
|
|
K14+
|
|
K10+
|
Basal superstratum
|
|
Hair Follicle
|
CD34+
|
The bulge region
|
All cells play an important role in HFs regeneration. Lgr5+, Lrig1+ and MTS24+ cells give rise to all cell lineages of cycling portion of hair follicle. Lgr6+ cells participate in long-term wound repair, including the formation of new HFs. K15+ cells generate new hair follicle and hair at the onset of anagen
|
|
K15+
|
|
Lgr5+
|
In the lower outer root sheath of anagen hair follicles
|
|
Lgr6+
|
The region above the follicle bulge
|
|
Lrig1+/MTS24+
|
Between the sebaceous glands and infundibulum
|
|
SG
|
Blimp1+
|
The base of the sebaceous glands
|
Sebaceous gland stem cells is an at least bipotent epidermal stem cell population. In addition to give raise to mature sebocytes, sebaceous gland stem cells can also differentiate other cells, such as involucrin positive cells.
|
|
SKPs
|
Sox2
|
DP is an enrichment niche of SKP. Beside DP, SKPs also exist in other hair follicular structures (such as bulge area) or extra follicular structures in dermis.
|
SKPs can clone and reconstruct dermis. They can maintain their multipotentiality and their ability to self-renew within their hair follicle niche, and that they can serially induce hair follicle formation,
|
|
Snail
|
|
Nestin1
|
|
PDGFRα
|
|
Slug
|
|
Twsit1
|
|
Versican
|
Table 1: Location, marker, position and regenerative potential of stem cells in skin.
STEM CELLS IN HAIR FOLLICLE
Hair Follicle (HF) consists of a bulge region, and a cycling proportion which undergoes cycles of growth (anagen), retraction (catagen), and resting (telogen) phases [5]. Many studies have found that Hair Follicle Stem Cells (HF-SCs) are resided in the bulge region, which were firstly described as Label Retaining Cells (LRCs) [6]. The majority of LRCs express CD34, which is one of the most compelling positive markers for murine HF-SCs [7]. Two distinct populations have been isolated in the CD34+ HF-SCs, which is based on α6 integrin expression [8]. Both the α6lowCD34+ and α6highCD34+ bulge cells exhibit the morphological and self-renewal features of stem cells when taken outside of their native niche and exposed to proliferation-inducing conditions, and they play an essential role in HF regeneration [3]. Lgr5, a marker of cycling stem cells in the intestine, is found to be expressed in actively cycling cells in the bulge and hair germ of telogen HFs and in the lower outer root sheath of anagen HFs [4]. In skin reconstitution assay, the Lgr5+ keratinocytes formed the most potent population in HFs regeneration and their regenerating efficiency is much higher than that of CD34+ cells [7]. Further study determined that the Lgr5+ cells in hair germ give rise to all cell lineages of cycling portion of HF, and the progeny provide a constant flux of stem cells downward in the direction of dermal papilla during the growth phase [4]. Keratin-15(K15) is highly expressed in the bulge region, but low expressed in the basal layers of the lower follicle outer-root sheath [7]. K15+ bulge cells contribute to generating new HF and hair at the onset of anagen during the normal HF cycling [9]. Nevertheless, K15+ bulge cell does not contribute to the regeneration process that HFs develops de novo following wounding [10]. Lgr6, as a marker for a distinct population of stem cells, was identified to generate all lineages of the skin. Lgr6+ cells are resided in a previously uncharacterized region directly above the follicle bulge, and these cells were shown to participate in long-term wound repair, including the formation of new HFs [11]. Lrig1, which is the first reported marker of the junctional zone of the HF bulge, SG and infundibulum, defines the HF junctional zone adjacent to the Sebaceous Glands (SG) and infundibulum. Lrig1+ cells can generate all cell types of the adult epidermal lineages in skin reconstitution assays [12]. Researchers found that the contribution of Lrig1+ cells to HFs is evident. These studies suggest that Lrig1+ cells play a potential role in HF regeneration [7]. Similarly, the cell-surface marker MTS24 is detected in a previously uncharacterized population of HF keratinocytes, which is located between the bulge and the SG. MTS24+ keratinocytes represent an important new committed progenitor or stem cell compartment within the HF [13] (Figure 1 and Table 1).
STEM CELLS IN SEBACEOUS GLAND
The pilosebaceous unit composed of Hair Follicle (HF) and Sebaceous Gland (SG), is formed during late embryogenesis and early postnatal stages [14]. According to several basic morphological criteria, the process of formation of the pilosebaceous unit has classified into eight stages and the SG is the last lineage to develop at stage 5 of HF morphogenesis [15,16]. The SG locates beneath the infundibulum and above the HF bulge stem cell compartment.
Sebum secretion is accompanied by the disintegration of glandular cells [17]. The constant sebocytes renewal requires a continuous source of cells to maintain the gland, which implies the importance of stem cells to fuel the degraded mature sebocytes. So far, there is evidence to support the existence of a unipotent sebaceous stem cell pool: the cells at the base of the SG should be marked by expression of Blimp1 [18]. Blimp1 (PRDM-1) is a transcriptional repressor that was first identified as a master regulator of differentiation of plasma cells from B cells [19]. Genetic lineage tracing experiments showed that the Blimp1-expressing cells are progenitors of all cells in the SG. However, Blimp1 is not only selectively and specifically expressed in sebaceous progenitors, but also expressed in terminal differentiation cells of IFE, SGs and HFs [20].
In addition, several stem and progenitor cell compartments distributed along the HF structure can also contribute to the continuous renewal of the SG [21]. Study has identified Lgr6 as a marker for a distinct stem cells population that produce all lineages of the skin [22]. The Lgr6+ cells close to the HF bulge can renew the sebaceous cells [22]. And the cell-surface marker MTS24 can identify a new reservoir of HF keratinocytes [23]. MTS24+keratinocytes can replenish the IFE, SG and/or HF lineages [23]. Furthermore, the transmembrane protein Lrig1 is a marker of human interfollicular epidermal stem cells and helps maintain stem cell quiescence; and it labelled the junctional zone close to the SG [24]. Previous studies support that the Lrig1+ cells of the junctional zone can replenish the IFE and SG. The Lrig1 expressing compartment has been identified as sebocyte precursor cells, which not only give rise to the SG by asymmetric cell fate decision but also drive SG morphogenesis at early postnatal stages [14].
SG-SCs is a bipotent epidermal stem cell population. In addition to give rise to mature sebocytes, SG-SCs can also differentiate into other cells. For example, a human sebocyte line Seb-E6E7 can differentiate into involucrin positive cells [25]. Clonogenic immortalized human sebaceous gland cells (SZ95 sebocytes) express involucrin and cornifin, which are the differentiation markers expressed in the interfollicular and HF inner root sheath [20]. However, when SZ95 sebocytes were injected into nude mice, the cells underwent epidermal differentiation and did not form HFs, so it was concluded that the SG-SCs had bipotent potential rather than multiple potential [20].
Further studies showed that activation of c-Myc contributes to differentiation of the IFE to SG, and leads to the existence of differentiated sebocytes in the IFE. The Hedgehog signaling can promote sebaceous glad differentiation in IFE (Figure 1 and Table 1).
SKPS
Skin-Derived Precursor Cells (SKPs) are an endogenous multipotent precursor cell present in human skin that can be isolated, expanded and differentiate into both neural and mesodermal cell types [26]. Endogenous SKPs can first be isolated from skin during embryogenesis and they persist into adulthood, with a niche in the papillae of hair and whisker follicles. Furthermore, lineage analysis indicates that both hair and whisker follicle dermal papillae contain neural-crest-derived cells, so that SKPs represent an endogenous embryonic precursor cell that arises in peripheral tissues such as skin during development and maintains multipotency into adulthood [27].
SKPs derive from Sox2+ follicle-associated precursors, and they can contribute dermal cells for tissue maintenance, wound-healing, and HF morphogenesis [28]. SKPs are conventionally formed from dissociated dermis; it is therefore unsurprising that a recent study has described an alternative technique whereby SKP-like structures are formed from monolayer dermal cultures (henceforth termed m-SKPs) [29]. In monolayer culture adult, human dermal fibroblast did not express the neural crest stem cell marker nestin or the undifferentiated mesenchymal stem cell marker versican. However, upon m-SKP formation both of these stem cell markers were up-regulated regardless of fibroblast passage number, body site or disease status [30]. Furthermore, Dermal Papilla Cells (DP) are an enrichment niche of SKPs that SKPs not only express embryonic transcription factors such as slug, snail and twist together, but also express specific DP markers such as nestin, versican and PDGFRα [24,27]. Besides DP, SKPs also exist in other hair follicular structures (such as bulge area) or extra follicular structures in dermis [23]. Ruetze et al., isolated SKPs from the skin of the abdomen without HFs. The results showed that the selected SKPs were mainly derived from the capillary circumference in the dermis [15]. In addition, SKPs could actively integrate into a HF niche [28]. In transplant experiments, some transplanted SKPs homed back to the follicle DP and Dermal Sheath (DS), where they appropriately expressed the DP markers or the DS markers. Interestingly, transplanted cells were also present in DP and DS of immature-appearing HFs, suggesting that SKPs contribute to new follicle formation in wounded skin [28].
Within adult dermal tissue, hair dense regions yield more m-SKPs than hair sparse [16]. Hill, R. P et al have found that enriched a-SMA expression within hair dense cultures, over hair sparse equivalents, correlated with an increased m-SKP forming potential. This may be due to a-SMA positive cells being more capable of focal adhesion during m-SKP formation. However, upon formation of m-SKPs a-SMA expression was lost, perhaps other adhesion molecules play a greater role in maintaining m-SKP integrity, or maybe it's more simply by the omission of FBS from SKP proliferation media [17,30].
Moreover, SKPs have the ability of self-proliferation and multi-directional differentiation [18]. Under certain conditions, SKPs can differentiate into neurons and mesodermal lineages, including neurons, glia, smooth muscle cells, osteoblasts and chondrocytes [19,28]. SKPs also can clone and reconstruct dermis. They can maintain the multipotentiality and self-renewal ability within their HF niche, and they can serially induce HF formation. It supports that SKPs display all of the properties predicted of dermal stem cells, as well as having ability to induce hair morphogenesis and maintain the dermis [28]. Because of the self-proliferation and pluripotency of SKPs, it has potential clinical application value in the damage repair of related tissues and organs, which makes SKPs become a research hotspot.
SKIN DERIVED STEM CELLS APPLICATION AND PERSPECTIVES
A comprehensive understanding of the niche, molecular identity and functions of the stem cells in skin such as IFE-SCs, HFSCs and SG-SCs, is an important step for their promising applications in clinic. IFE-SCs produce highly proliferative juvenile TA cells through their self-renewal activity, during skin injury TA cells play an immediate role in reconstituting the damaged tissue. This highlight a novel therapeutic approach of epidermal degenerative diseases such as chronic wounding, as the cultured TA cells could be used to promote the healing of chronic wound [20]. K15+ and Lgr5+ bulge cells are proved to be activated and participated in epidermal reepithelization during skin injury [9,21]. In addition, recent studies found that cultured HFSCs can accelerate skin wound healing, which provide an alternative cell source to treat wound healing [22]. In addition to promote wound healing, both freshly isolated and cultured mouse HFSCs are capable to regenerate de novo functional hair follicles and SGs. This is promising in regenerating human hair follicle to treat disease such as alopecia [14]. Moreover, mouse pluripotent stem cells can give raise to functional hair follicle structure in 3D organoids culture. This provides a valuable approach which not only is promising in regenerated functional hair follicle, but also provides a unique model for testing drug in treating skin and hair follicle related disease [25]. Furthermore, SKPs showed long term in vitro self-renewal capacity; and this highlight their clinical application to be used for skin and hair follicle regeneration, also possibly be used to other regeneration medicine, such as be transdifferentiated to neural cells.
CONCLUSION
We summarized four kinds of stem cells in skin tissue: IFE-SCs, HF-SCs, SG-SCs and SKPs. The main markers of IFE-SCs in Basal layer are K5 and K14, while those in Basal superstratum are K10. IFE-SCs have infinite proliferative capacity and can differentiate into various functional cells in epidermis. HF-SCs mainly located in bulge region and a junctional zone between the HF bulge and SGs. The main markers of HF-SCs in HF bulge are CD34 and Lgr5, and HF-SCs above HF bulge express Lgr6 and Lrig1. HF-SCs play an important role in HF regeneration. SG-SCs, which can maintain the continuous renewal of adipocytes in glands, is located below the infundibulum and above the HF bulge stem cell compartment. The most representative marker of SG-SCs is Blimp1, in addition to Lgr6 and Lrig1. SKPs are an endogenous multipotent precursor cell present in human skin that can be isolated and expanded and differentiate into both neural and mesodermal cell types. SKPs not only express embryonic transcription factors such as slug, snail and twist, but also express specific DP markers such as nestin, versican and PDGFRα. Even certain progresses have achieved in exploring the stem cells reside in skin tissue, further study is still desired for a better understanding of stem cell niche and their characterizations in skin. With better understanding of above knowledge about stem cells in skin, more solutions could be developed to circumvent the challenges in skin diseases, such as wound healing, alopecia and skin tumors.
ACKNOWLEDGEMENT
There are many excellent studies in stem cell research field that we were unable to cover due to space constraints. We apologize to those authors whose work we have omitted.
REFERENCES
- Gonzales KAU, Fuchs E (2017) Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev Cell 43: 387-401.
- Beverdam A, Claxton C, Zhang X, James G, Harvey KF, et al. (2013) Yap Controls Stem/Progenitor Cell Proliferation in the Mouse Postnatal Epidermis. J Invest Dermatol 133: 1497-1505.
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635-648.
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, et al. (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291-1299.
- Alonso L, Fuchs E (2006) The hair cycle. J Cell Sci 119: 391-393.
- Wang X (2019) Stem cells in tissues, organoids, and cancers. Cell Mol Life Sci Pg no: 1-28.
- Wang X, Tredget EE, Wu Y (2012) Dynamic signals for hair follicle development and regeneration. Stem Cells Dev 21: 7-18.
- Soteriou D, Kostic L, Sedov E, Yosefzon Y, Steller H, et al. (2016) Isolating Hair Follicle Stem Cells and Epidermal Keratinocytes from Dorsal Mouse Skin. J Vis Exp 110: 53931.
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, et al. (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411-417.
- Ito M, Yang Z, Andl T, Cui C, Kim N, et al. (2007) Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316-320.
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, et al. (2010) Lgr6 Marks Stem Cells in the Hair Follicle That Generate All Cell Lineages of the Skin. Science 327: 1385-1389.
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, et al. (2009) Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4: 427-439.
- Nijhof JG, Braun KM, Giangreco A, van Pelt C, Kawamoto H, et al. (2006) The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133: 3027-3037.
- Wang X, Liu J, Cai T, Guo L, Wang S, et al. (2016) Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors. Stem Cells Transl Med 5: 1695-1706.
- Ruetze M, Knauer T, Gallinat S, Wenck H, Achterberg V, et al. (2013) A novel niche for skin derived precursors in non-follicular skin. J Dermatol Sci 69: 132-139.
- Biernaskie JA, McKenzie IA, Toma JG, Miller FD (2006) Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc 1: 2803-2812.
- Steinbach SK, El-Mounayri O, DaCosta RS, Frontini MJ, Nong Z, et al. (2011) Directed Differentiation of Skin-Derived Precursors Into Functional Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 31: 2938-2948.
- Fan Z, Chu W, Wu Y (2019) The influence of keratinocytes conditioned medium on skin-derived precursors. Journal of Yunnan University. Natural Science 41: 411-415.
- Agabalyan NA, Rosin NL, Rahmani W, Biernaskie J (2017) Hair follicle dermal stem cells and skin-derived precursor cells: Exciting tools for endogenous and exogenous therapies. Exp Dermatol 26: 505-509.
- Senoo M (2013) Epidermal Stem Cells in Homeostasis and Wound Repair of the Skin. Adv Wound Care (New Rochelle) 2: 273-282.
- Wang X, Chen H, Tian R, Zhang Y, Drutskaya MS, et al. (2017) Macrophages induce AKT/β-catenin-dependent Lgr5+ stem cell activation and hair follicle regeneration through TNF. Nat Commun 8: 14091.
- Heidari F, Yari A, Rasoolijazi H, Soleimani M, Dehpoor A, et al. (2016) Bulge Hair Follicle Stem Cells Accelerate Cutaneous Wound Healing in Rats. Wounds 28: 132-141.
- Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, et al. (2006) Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol 175: 1005-1015.
- Chen RS, Miao Y, Hu ZQ (2017) [Research progress of skin-derived precursor cells]. Nan Fang Yi Ke Da Xue Xue Bao 37: 420-422.
- Toyoshima K, Ogawa M, Tsuji T (2019) Regeneration of a bioengineered 3D integumentary organ system from iPS cells. Nature Protocols 14: 1323-1338.
- Toma JG, McKenzie IA, Bagli D, Miller FD (2005) Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 23: 727-737.
- Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, et al. (2004) A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 6: 1082-1093.
- Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, et al. (2009) SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells. Cell Stem Cell 5: 610-623.
- Wenzel V, Roedl D, Gabriel D, Gordon LB, Herlyn M, et al. (2012) Naive adult stem cells from patients with Hutchinson-Gilford progeria syndrome express low levels of progerin in vivo. Biol Open 1: 516-526.
- Hill RP, Gledhill K, Gardner A, Higgins CA, Crawford H, et al. (2012) Generation and Characterization of Multipotent Stem Cells from Established Dermal Cultures. Plos One 7: 50742.

 Figure 1: Stem cells and progenitors in skin. i. K5+ and K14+ mainly exist in basal layer, while K10+are in Basal super stratum. K5, Keratin-5. K14, Keratin-14. K10, Keratin-10 ii. CD34+ exists in the bulge region and Lgr5+ exists in the lower outer root sheath of anagen hair follicles. Lgr, Leucine-rich repeat-containing GPCR. iii. Lgr6+ exists in the region above the follicle bulge. iv. Lrig1+/MTS24+ exist in the region between the sebaceous glands and infundibulum. Lrig1, Leucine-rich repeats and immunoglobulin-like domains protein 1. v. Blimp1+exists in the base of the sebaceous glands. Blimp1, B-lymphocyte-induced maturation protein 1.
Figure 1: Stem cells and progenitors in skin. i. K5+ and K14+ mainly exist in basal layer, while K10+are in Basal super stratum. K5, Keratin-5. K14, Keratin-14. K10, Keratin-10 ii. CD34+ exists in the bulge region and Lgr5+ exists in the lower outer root sheath of anagen hair follicles. Lgr, Leucine-rich repeat-containing GPCR. iii. Lgr6+ exists in the region above the follicle bulge. iv. Lrig1+/MTS24+ exist in the region between the sebaceous glands and infundibulum. Lrig1, Leucine-rich repeats and immunoglobulin-like domains protein 1. v. Blimp1+exists in the base of the sebaceous glands. Blimp1, B-lymphocyte-induced maturation protein 1.
