
Journal of Stem Cells Research Development & Therapy Category: Medical
Type: Research Article
The Biologic Treatment of Osteoarthritis with Mesenchymal Stem Cell Exosomes: The Future is Now
*Corresponding Author(s):
Kenneth A Pettine M.DPaisley Laboratories, 2552 Barry Ln., Ft. Collins, Colorado, United States
Email:ken@paisleylaboratories.com
Received Date: Feb 15, 2019
Accepted Date: Mar 06, 2019
Published Date: Mar 13, 2019
Abstract
Over the last several years it has become increasingly understood by researchers and clinicians that the clinical efficacy of utilizing Mesenchymal Stem Cells (MSCs) to treat Osteoarthritis (OA) is not dependent on the cells differentiating into articular cartilage but entirely on their paracrine release of Growth Factors (GFs) and exosomes. Living MSCs are not required to accomplish the release of GFs and exosomes into an arthritic joint. The purpose of this paper is to introduce the concept of using acellular MSC exosomes to treat OA and the rationale of why acellular will replace all current cellular therapies both autogenous and allogeneic.
Keywords
Bone marrow concentrate; Cell-based therapy; Exosomes; Mesenchymal stem cells; Stem cells
WHAT CAUSES THE PAIN IN OSTEOARTHRITIS?
In patients with Osteoarthritis (OA), why is the correlation between what a patients imaging studies look like and their report of pain so low? Articular cartilage has no direct nerve or blood supply. Without direct innervation, cartilage is incapable of generating pain [1].
In contrast, the synovium and joint capsule are richly innervated and are likely the primary source of the pain in OA [2]. The synovial reaction in OA includes synovial hyperplasia, fibrosis, thickening of the synovial capsule, activated synoviocytes and in some cases lymphocytic infiltrate (B- and T-cells as well as plasma cells) [3-7]. The synovium is of obvious relevance as one of the most densely innervated structures of the joint. The white adipose tissue of the fat pad can also show evidence of inflammation and act as a rich source of inflammatory adipokines [8]. Synovial causes of pain include irritation of sensory nerve endings within the synovium from osteophytes and synovial inflammation that is due, at least in part, to the release of prostaglandins, leukotrienes, proteinases, neuropeptides and cytokines. Pro-inflammatory examples include Interleukins 1,6 and 8 along with various tumor necrosis factors. A semi-quantitative measure of synovitis from the infrapatellar fat pad is associated with pain severity. Any decrease in synovitis is associated with a reductionin OA pain severity [9].
In contrast, the synovium and joint capsule are richly innervated and are likely the primary source of the pain in OA [2]. The synovial reaction in OA includes synovial hyperplasia, fibrosis, thickening of the synovial capsule, activated synoviocytes and in some cases lymphocytic infiltrate (B- and T-cells as well as plasma cells) [3-7]. The synovium is of obvious relevance as one of the most densely innervated structures of the joint. The white adipose tissue of the fat pad can also show evidence of inflammation and act as a rich source of inflammatory adipokines [8]. Synovial causes of pain include irritation of sensory nerve endings within the synovium from osteophytes and synovial inflammation that is due, at least in part, to the release of prostaglandins, leukotrienes, proteinases, neuropeptides and cytokines. Pro-inflammatory examples include Interleukins 1,6 and 8 along with various tumor necrosis factors. A semi-quantitative measure of synovitis from the infrapatellar fat pad is associated with pain severity. Any decrease in synovitis is associated with a reductionin OA pain severity [9].
WHAT IS THE FUTURE FOR USING MSC EXOSOMES TO TREAT OA?
The MSC has always been the primary cell for orthopedics because only it can become a chondroblast, osteoblast or fibroblast. Recently we have begun to appreciate and realize the MSC may be the most critical cell in your body because of what it releases to communicate with other cells. The MSC modulates your immune system to control inflammation by releasing exosomes, secretomes, growth factors, cytokines, and chemokines. These proteins are what is essential in regenerative medicine, NOT the MSC itself. Caplan even suggests changing the name to Medicinal Signaling Cells [10].
Arnold Caplan, the Ph.D. responsible for naming the Mesenchymal Stem Cell (MSC) states, “Now that Mesenchymal Stem Cells (MSCs) have been shown to be perivascular in vivo, the existing traditional view that focuses on the multipotent differentiation capacity of these cells should be expanded to include their equally interesting role as cellular modulators that brings them into a broader therapeutic scenario. We discuss existing evidence that leads us to propose that during local injury, MSCs are released from their perivascular location, become activated, and establish a regenerative microenvironment by secreting bioactive molecules and regulating the local immune response. These trophic and immunomodulatory activities suggest that MSCs may serve as site-regulated ‘‘drugstores’’ in vivo” [11]. Extensive research has shown Caplan to be correct.
The MSC produces numerous growth factor proteins to treat orthopedic pathology. But the most crucial paracrine method by which the MSC functions may be through the creation of the acellular structure named the exosome [12-15]. The exosome is a tiny 30 to 150 nanometer-sized (1 billionth of a meter) bi-phospholipid membrane-enclosed structure created by the Golgi body or apparatus. An MSC (12 to 18 microns) is 1,000 times larger than an exosome. The diameter of a hair is 80,000 nanometers. Exosomes contain growth factors, signaling lipids and micro, and messenger RNA. The RNA contents in exosomes mediate most of their anti-inflammatory effects. The RNA is placed into an exosome along with numerous peptide growth factors and signaling lipids by the Golgi bodies within the donor MSC. The exact type and amount of growth factor proteins, signaling lipids, and RNA placed into an exosome are dependent on the surrounding microenvironment of the MSC. The exosomes are released into the extracellular matrix and taken up by a receptor cell. The exosome RNA is then taken into the receptor cell ribosome where the RNA is translated to create numerous anti-inflammatory growth factors, chemokines, cytokines, and secretomes. This processis illustrated in figure 1. Exosomes do not elicit acute immune rejection, and there is no risk for tumor formation. The effect of exosome RNA may last months or longer as the receptor cell ribosomes continue to translate the donor RNA [16-20]. BEES MAKE HONEY AND MSCs MAKE EXOSOMES [21].
Arnold Caplan, the Ph.D. responsible for naming the Mesenchymal Stem Cell (MSC) states, “Now that Mesenchymal Stem Cells (MSCs) have been shown to be perivascular in vivo, the existing traditional view that focuses on the multipotent differentiation capacity of these cells should be expanded to include their equally interesting role as cellular modulators that brings them into a broader therapeutic scenario. We discuss existing evidence that leads us to propose that during local injury, MSCs are released from their perivascular location, become activated, and establish a regenerative microenvironment by secreting bioactive molecules and regulating the local immune response. These trophic and immunomodulatory activities suggest that MSCs may serve as site-regulated ‘‘drugstores’’ in vivo” [11]. Extensive research has shown Caplan to be correct.
The MSC produces numerous growth factor proteins to treat orthopedic pathology. But the most crucial paracrine method by which the MSC functions may be through the creation of the acellular structure named the exosome [12-15]. The exosome is a tiny 30 to 150 nanometer-sized (1 billionth of a meter) bi-phospholipid membrane-enclosed structure created by the Golgi body or apparatus. An MSC (12 to 18 microns) is 1,000 times larger than an exosome. The diameter of a hair is 80,000 nanometers. Exosomes contain growth factors, signaling lipids and micro, and messenger RNA. The RNA contents in exosomes mediate most of their anti-inflammatory effects. The RNA is placed into an exosome along with numerous peptide growth factors and signaling lipids by the Golgi bodies within the donor MSC. The exact type and amount of growth factor proteins, signaling lipids, and RNA placed into an exosome are dependent on the surrounding microenvironment of the MSC. The exosomes are released into the extracellular matrix and taken up by a receptor cell. The exosome RNA is then taken into the receptor cell ribosome where the RNA is translated to create numerous anti-inflammatory growth factors, chemokines, cytokines, and secretomes. This processis illustrated in figure 1. Exosomes do not elicit acute immune rejection, and there is no risk for tumor formation. The effect of exosome RNA may last months or longer as the receptor cell ribosomes continue to translate the donor RNA [16-20]. BEES MAKE HONEY AND MSCs MAKE EXOSOMES [21].
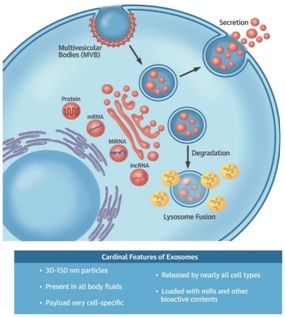
Figure 1: (Top) Schematic of exosome biogenesis. Exosomes arise from the fusion of surface membrane invaginations (multi-vesicular bodies) and the products of the Golgi apparatus. The resulting vesicles are either degraded by lysosomes or secreted as exosomes. (Bottom) Cardinal features of exosomes.
The amount of scientific research on exosomes has dramatically increased since 2008 when it was discovered exosomes contain DNA. There were 28 scientific citations on exosomes in 1996 and 24,765 in 2016 [22]. Exosome research has created a renaissance in our understanding of cellular communication. Cells communicate near and far by a dynamic of exosome secretion and uptake. Extensive research is ongoing for utilizing exosomes for the treatment of various autoimmune conditions, the diagnosis and cure for various cancers and in treating cardiac diseases.
Phinney recently published an excellent concise-review on MSC-derived exosomes for cell-free therapy and stated: “The majority of the published MSC exosome literature recapitulates in large part the nature and scope of that previously devoted to the study of MSC action in animal models of disease. For example, various groups have confirmed that MSC-derived exosomes exhibit cardio and renal-protective activity, are efficacious in animal models of myocardial infarction, stroke, perinatal hypoxic-ischemic brain injury, and hind-limb ischemia. The MSC-derived exosomes also ameliorated carbon tetrachloride-induced liver fibrosis and conferred cytoprotective effects in models of necrotizing enterocolitis. In lung studies, the mouse MSC exosomes were effective in improving pulmonary hypertension, silicosis and human MSC-exosomes improved endotoxin-induced pulmonary edema and cleared alveolar fluid from human lungs. Other studies have shown that MSC-derived exosomes also promoted re-epithelialization of cutaneous wounds by inducing epithelial cell proliferation and angiogenesis, activated collagen and elastin secretion by fibroblasts and prevented myofibroblast formation thereby reducing scarring. The MSC-derived exosomes also promoted muscle regeneration, protected against experimental colitis and exhibited potent neuroprotective activities in neurons and in models of traumatic brain injury. MSC-derived exosomes are also immunologically active based on evidence that they suppressed proliferation and IFN-γ secretion by T cells stimulated with anti-CD3 and anti-CD28 antibodies and also enhanced the survival of allogeneic skin grafts in mice by enhancing T cell polarization to a regulatory phenotype. A growing number of studies suggest that MSC-derived exosomes mimic the ability of MSCs to influence the activity of immune effector cells including B, T, NK, dendritic cells, and macrophages although not all studies show positive effects. Collectively, these studies readily demonstrate that MSC-derived exosomes recapitulate to a large extent the immensely broad therapeutic effects previously attributed to MSCs” [23].
This is illustrated in figure 2. “Think of the extracellular space as a sea containing trillions of messages in a bottle, quickly read and answered, always turning over, and you begin to get a sense of what is going on inside us every moment of every day” [24].
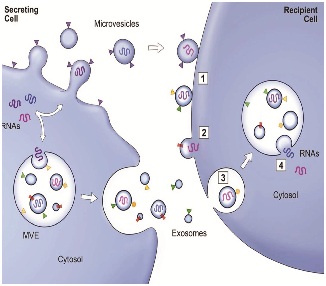 Figure 2: Exosome secretion and uptake.
Figure 2: Exosome secretion and uptake.WHAT IS THE FUTURE FOR USING MSC EXOSOMES TO TREAT OSTEOARTHRITIS?
MSCs direct the anti-inflammatory function of other cells by releasing exosomes into the ECM. The future of using MSCs to treat OA will be to expand the MSCs in a defined growth media. The cells are then subjected to 48 hrs. of “stress” conditions of hypoxia, low glucose and low pH to maximize their release of anti-inflammatory exosomes. The growth media is collected, and the exosomes separated and stored for future use [24]. The future of regenerative medicine is the use of ACELLULAR vs. cellular products. Acellular MSC derived exosomes can provide a consistent product that can have proteomic analysis and RNA sequencing. Every growth factor can be identified and quantified. Every micro and messenger RNA can be characterized. Think of acellular MSC exosomes as a bio-pharmacological quality product that can be standardized and tested regarding dose and biological activity.
None of this is possible with a cellular product. Perhaps most important is that an acellular product will not introduce extensive foreign DNA into the recipient patient that an allogeneic cellular source does. No one knows the long-term effects of having foreign DNA. Is it carcinogenic? Replacing the administration of live cells with acellular exosomes will mitigate the safety concerns and limitations associated with the transplantation of viable replicating cells [25].
MSC derived exosomes do not have any of the immunogenic concerns related to the administration of allogeneic cellular products. Acellular exosome ‘off-the-shelf’ products have no immunogenicity [26]. There have been measured to be 1010 exosomes per cc of conditioned growth media [27]. Exosomes can be stored at room temperature for up to three years with no loss to their biological activity [28,29]. In contrast to cell-based therapy, MSC derived exosomes provide an ‘off-the-shelf’ therapeutic product that has safety and may have clinical efficacy superior to any allogeneic MSC treatment for orthopedics and spine [23,30].
None of this is possible with a cellular product. Perhaps most important is that an acellular product will not introduce extensive foreign DNA into the recipient patient that an allogeneic cellular source does. No one knows the long-term effects of having foreign DNA. Is it carcinogenic? Replacing the administration of live cells with acellular exosomes will mitigate the safety concerns and limitations associated with the transplantation of viable replicating cells [25].
MSC derived exosomes do not have any of the immunogenic concerns related to the administration of allogeneic cellular products. Acellular exosome ‘off-the-shelf’ products have no immunogenicity [26]. There have been measured to be 1010 exosomes per cc of conditioned growth media [27]. Exosomes can be stored at room temperature for up to three years with no loss to their biological activity [28,29]. In contrast to cell-based therapy, MSC derived exosomes provide an ‘off-the-shelf’ therapeutic product that has safety and may have clinical efficacy superior to any allogeneic MSC treatment for orthopedics and spine [23,30].
THE ACELLULAR MSC PARACRINE TREATMENT FOR OA
All di-arthrodial joints have a synovial lining and a joint capsule. The synovial capsules contain numerous synovial MSCs (more than found in bone marrow or adipose). These MSCs have more chondrogenic potential than bone or adipose MSCs [6,7]. During the development of OA, pro-inflammatory growth factors are produced by these synovial MSCs. This creates a chronically inflamed painful joint environment. Bone Marrow Concentrate (BMC) contains on average only about 2,500 MSCs per cc [27]. Despite the incredibly small number of MSCs found in BMC; there is extensive literature reporting clinical efficacy in animals and humans using BMC for the treatment of OA. This effect cannot be dependent upon BMC/MSC cell survival or differentiation. The efficacious effect must be from the release of acellular paracrine factors. The future of the biologic treatment of OA will be the utilization of acellular MSC derived growth factors, secretomes, chemokines, cytokines and especially exosomes. These paracrine factors can be placed into the knee joint in concentrations of 100,000 or more times that of any cellular MSC treatment. These proteins and exosomes will function in a paracrine fashion to, directly and indirectly, alter the inflammatory environment of any painful arthritic joint to a normal non-painful physiologic environment. Zhang et al., [31] investigated the cellular processes modulated by MSC exosomes and the mechanism of action underlying the exosome-mediated responses in cartilage repair. They observed that exosome-mediated repair of osteochondral defects was characterized by increased cellular proliferation and infiltration, enhanced matrix synthesis and a regenerative immune phenotype. Using chondrocyte cultures, they could attribute the rapid cellular proliferation and infiltration during exosome-mediated cartilage repair to exosomal CD73-mediated adenosine activation of AKT and ERK signaling. Inhibitors of AKT or ERK phosphorylation suppressed an exosome-mediated increase in cell proliferation and migration but not matrix synthesis. The role of exosomal CD73 was confirmed by the attenuation of AKT and ERK signaling by AMPCP, a CD73 inhibitor, and theophylline, an adenosine receptor antagonist. Exosome-treated defects also displayed a regenerative immune phenotype characterized by a higher infiltration of CD163+ regenerative M2 macrophages over CD86+ M1 macrophages, with a concomitant reduction in pro-inflammatory synovial cytokines IL-1β and TNF-α. Together, these observations demonstrated that the efficient osteochondral regeneration by MSC exosomes was effected through a coordinated mobilization of multiple cell types and activation of several cellular processes.
The future acellular treatment for OA will involve a two-front attack. First, highly concentrated anti-inflammatory MSC derived growth factors are injected into the arthritic joint. These growth factors will enter the nucleus of the recipient synovial MSC. The donor growth factors will stimulate DNA transcription of mRNA containing instructions for the production of continuous anti-inflammatory secretomes, chemokines, and cytokines. These will be released from the recipient synovial MSC into the synovial fluid. Second, the highly concentrated donor exosomes will enter recipient synovial MSCs to deliver their mRNA. This delivered mRNA will directly undergo translation in the recipient synovial MSC ribosomes to produce anti-inflammatory secretomes, cytokines, and chemokines. These salubrious effects could last months or years. This acellular biologic treatment can all be achieved with a single arthritic joint injection, not requiring the morbidity and cost of obtaining autogenous MSCs. The future of regenerative medicine in orthopedics and spine may well be the utilization of highly concentrated acellular MSC derived growth factors and especially EXOSOMES [32-34]. Figure 3 illustrates some of the mechanisms on how a single injection of bone marrow-derived MSC exosomes can have a salubrious effect on returning the inflamed OA joint to a normal physiologic state.
The future acellular treatment for OA will involve a two-front attack. First, highly concentrated anti-inflammatory MSC derived growth factors are injected into the arthritic joint. These growth factors will enter the nucleus of the recipient synovial MSC. The donor growth factors will stimulate DNA transcription of mRNA containing instructions for the production of continuous anti-inflammatory secretomes, chemokines, and cytokines. These will be released from the recipient synovial MSC into the synovial fluid. Second, the highly concentrated donor exosomes will enter recipient synovial MSCs to deliver their mRNA. This delivered mRNA will directly undergo translation in the recipient synovial MSC ribosomes to produce anti-inflammatory secretomes, cytokines, and chemokines. These salubrious effects could last months or years. This acellular biologic treatment can all be achieved with a single arthritic joint injection, not requiring the morbidity and cost of obtaining autogenous MSCs. The future of regenerative medicine in orthopedics and spine may well be the utilization of highly concentrated acellular MSC derived growth factors and especially EXOSOMES [32-34]. Figure 3 illustrates some of the mechanisms on how a single injection of bone marrow-derived MSC exosomes can have a salubrious effect on returning the inflamed OA joint to a normal physiologic state.
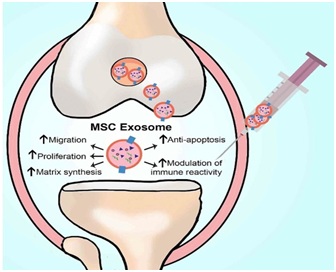 Figure 3: Diagram of the MSC Exosome treatment for OA [31]
Figure 3: Diagram of the MSC Exosome treatment for OA [31]CONCLUSION
The pain of osteoarthritis is mostly the result of inflammation of the synovial capsule. This synovitis is perpetuated by the intra-articular synovial mesenchymal stem cells. Bone marrow MSCs create intracellular exosomes that are filled with hundreds of various anti-inflammatory growth factors along with micro and messenger RNA. The anti-inflammatory growth factors and RNA redirect the synovial MSC's from inflammation to a physiologic state. Autogenous cellular treatments have no consistency between donors. Allogeneic cellular products introduce large amounts of foreign DNA and after cell death, unwanted cellular debris including membranes, mitochondria, Golgi bodies, cytoplasm, etc., all within the joint creating inflammation. Acellular products can be produced to have a consistent known quantity of GFs and exosomes along with proteomic analysis of the GF proteins and a genetic analysis of the RNA. This represents a bio-pharmacological quality product. Acellular MSC exosomes deliver the positive aspects of cellular therapy without all the negative aspects of cellular therapy.
REFERENCES
- Felson DT (2005) The sources of pain in knee osteoarthritis. Curr Opin Rheumatol 17: 624-628.
- Roach HI, Aigner T, Soder S, Haag J, Welkerling H (2007) Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets 8: 271-282.
- De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44: 1928-1942.
- Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, et al. (2012) Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res 30: 943-949.
- Hunziker EB, Kapfinger E, Geiss J (2007) The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage 15: 403-413.
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52: 2521-2529.
- Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, et al. (2006) Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum 54: 843-853.
- Ushiyama T, Chano T, Inoue K, Matsusue Y (2003) Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis 62: 108-112.
- Hill C, Hunter D, Niu J, Clancy M, Guermazi A, et al. (2005) Changes in synovitis are associated with changes in pain in knee osteoarthritis. Arthritis & Rheumatism 52: 71.
- Caplan AI, Correa D (2011) The MSC: An injury drugstore. Cell Stem Cell 9: 11-15.
- Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076-1084.
- Yeo RWY, Lai RC, Tan KH, Lim SK (2013) Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell. Journal of Circulating Biomarkers 1: 1-12.
- Théry C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3: 15.
- De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC (2014) Extracellular vesicles: potential roles in regenerative medicine. Front Immunol 5: 608.
- Bang C, Thum T (2012) Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol 44: 2060-2064.
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, et al. (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20: 1053-1067.
- Neviani P, Fabbri M (2015) Exosomic microRNAs in the tumor microenvironment. Front Med 2: 47.
- Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, et al. (2014) Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep 8: 1432-1446.
- Dai L, Zhang X, Hu X, Liu Q, Man Z, et al. (2015) Silencing of miR-101 Prevents Cartilage Degradation by Regulating Extracellular Matrix–related Genes in a Rat Model of Osteoarthritis. Mol Ther 23: 1331-1340.
- Clancy S, Brown W (2008) Translation: DNA to mRNA to Protein. Nature Education 1: 101.
- Marban E (2018) The Secret Life of Exosomes. Journal of the American College of Cardiology 71: 193-200.
- Dodson BP, Levine AD (2015) Challenges in the translation and commercialization of cell therapies. BMC Biotechnol 15: 70.
- Phinney DG, Pittenger MF (2017) Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 35: 851-858.
- Yeo RWY, Lai RC, Tan KH, Lim SK (2013) Exosome: A Novel and Safer Therapeutic Refinement of Mesenchymal Stem Cell. Journal of Circulating Biomarkers 1: 1-12.
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820: 940-948.
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, et al. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654-659.
- Pettine KA, Murphy MB, Suzuki RK, Sand TT (2015) Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33: 146-156.
- Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, et al. (2017) Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med 40: 834-844.
- Vishnubhatla I, Corteling R, Stevanato L, Hicks C, Sinden J (2014) The Development of Stem Cell-Derived Exosomes as a Cell-Free Regenerative Medicine. Journal of Circulating Biomarkers 3: 1-14.
- Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, et al. (2016) Stem Cell-Derived Exosomes: A Potential Alternative Therapeutic Agent in Orthopedics. Stem Cells Int 2016: 6.
- Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK et al. (2018) MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 156: 16-27.
- Li Z, Wang Y, Xiao K, Xiang S, Li Z, et al. (2018) Emerging Role of Exosomes in the Joint Diseases. Cell Physiol Biochem 47: 2008-2017.
- Chang YH, Wu KC, Harn HJ, Lin SZ, Ding DC (2018) Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant 27: 349-363.
- Cheng L, Zhang K, Wu S, Cui M, Xu T (2017) Focus on Mesenchymal Stem Cell-Derived Exosomes: Opportunities and Challenges in Cell-Free Therapy. Stem Cells Int 2017: 6305295.
Citation: Pettine KA, Dordevic M (2019) The Biologic Treatment of Osteoarthritis with Mesenchymal Stem Cell Exosomes: The Future is Now. J Stem Cell Res Dev Ther: S1001.
Copyright: © 2019 Kenneth A Pettine M.D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2024, Copyrights Herald Scholarly Open Access. All Rights Reserved!
