
The Efficacy of Liposomal Bupivacaine in Lumbar Spine Surgery
*Corresponding Author(s):
Emmett J GannonDepartment Of Orthopaedic Surgery And Rehabilitation, University Of Nebraska Medical Center, Omaha, Nebraska, United States
Tel:+1 4025598000,
Email:emmett.gannon@unmc.edu
Abstract
Background
Pain management is an important portion of a patient’s postoperative care. Improved pain control can lead to improved outcomes, earlier mobilization, and shortened hospital stays. With the large side-effect profile of narcotics, numerous alternative methods to decrease opioid consumption, attain better pain-control and improve outcomes have been used. The emergence of the long-acting local anesthetic agent, Liposomal Bupivacaine (LB), has shown promise in improving pain control and decreasing opioid consumption.
Study Design
This is a retrospective study.
Objective
This study aims to determine the efficacy of LB in lumbar spine surgery.
Methods
A total of 54 consecutive lumbar spine cases were performed in which LB was administered in all cases. This was then matched to a cohort of 54 patients who underwent lumbar procedures in the period immediately preceding the initial use of LB. Demographic data and hospital Length of Stay (LOS) were recorded. Pain scores were recorded using the Visual Analog Scale (VAS) and were recorded as an average over 8 hour shifts for 72 hours postoperatively. Daily narcotic consumption was also measured and converted to oral morphine equivalents.
Results
There was a statistically significant difference in the mean LOS between the two groups (p=0.0071). The mean LOS for the LB group was 2.04 days and 2.73 days for the control group. A significant difference was also found between the two groups in VAS scores (p=0.016) and narcotic use (p=0.048). The LB group was found to have lower VAS scores and opioid consumption compared to the control group.
Conclusion
The use of liposomal bupivacaine shows promise as an adjuvant for postoperative analgesia in lumbar spine surgery with diminished pain scores, length of stay, and narcotic use. Further investigation with a larger prospective randomized study is warranted to better understand its potential benefit.
INTRODUCTION
Postoperative pain control is an important portion of a patient’s postoperative management. Expectation of outcomes following spinal procedures often focuses on the long-term relief offered by the intervention and the potential severity of the temporal immediate to short-term postoperative pain can be overlooked. Post-surgical pain has been found to be significant enough in some cases to alter the course of rehabilitation and secondarily the long-term result of surgery itself [1]. Adequate pain control has been linked to improved quality of life, patient satisfaction, and clinical outcomes [2]. Obtaining sufficient postoperative pain control can also allow for earlier mobilization and has the ability to shorten a patient’s LOS thereby decreasing hospital costs [3,4]. The current standard therapy for postoperative pain management consists of opioid medications, however, with the current epidemic of overdoses and abuse, physicians must consider the potential risks when formulating a postoperative pain regimen [5,6]. The importance of postoperative pain control and potential detrimental side-effects of opioid medications lends to the importance of finding alternative analgesic interventions with improved adverse-effect profiles [7]. Local anesthetic infiltration is one such modality that has been used as an adjunct postoperative analgesic [3]. The use of standard bupivacaine produces an anesthetic effect that lasts about 9 hours [8]. Due to its relative short duration, there has been an emergence of formulations that can prolong the local anesthetic effect, such as Liposomal Bupivacaine (LB; Exparel, Pacira Pharmaceuticals Inc., Parsippany, NJ, USA). LB consists of 3% free bupivacaine for immediate effects and 97% liposomal-bound bupivacaine, which allows for a slow release and prolonged anesthetic effect lasting up to 72 hours after administration [8,9]. The use of LB in Total Knee Arthroplasty (TKA) has been heavily investigated with studies demonstrating varying results. A few studies have demonstrated an efficacy of LB close to that of regional femoral blockade and others have shown that it can significantly decrease postoperative narcotic use, length of stay, and postoperative Visual Analog Scale (VAS) scores [10-23]. Although many studies have shown the potential advantages of its use, many other studies have exhibited no benefit of its use in TKA [24-30]. The use of LB in the spine has not been investigated near the extent as it has been in TKA and the results from these studies have yet to yield a definitive conclusion.
This study aims to determine the efficacy of LB in postoperative analgesia for patients undergoing lumbar spine surgery and report any adverse outcomes or complications that may be specific to its use around the lumbosacral spine. Decreasing the consumption of opioids, earlier mobilization, decreased hospital costs, and decreased length of stay are all potential benefits of its use.
METHODS AND MATERIALS
The study was a retrospective review of lumbar procedures performed by a single surgeon. LB was used beginning in July 2014 to September 2014. During this period, a total of 54 consecutive lumbar spine cases were performed and LB was administered in all cases. This was then matched to the 54 patients who underwent open lumbar procedures in the period immediately preceding the initial use of LB, ultimately beginning in April 2014. Exclusion criteria were patients with a history of adverse reactions to local anesthetics, chronic use of narcotic agents defined as prescriptions provided for a continuous period exceeding 12 months, or those under the age of 19. Of the 54 patients who received LB, 52 met criteria for inclusion with one being excluded for chronic narcotic use and one patient being less than 19 years old. Of the 54 patients in the control group, 52 met criteria for inclusion with the two exclusions being secondary to chronic narcotic use.
Preparation of the LB consisted of one 20 ml single-dose vial of LB containing 266 mg of bupivacaine and further dilution in an additional 20 ml of normal saline. The entire 40 ml solution was administered in the experimental group immediately prior to closure into the skin and subcutaneous wound bed up to the fascial margin of the erector spinae musculature in multiple small wheals. All wounds were subsequently closed in the same fashion with interrupted absorbable suture with a majority also including a drain exiting through a separate skin incision.
Demographic data and hospital Length of Stay (LOS) defined by number of midnights spent in an inpatient ward were recorded. Pain scores on a standard Visual Analogue Scale (VAS) were recorded as an average over 8 hour shifts for 72 hours postoperatively. Each shift was defined by the hour at which the floor nurse documented arrival of the patient to the ward. Daily total narcotic administration was also collected and converted to oral morphine equivalents using the opioid calculator developed by the Washington State Agency Medical Directors’ Group (AMDG) in the 2015 Interagency Guideline on Prescribing Opioids for Pain [31]. A full neurological exam was performed daily to monitor for any potential complications. This included the documentation of any sensory or motor changes compared to preoperative examination. Both cohorts were separated into decompression alone (laminectomy, foraminotomy, microdiscectomy) or fusion procedures. The procedures involving a fusion were further stratified by number of levels operated on (one-level, two-level, and three or more levels). All fusions were performed in a posterolateral instrumented fashion with or without inter body instrumentation. The decompression group was not further divided by number of levels involved.
Descriptive statistics were used to summarize the distributions of the enrolled patients. Fisher’s exact test was used to compare categorical variables between the two groups. The independent sample t-test was used to compare continuous variables between the two groups. VAS scores and opioid consumption in morphine equivalents were summarized daily by taking the average of the three shifts for each measurement. For example, day 1 VAS score was the average of the VAS score for each of the three shifts on day 1 for a particular patient. The change in opioid consumption and VAS scores over the three day period (i.e., day 1 average VAS, day 2 average VAS and day 3 average VAS) was compared between the two groups using a linear mixed effect model with AR (1) structure to account for the within-patient correlation of repeated measures. Surgery type was also considered as a covariate. Interaction terms were explored as multiple variables were involved. For example, evaluating whether the pattern of change in VAS score over the three-day period differed by group.
RESULTS
A total of 104 patients met inclusion criteria and were analyzed in this study. All of which underwent a lumbar spine procedure by a single surgeon. 52 of these patients received LB and 52 did not receive a local anesthetic. The average age of the LB group was 58.54 and 59.54 for the control group. In the LB group, 28 (53.85%) were female and 24 (46.15%) were male. The control group consisted of 30 (57.69%) females and 22 (42.31%) males. There was no significant difference in mean age or gender between the LB and control groups (p=0.71 and 0.8436). There was a statistically significant difference in the mean LOS between the two groups (p=0.0071). The mean LOS was found to be 2.04 days for the LB group and 2.73 days for the control group (Table 1). When analyzing the LOS by type of procedure, it was found that the average LOS was shorter for the LB compared to the control group for all procedure types except for single level fusions. This procedure group was found to have the same mean LOS, 2.62 days, in both the LB and control groups (Table 1).
|
Length of Stay by Procedure Type and Treatment Group |
|||||
|
Type of Procedure |
Treatment Group |
Total Number Observed |
Average LOS (Days) |
Minimum LOS (Days) |
Maximum LOS (Days) |
|
Isolated Decompression |
LB |
16 |
0.56 |
0 |
2 |
|
Control |
9 |
1.33 |
0 |
3 |
|
|
Single-Level Fusion |
LB |
16 |
2.62 |
1 |
4 |
|
Control |
21 |
2.62 |
1 |
4 |
|
|
Two-Level Fusion |
LB |
10 |
2.50 |
1 |
4 |
|
Control |
13 |
3.08 |
2 |
5 |
|
|
≥ Three-Level Fusion |
LB |
10 |
3.00 |
1 |
5 |
|
Control |
9 |
3.89 |
2 |
5 |
|
|
Total |
LB |
52 |
2.04 |
0 |
5 |
|
Control |
52 |
2.73 |
0 |
5 |
|
Table 1: Length of stay in days by procedure type and treatment group. A significant difference was found between the two groups (p-value=0.0071) with an average LOS of 2.73 days for the control group and 2.04 days for the LB group.
There was no significant interaction found between hospital day, treatment group and type of procedure. More specifically, there was no significant difference found between the treatment groups and the types of procedure performed (p=0.4899, Table 2). In addition, the type of procedure was not associated with VAS score after adjusting for hospital day and treatment group (p=0.66). Hospital day was also not found to be associated with VAS score after adjusting for treatment group and surgery type (p=0.66). In other words, overall VAS scores did not change significantly over the three-day study period. The treatment group, however, was found to be significantly associated with VAS scores after adjusting to hospital day and type of surgery (Table 3). The average VAS score across the three-day period was found to be lower in the LB group compared to the control group (p=0.016) (Figure 1). It was also noted that the mean VAS score was lower in the LB compared to the control group in all procedure types for all three measured hospital days (Table 4).
|
Procedures by Treatment Group |
||||
|
Procedure |
Frequency Percentage |
Treatment Group |
||
|
LB |
Control |
Total |
||
|
Isolated Decompression |
n |
16 |
9 |
25 |
|
Single Level Fusion |
n |
16 |
21 |
37 |
|
Two Level Fusion |
n |
10 |
13 |
23 |
|
Three Level Fusion |
n |
7 |
5 |
12 |
|
> Three Level Fusion |
n |
3 |
4 |
7 |
|
Total |
n |
52 |
52 |
104 |
Table 2: Type and number of procedures performed for both the LB and control groups. No significant difference was found between procedure type and treatment group (p-value=0.4899).
|
VAS score by Hospital Day and Treatment Group |
||||||||
|
Hospital Day |
Group |
Total Observed |
Number Remaining |
Mean |
Standard Deviation |
Median |
Minimum |
Maximum |
|
1 |
LB |
52 |
43 |
4.13 |
1.93 |
4.25 |
0 |
7.92 |
|
Control |
52 |
49 |
5.13 |
2 |
5 |
1.06 |
8.83 |
|
|
2 |
LB |
52 |
35 |
4.16 |
1.92 |
4.06 |
0 |
8 |
|
Control |
52 |
45 |
4.96 |
1.98 |
5.33 |
0.76 |
8.43 |
|
|
3 |
LB |
52 |
21 |
4.24 |
1.85 |
4.5 |
0 |
7.67 |
|
Control |
52 |
36 |
4.96 |
1.79 |
5.05 |
0 |
8.17 |
|
Table 3: VAS scores for both treatment groups by hospital day. The mean VAS score was noted to be lower in the LB group for all three hospital days. The average VAS score across the three-day period was found to be significantly lower in the LB compared to the control group (p-value=0.016).
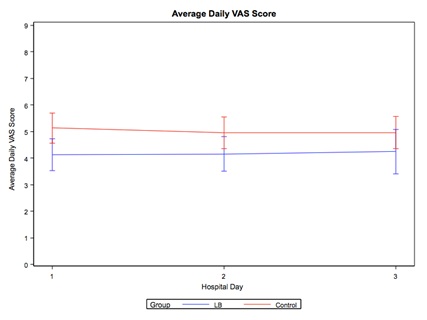 Figure 1: The mean VAS scores (with 95% confidence limit bars) of the LB and Control Group over the three days postoperatively.
Figure 1: The mean VAS scores (with 95% confidence limit bars) of the LB and Control Group over the three days postoperatively.
|
VAS score by Procedure Type, Hospital Day, and Treatment Group |
||||||||
|
Type of Surgery |
Hospital Day |
Group |
Total Observed |
Mean |
Standard Deviation |
Median |
Minimum |
Maximum |
|
Isolated Decompression |
1 |
LB |
16 |
4.09 |
2.39 |
3.63 |
1.02 |
7.92 |
|
Control |
9 |
4.31 |
2.23 |
5.12 |
1.06 |
6.93 |
||
|
2 |
LB |
16 |
2.83 |
4.01 |
2.83 |
0 |
5.67 |
|
|
Control |
9 |
5.21 |
2.77 |
5.87 |
1.33 |
7.78 |
||
|
3 |
LB |
16 |
. |
. |
. |
. |
. |
|
|
Control |
9 |
5.61 |
0.97 |
6.11 |
4.5 |
6.23 |
||
|
Single-Level Fusion |
1 |
LB |
16 |
4.3 |
1.65 |
4.15 |
1.67 |
7.06 |
|
Control |
21 |
4.76 |
1.73 |
4.44 |
1.64 |
8.25 |
||
|
2 |
LB |
16 |
4.34 |
1.85 |
3.89 |
0.89 |
8 |
|
|
Control |
21 |
4.45 |
2.01 |
4 |
0.76 |
8.16 |
||
|
3 |
LB |
16 |
4.06 |
1.77 |
4.06 |
1.64 |
7.67 |
|
|
Control |
21 |
4.14 |
1.87 |
4.33 |
0 |
7 |
||
|
Two-Level Fusion |
1 |
LB |
10 |
4.36 |
2.18 |
4.75 |
0 |
6.53 |
|
Control |
13 |
5.51 |
1.97 |
5.92 |
2.22 |
8.43 |
||
|
2 |
LB |
10 |
4.8 |
2.01 |
5.89 |
1.33 |
6.39 |
|
|
Control |
13 |
5 |
1.42 |
5.03 |
2.38 |
7.17 |
||
|
3 |
LB |
10 |
4.19 |
2.33 |
4.74 |
0 |
6.75 |
|
|
Control |
13 |
5.07 |
1.7 |
5.29 |
1.17 |
8.17 |
||
|
≥ Three-Level Fusion |
1 |
LB |
10 |
3.65 |
1.96 |
4.17 |
0.67 |
5.89 |
|
Control |
9 |
6.01 |
2.37 |
5.75 |
2.44 |
8.83 |
||
|
2 |
LB |
10 |
3.51 |
1.48 |
3.5 |
1.47 |
6.11 |
|
|
Control |
9 |
5.86 |
2.23 |
5.72 |
0.98 |
8.43 |
||
|
3 |
LB |
10 |
4.57 |
1.75 |
4.99 |
1.17 |
6 |
|
|
Control |
9 |
6.14 |
1.37 |
6.11 |
4.43 |
8 |
||
Table 4: VAS scores for both treatment groups by procedure type and hospital day. The LB group was noted to have a lower mean VAS score for all three postoperative hospital days for all procedure types.
The procedure type was not associated with opioid consumption (calculated as morphine equivalents) after adjusting for hospital day and treatment group (p=0.24). Day of evaluation was significantly associated with average opioid consumption after adjusting for treatment group and surgery type (p<0.0001). In other words, there was a significant change in average opioid consumption over the three-day period as it was consistently highest on the second day (Table 5). This pattern of change was also found to be similar between the two treatment groups. The treatment group was also found to be significantly associated with opioid consumption after adjusting for hospital day and type of surgery. It was shown that the average opioid consumption across the three-day period was lower in the LB compared to the control group (p=0.048, Table 6 and Figure 2).
|
Opioid Consumption in Morphine Equivalents |
|||||||
|
Hospital Day |
Treatment Group |
Total Number Observed |
Mean |
Standard Deviation |
Median |
Minimum |
Maximum |
|
1 |
LB |
52 |
45.81 |
35.74 |
37.5 |
0 |
122.5 |
|
Control |
52 |
59.91 |
39.26 |
51 |
4 |
144 |
|
|
2 |
LB |
52 |
60.24 |
37.75 |
55 |
0 |
165 |
|
Control |
52 |
79.88 |
47.89 |
66 |
10 |
220 |
|
|
3 |
LB |
52 |
52.05 |
36.6 |
51.25 |
0 |
150 |
|
Control |
52 |
65.84 |
42.26 |
53.5 |
10 |
197.5 |
|
Table 5: Opioid consumption in oral morphine equivalents in milligrams for both treatment groups for the three postoperative hospital days. Opioid consumption was found to be significantly higher on hospital day two for both treatment groups (p-value < 0.0001). In addition, total opioid consumption was found to be significantly lower in the LB compared to the control group across the three-day period (p-value=0.048).
|
Opioid Consumption in Morphine Equivalents |
||||||||
|
Type of Procedure |
Hospital Day |
Treatment Group |
Total Number Observed |
Mean |
Standard Deviation |
Median |
Minimum |
Maximum |
|
Isolated Decompression |
1 |
LB |
16 |
46.14 |
30.8 |
40 |
4 |
90 |
|
Control |
9 |
23.58 |
19.09 |
20 |
4 |
53.5 |
||
|
2 |
LB |
16 |
30 |
0 |
30 |
30 |
30 |
|
|
Control |
9 |
68.88 |
21.58 |
72 |
40 |
91.5 |
||
|
3 |
LB |
16 |
. |
. |
. |
. |
. |
|
|
Control |
9 |
41.67 |
36.12 |
34 |
10 |
81 |
||
|
Single-Level Fusion |
1 |
LB |
16 |
54.72 |
40.02 |
46.25 |
0 |
122.5 |
|
Control |
21 |
68.12 |
41.86 |
60 |
15 |
144 |
||
|
2 |
LB |
16 |
72.07 |
40.65 |
60.5 |
15 |
165 |
|
|
Control |
21 |
85.5 |
54.8 |
62.5 |
10.5 |
220 |
||
|
3 |
LB |
16 |
54 |
44.54 |
52.5 |
5 |
150 |
|
|
Control |
21 |
66.44 |
54.98 |
44.25 |
17.5 |
197.5 |
||
|
Two-Level Fusion |
1 |
LB |
10 |
41.35 |
44.55 |
18.75 |
0 |
120 |
|
Control |
13 |
59.54 |
38.34 |
51 |
7.5 |
137 |
||
|
2 |
LB |
10 |
60.28 |
42.62 |
60 |
0 |
140 |
|
|
Control |
13 |
79.12 |
44.5 |
66 |
25 |
170 |
||
|
3 |
LB |
10 |
47.08 |
30.43 |
51.25 |
0 |
90 |
|
|
Control |
13 |
68 |
23.47 |
62.5 |
37.5 |
115 |
||
|
≥ Three-Level Fusion |
1 |
LB |
10 |
35.8 |
20 |
37.25 |
0 |
70 |
|
Control |
9 |
65.5 |
35.13 |
56 |
24 |
120 |
||
|
2 |
LB |
10 |
47.22 |
25.84 |
45 |
15 |
107.5 |
|
|
Control |
9 |
74 |
50.34 |
70 |
10 |
168 |
||
|
3 |
LB |
10 |
53.75 |
33.01 |
52.5 |
7.5 |
90 |
|
|
Control |
9 |
71.56 |
43.16 |
56 |
20 |
154.5 |
||
Table 6: Opioid consumption in oral morphine equivalents in milligrams for the two treatment groups by hospital day and type of procedure. Opioid consumption was noted to be lower in the LB compared to the control group for all procedure types on all hospital days, except hospital day one in the isolated decompression group.
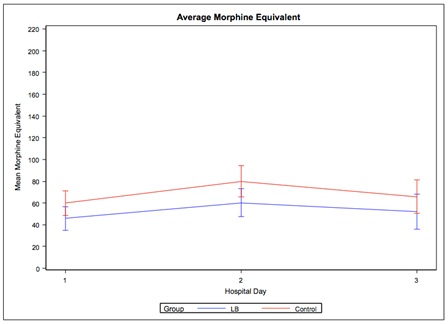
Figure 2: The mean morphine equivalents consumed (with 95% confidence limit bars) per patient by the LB and control group for the three days postoperatively.
DISCUSSION
The results from this study suggest that the use of LB may be beneficial for use in lumbar spinal surgery. The LB treatment group was found to have a significant decrease in LOS, VAS scores, and opioid consumption when compared to the control group. These findings would suggest that LB may be worth the increased cost, as being able to control a patient’s pain can not only lead to improved outcomes, but also lead to cost savings by decreasing the LOS and consumption of narcotics. When comparing the two groups by the procedure performed, the LB group was found to have a lower mean VAS score for all hospital days measured (Table 4). This was also true for opioid consumption as it was noted to be consistently lower in the LB for all hospital days except for hospital day one in patients undergoing an isolated decompression (Table 6). Although not measured, given the improved VAS scores and decreased LOS, LB may also lead to earlier mobilization and activity, which could contribute to the observed shortened LOS. A significant increase in opioid consumption was also found on the second hospital day for both treatment groups. This finding would suggest that the second hospital day is an important time for added pain control postoperatively. As standard bupivacaine produces an anesthetic effect that lasts only 9 hours, LB may be more beneficial as it will still be effective during this critical time period [8,9].
There are many limitations with this study. The design itself is retrospective in nature and at the time the patients were undergoing the procedures in both treatment groups, there was not a standard analgesic regimen. Access to narcotics and dosing was also variable between patients. In addition, some patients had access to intravenous narcotics on an as needed basis with varying dosing increments while others did not. Furthermore, in some cases, inconsistent multi-modal pharmaceutical analgesia was administered with use of benzodiazepines and single doses of non-steroidal anti-inflammatories. In addition, patients in the LB group were not formally blinded, which could have led to a placebo effect. It was also noted that more patients underwent an isolated decompression in the LB group, which may have played a role in the significant difference found in the LOS between the two groups. There was, however, no significant difference found in the type and number of procedures performed between the treatment groups. In addition, the VAS scores and opioid consumption were consistently lower in the LB group when comparing by procedure. This was also true for LOS, except for single-level fusion, in which the LB and control group shared the same average LOS. The same measured LOS in single-level fusions, however, also leads to concerns on the consistency and validity of the results.
Another limitation with this study is that the control group received no local anesthetic. Recently there have been studies looking into the benefit of LB over bupivacaine alone. In a recent prospective randomized control study, there was found to be no benefit of using liposomal bupivacaine over bupivacaine alone in patients undergoing TKA [32]. In addition, this study found no significant difference in postoperative narcotic use, LOS or VAS scores between those receiving LB or bupivacaine alone. In a retrospective cohort-matched review, Grieff et al., compared the efficacy of bupivacaine alone against LB in the management of posterior cervical and lumbar decompression and fusions. This study showed that patients who received bupivacaine alone required approximately twice the number of narcotics per day compared to the LB group [33]. Despite these results, they were unable to find a significant difference in either the cervical or lumbar group. This same study also showed no significant difference in LOS between the two treatment groups. Kim et al., compared the use of LB and bupivacaine alone via local infiltration in patients undergoing unilateral, single-level transforaminal lumbar interbody fusions and found that the LB group had a significantly shorter LOS compared to those that received bupivacaine alone [34]. The LB group also consumed significantly fewer narcotics between12-24 hours postoperatively and had significantly lower VAS scores during the first 24 hours postoperatively. There were, however, no other time periods that demonstrated a significant difference between the two groups in regards to VAS scores and narcotic consumption.
There have been a very limited number of other studies investigating the role of LB in spinal surgery. In addition to the studies mentioned previously, there was another study investigating LB use in spine surgery that observed a significant difference in IV narcotic use in patients receiving LB compared to a control group. In a mixed prospective/retrospective analysis, Puffer et al., found that patients undergoing a single-level microdiscectomy who received LB used significantly less time using IV narcotics when compared to a control group who did not receive a local anesthetic. This same study, however, found no significant difference in VAS scores or total opioid consumption [3].
There was one known complication in the study, a single post-operative seroma, which occurred in the LB group. This was felt to be unrelated to the administration of the local anesthetic; however, a further large-scale study is warranted to investigate any potential increased risks with its use around the spine. No other complications were noted for either treatment group.
CONCLUSION
The use of LB shows promise as an adjuvant for postoperative analgesia in lumbar spine surgery by potentially decreasing pain scores, LOS, and narcotic use. The recent findings from studies investigating the use of LB in spinal surgery, along with the results from this study demonstrate the need for further investigation, namely a larger prospective randomized control study that includes the use of a local anesthetic without LB.
CONFLICTS OF INTEREST AND SOURCE OF FUNDING:
None declared for all contributing authors
STUDY HAS BEEN IRB APPROVED
IRB # 737-14-EP
REFERENCES
- Atchabahian A, Schwartz G, Hall CB, Lajam CM, Andreae MH (2015) Regional analgesia for improvement of long-term functional outcome after elective large joint replacement. Cochrane Database Syst Rev 8: 010278.
- Wells N, Pasero C, McCaffery M (2008) Improving the quality of care through pain assessment and management. In: Hughes RG (ed.). Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US), Rockville, Maryland, USA.
- Puffer RC, Tou K, Winkel RE, Bydon M, Currier B, et al. (2016) Liposomal bupivacaine incisional injection in single-level lumbar spine surgery. Spine J 16: 1305-1308.
- Chelly JE, Greger J, Al Samsam T, Gebhard R, Masson M, et al. (2001) Reduction of operating and recovery room times and overnight hospital stays with interscalene blocks as sole anesthetic technique for rotator cuff surgery. Minerva Anestesiol 67: 613-619.
- Cauley CE, Anderson G, Haynes AB, Menendez M, Bateman BT, et al. (2017) Predictors of in-hospital postoperative opioid overdose after major elective operations: a nationally representative cohort study. Ann Surg 265: 702-708.
- Centers for Disease Control and Prevention (2012) CDC grand rounds: Prescription drug overdoses - A U.S. epidemic. MMWR Morb Mortal Wkly Rep 61: 10-13.
- Hutchison RW (2007) Challenges in acute post-operative pain management. Am J Health Syst Pharm 64: 2-5.
- Tong YC, Kaye AD, Urman RD (2014) Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol 28: 15-27.
- Richard BM, Rickert DE, Newton PE, Ott LR, Haan D, et al. (2011) Safety evaluation of EXPAREL (DepoFoam Bupivacaine) administered by repeated subcutaneous injection in rabbits and dogs: Species comparison. J Drug Deliv 2011: 467429.
- Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, et al. (2006) Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am 88: 959-963.
- Andersen LØ, Husted H, Otte KS, Kristensen BB, Kehlet H (2008) High-volume infiltration analgesia in total knee arthroplasty: A randomized, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand 52: 1331-1335.
- Andersen LØ, Husted H, Kristensen BB, Otte KS, Gaarn-Larsen L, et al. (2010) Analgesic efficacy of subcutaneous local anaesthetic wound infiltration in bilateral knee arthroplasty: A randomised, placebo-controlled, double-blind trial. Acta Anaesthesiol Scand 54: 543-548.
- Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, et al. (2010) Reduced morphine consumption and pain intensity with Local Infiltration Analgesia (LIA) following total knee arthroplasty. Acta Orthop 81: 354-360.
- Zhang S, Wang F, Lu ZD, Li YP, Zhang L, et al. (2011) Effect of single-injection versus continuous local infiltration analgesia after total knee arthroplasty: A randomized, double-blind, placebo-controlled study. J Int Med Res 39: 1369-1380.
- Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, et al. (2006) A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am 88: 282-289.
- Cien AJ, Penny PC, Horn BJ, Popovich JM, Taunt CJ (2015) Comparison between liposomal bupivacaine and femoral nerve block in patients undergoing primary total knee arthroplasty. J Surg Orthop Adv 24: 225-229.
- Chughtai M, Cherian JJ, Mistry JB, Elmallah RD, Bennett A, et al. (2016) Liposomal bupivacaine suspension can reduce lengths of stay and improve discharge status of patients undergoing total knee arthroplasty. J Knee Surg 29: 224-227.
- Sporer SM, Rogers T (2016) Postoperative pain management after primary total knee arthroplasty: The value of liposomal bupivacaine. J Arthroplasty 31: 2603-2607.
- Singh PM, Borle A, Trikha A, Michos L, Sinha A, et al. (2017) Role of periarticular liposomal bupivacaine infiltration in patients undergoing total knee arthroplasty-A meta-analysis of comparative trials. J Arthroplasty 32: 675-688.
- Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR (2017) No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res 475: 94-105.
- Snyder MA, Scheuerman CM, Gregg JL, Ruhnke CJ, Eten K (2016) Improving total knee arthroplasty perioperative pain management using a periarticular injection with bupivacaine liposomal suspension. Arthroplasty Today 2: 37-42.
- Heim EA, Grier AJ, Butler RJ, Bushmiaer M, Queen RM, et al. (2015) Use of liposomal bupivacaine instead of an epidural can improve outcomes following total knee arthroplasty. J Surg Orthop Adv 24: 230-234.
- Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo DJ (2018) Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty 33: 90-96.
- Amundson AW, Johnson RL, Abdel MP, Mantilla CB, Panchamia JK, et al. (2017) A three-arm randomized clinical trial comparing continuous femoral plus single-injection sciatic peripheral nerve blocks versus periarticular injection with ropivacaine or liposomal bupivacaine for patients undergoing total knee arthroplasty. Anesthesiology 126: 1139-1150.
- Meneghini RM, Bagsby D, Ireland PH, Ziemba-Davis M, Lovro LR (2017) Liposomal bupivacaine injection technique in total knee arthroplasty. J Knee Surg 30: 88-96.
- Collis PN, Hunter AM, Vaughn MD, Carreon LY, Huang J, et al. (2016) Periarticular injection after total knee arthroplasty using liposomal bupivacaine vs a modified ranawat suspension: A prospective, randomized study. J Arthroplasty 31: 633-636.
- Jain RK, Porat MD, Klingenstein GG, Reid JJ, Post RE, et al. (2016) The AAHKS clinical research award: liposomal bupivacaine and periarticular injection are not superior to single-shot intra-articular injection for pain control in total knee arthroplasty. J Arthroplasty 31: 22-25.
- Klug MJ, Rivey MP, Carter JT (2016) Comparison of intraoperative periarticular injections versus liposomal bupivacaine as part of a multimodal approach to pain management in total knee arthroplasty. Hosp Pharm 51: 305-311.
- Schwarzkopf R, Drexler M, Ma MW, Schultz VM, Le KT, et al. (2016) Is there a benefit for liposomal bupivacaine compared to a traditional periarticular injection in total knee arthroplasty patients with a history of chronic opioid use? J Arthroplasty 31: 1702-1705.
- Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME (2015) Does extended-release liposomal bupivacaine better control pain than bupivacaine after Total Knee Arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty 30: 64-67.
- AHRQ (2015) Interagency Guideline on Prescribing Opioids for Pain. AHRQ, Rockville, Maryland, USA.
- Alijanipour P, Tan TL, Matthews CN, Viola JR, Purtill JJ, et al. (2017) Periarticular injection of liposomal bupivacaine offers no benefit over standard bupivacaine in total knee arthroplasty: A prospective, randomized, controlled trial. J Arthroplasty 32: 628-634.
- Grieff AN, Ghobrial GM, Jallo J (2016) Use of liposomal bupivacaine in the postoperative management of posterior spinal decompression. J Neurosurg Spine 25: 88-93.
- Kim J, Burke SM, Kryzanski JT, Roberts RJ, Roguski M, et al. (2016) The role of liposomal bupivacaine in reduction of postoperative pain after transforaminal lumbar interbody fusion: A clinical study. World Neurosurg 91: 460-467.
Citation: Gannon EJ, Cornett CA, Larson EP, Lyden ER (2018) The Efficacy of Liposomal Bupivacaine in Lumbar Spine Surgery. J Surg Curr Trend Innov 2: 005.
Copyright: © 2018 Emmett J Gannon, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

