An Unusual Case of Sudden Sensorineural Hearing Loss after Cycling Class
*Corresponding Author(s):
Vivian F KauDepartment Of Otolaryngology Head And Neck Surgery, Icahn School Of Medicine At Mount Sinai, New York, United States
Tel:+1 7815919202,
Email:Vivian.zhu@mountsinai.org
Abstract
In this case report, our patient developed Sudden Sensorineural Hearing Loss (SSNHL) after loud noise exposure during a cardiovascular group exercise class at ‘Soul Cycle.’ To increase awareness among all healthcare professionals of the effects of these modern-day group fitness classes on hearing loss, we describe this case and review the current literature on SSNHL and its management. A 35-year old man developed SSNHL in the setting of loud noise exposure during a high intensity aerobic exercise class. After a short course of oral steroids with no improvement, intratympanic steroids were administered weekly for three weeks. The patient showed minimal improvement; thus, hyperbaric oxygen therapy was conducted. Serial audiograms continued to show severe to profound mixed hearing loss in the right ear. In conclusion, individuals who participate in loud, high-intensity aerobic group-exercise classes should be careful of the potential for noise-induced hearing loss. Aerobic exercise may make these individuals more susceptible to noise-induced hearing loss. Early intervention is critical for any chance of recovery.
Keywords
INTRODUCTION
However, data suggests that risk of noise-induced hearing damage in these classes may mimic that of a nightclub [4]. Sudden loud noise exposure is known to cause acoustic trauma that can induce sensorineural hearing loss [5]. Prolonged exposure to loud noises can pose a similar risk of noise-induced hearing loss [6]. A typical fitness class with music ranges from 40 to 60 minutes with an average noise level of 101 dB and a maximum noise level up to 116 dB [7]. Although this noise level is below the instantaneous exposure guideline of the National Institute for Occupational Safety and Health (NIOSH) of 140 dB, it does exceed the 15-minute exposure or less guideline of 100 dB per day [8,9]. In addition, hearing loss risk can be exacerbated by concurrent high intensity aerobic activity [10]. Previous studies hypothesize that aerobic exercise predisposes the body to hearing loss by inducing a global state of ischemia that can depress stapedius muscle reflexes [10]. We present a case of Sudden Sensorineural Hearing Loss (SSNHL) following cycling class and a review of the literature.
CASE PRESENTATION
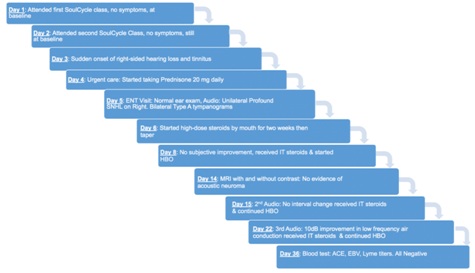
Figure 1: Timeline of the patient’s disease course and treatment.
DISCUSSION
Currently the mechanism for TTS is not fully understood. When the auditory system is over-stimulated with high-intensity noise, there can be a temporary, decreased hearing sensitivity which then returns to normal. One current proposed theory is that the Outer Hair Cells (OHC) in the organ of Corti have temporarily diminished activity either through decreased current or reduced action potentials in the OHC [17]. While there is evidence of altered blood flow in the stria vascularis manifesting in hearing loss, there is no evidence in the literature that vasospasm may cause a TTS [16]. In summary, there is no consensus on how the cardiovascular system affects hearing in the short or long-term. While continued research is conducted on the relationship between cardiovascular health and hearing, loud-noise exposure is widely-accepted as detrimental to hearing.
The patient in our case report experienced SSNHL, as defined by the clinical practice guidelines as sensineural hearing loss meeting audiometric criteria ?30 dB in at least 3 consecutive frequencies [11]. SSNHL must have a rapid onset occurring over or less than 72 hours in one or both ears. While the spontaneous recovery rate can be variable 32-65%, there is limited success in treatment options. Patients are often offered oral steroids, intra-tympanic steroid injections, antiviral medications, diuretics, HBO, middle-ear surgical exploration, or conservative management with observation [11]. Our patient received the standard of care with oral and intratympanic steroids as well as a course of HBO, but did not improve in hearing. This could be perhaps due to confounding factors, such as a sub-clinical viral illness or latent herpes simplex virus, contributing to his hearing loss.
Noise induced hearing loss is defined as unwanted sound adversely affecting one’s hearing from exposure to intermittent or continuous loud noise [20]. Sinha et al., used the Sound Meter Pro application on iPhone and iPod devices to measure the noise level in different group fitness cycling classes. They found that the average high maximum sound levels reached 113 dB where the majority of the class was spent > 100 dB [7]. According to the NIOSH guidelines, the participants would exceed the recommended daily noise exposure by greater than eight times after attending one 45-minute class [8,9]. This was not exclusive to Soul Cycle classes. Yaremchuk and Kaczor reviewed 125 group fitness classes of all types at five American health clubs and found that the majority had an average noise level greater than 90 dB, and 50% of the participants self-reported decreased hearing and tinnitus [21]. Group fitness classes ranged from group yoga to cycling. Among all the group fitness classes, cycling tended to be the loudest, with an average sound level of 94 dB [22]. Cyclists in these loud group classes may arguably have less noise exposure if they performed the sport in nature. Seidman et al., looked at the noise-induced trauma from wind on cyclists. By using a simulation wind-tunnel, a single cyclist was asked to pedal while tilting their head at varying degrees relative to oncoming differing wind speeds [23]. They found that the noise level reached a maximum of 120.3 dB when the ear was 90 degrees away from the wind traveling at 60 miles per hour [23]. Natural environments may not always be a safe alternative for acoustic safety during exercise.
The present case supports prior reports of SSNHL following high volume, high-intensity exercise classes. It is possible that overall improved fitness may be otoprotective, concurrent, high-decibel sound exposure during exercise may, confer additional hearing loss risk. Compared to noise exposure alone, combined noise exposure during physical activity has been shown to induce a greater TTS [24]. The definitive mechanism of which is unknown, but the cochlea can have vascular changes from noise and exercise [25]. The inner ear is an end-organ with a potentially tenuous vascular supply; the cochlea is supplied by the terminating spiral modiolar artery, a branch of the anterior inferior cerebellar artery [26]. There has been abundant research proving that sympathetic stimulation of the cochlear vessels through the stellate ganglion, does decrease blood flow in the cochlea [27,28]. Interestingly, it appears that noise can induce a sympathetic response similar to that of exercise. Noise as a stressor can elevate heart rate and blood pressure [29,30]. Noise exposure also reduces the red blood cell velocity in cochlear microcirculation [31]. Additionally, Axelsson et al., shows that noise exposure in guinea pigs lead to vasoconstriction in the stria vascularis of the cochlear duct [32]. Hawkins et al., observed this finding on a molecular level, showing that there was vascular damage in the cochlea. Twelve guinea pigs were exposed to 118-120 dB for 8-110 hours. Histological sectioning of their cochlear showed microscopic changes of inhibited blood flow and oxygen delivery [33]. This level of noise used in these guinea pig models is similar to the level which participants at Soul Cycle experience [7]. Together, the exercise-induced vasoconstriction and noise-induced vasoconstriction of the cochlea could lead to a damaging, hypoxic state, potentially increasing the risk of permanent SNHL.
This case report and other emerging data supports a need for increased awareness of hearing loss risk among participants in high-intensity, high decibel exercise classes. Since outcomes have been linked to timing of treatment, individuals should be encouraged to seek medical attention for any sudden-onset hearing loss, especially after repeated exercise and noise exposure. Even with prompt attention and multi-modality treatment, such as oral and IT steroids and HBO in this case, hearing loss may be refractory to treatment. According to the Occupational Safety and Health Administration (OSHA) guideline a noise level of 85 dB or higher is considered “action” level, and requires employees exposed to this level of noise to wear hearing protection and undergo annual audiograms [34]. Hearing protection should also be offered to all participants exposed to high-decibel music during exercise classes. Ear plugs are offered at certain boutique cardiovascular group workout classes, but not promoted on their websites [3]. Participants should be encouraged to participate in wearing ear plugs. Perhaps these classes can also provide an option to modify the noise exposure during class. This could be another way to temporize the noise-induced environment and promote acoustic safety.
Additional research is needed on the effects of noise and exercise on hearing loss. Specifically, the relationship between high-decibel music and how it affects individuals during high-intensity cardiovascular group fitness classes. More research on the cellular and molecular changes which occur during a global state of exercise in combination with loud has not been fully elucidated. Further information on the temporal relationship of the loud music and its effects on short and long-term hearing are also needed. This is imperative because it can shape the way people currently work out and maintain their own hearing health from a young age. Perhaps wearing protective equipment for acoustic safety during exercise is adequate, but at this time, further research on hearing and this popular new way to exercise is needed.
CONCLUSION
FUNDING
CONFLICTS OF INTEREST
REFERENCES
- Nielsen Consumer Fitness Trends Statistics &Insights for Fitness Facilities.
- O’Connor C (2015) As soul cycle preps for IPO, stats show boutique fitness isn’t just a fad.
- Soul Cycle.
- Beach E, Williams W, Gilliver M (2013) Estimating young Australian adults' risk of hearing damage from selected leisure activities. Ear Hear 34: 75-82.
- Nottet JB, Moulin A, Brossard N, Suc B, Job A (2006) Otoacoustic emissions and persistent tinnitus after acute acoustic trauma. Laryngoscope 116: 970-975.
- Neitzel R, Fligor B (2017) Determination of risk of noise-inducted hearing loss due to recreational sound: Review. WHO Pg no: 24.
- Sinha S, Kozin ED, Naunheim MR, Barber SR, Wong K, et al. (2017) Cycling exercise classes may be bad for your (hearing) health. Laryngoscope 127: 1873-1877.
- Johnson PT Noise exposure: Explanation of OSHA and NIOSH safe-exposure limits and the importance of noise dosimetry. Pg no: 1-8.
- Sriwattanatamma P, Breysse P (2000) Comparison of NIOSH noise criteria and OSHA hearing conservation criteria. Am J Ind Med 37: 334-338.
- Vittitow M, Windmill IM, Yates JW, Cunningham DR (1994) Effect of simultaneous exercise and noise exposure (music) on hearing. J Am Acad Audiol 5: 343-348.
- Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, et al. (2012) American Academy of, O.-H. In: Neck S (eds.). Clinical practice guideline: Sudden hearing loss. Otolaryngol Head Neck Surg 146: 1-5.
- Lin RJ, Krall R, Westerberg BD, Chadha NK, Chau JK (2012) Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope 122: 624-635.
- Marron KH, Sproat B, Ross D, Wagner S, Alessio H (2014) Music listening behavior, health, hearing and otoacoustic emission levels. Int J Environ Res Public Health 11: 7592-7607.
- Gispen FE, Chen DS, Genther DJ, Lin FR (2014) Association between hearing impairment and lower levels of physical activity in older adults. J Am Geriatr Soc 62: 1427-1433.
- Tan HE, Lan NSR, Knuiman MW, Divitini ML, Swanepoel DW (2018) Associations between cardiovascular disease and its risk factors with hearing loss-A cross-sectional analysis. Clin Otolaryngo l 43: 172-181.
- Oron Y, Elgart K, Marom T, Roth Y (2014) Cardiovascular risk factors as causes for hearing impairment. Audiol Neurootol 19: 256-260.
- Kolkhorst FW, Smaldino JJ, Wolf SC, Battani LR, Plakke BL et al. (1998) Influence of fitness on susceptibility to noise-induced temporary threshold shift. Med Sci Sports Exerc 30: 289-293.
- Ismail AH, Corrigan DL, MacLeod DF, Anderson VL, Kasten RN et al. (1973) Biophysiological and audiological variables in adults. Arch Otolaryngol 97: 447-451.
- Manson J, Alessio HM, Cristell M, Hutchinson KM (1994) Does cardiovascular health mediate hearing ability? Med Sci Sports Exerc 26: 866-871.
- Seidman MD, Standring RT (2010) Noise and quality of life. Int J Environ Res Public Health 7: 3730-3738.
- Yaremchuk K, Dickson L, Burk K, Shivapuja BG (1997) Noise level analysis of commercially available toys. Int J Pediatr Otorhinolaryngol 4: 187-197.
- Beach EF, Nie V (2011) Noise levels in fitness classes are still too high: Evidence from 1997-1998 and 2009-2011. Arch Environ Occup Health 69: 223-230.
- Seidman MD, Wertz AG, Smith MM, Jacob S, Ahsan SF (2017) Evaluation of Noise exposure secondary to wind noise in cyclists. Otolaryngol Head Neck Surg 157: 848-852.
- Miani C, Bertino G, Francescato MP, di Prampero PE, Staffieri A (1996) Temporary threshold shift induced by physical exercise. Scand Audiol 25: 179-186.
- Torre P3rd, Howell JC (2008) Noise levels during aerobics and the potential effects on distortion product otoacoustic emissions. J Commun Disord 41: 501-511.
- Shi X (2011) Physiopathology of the cochlear microcirculation. Hear Res 282: 10-24.
- Ren T, Laurikainen E, Quirk WS, Miller JM, Nuttall AL (1993) Effects of stellate ganglion stimulation on bilateral cochlear blood flow. Ann Otol Rhinol Laryngol 102: 378-384.
- Ren TY, Laurikainen E, Quirk WS, Miller JM, Nuttall AL (1993) Effects of electrical stimulation of the superior cervical ganglion on cochlear blood flow in guinea pig. Acta Otolaryngol 113: 146-151.
- Lu SY, Lee CL, Lin KY, Lin YH (2018) The acute effect of exposure to noise on cardiovascular parameters in young adults. J Occup Health 60: 289-297.
- Lindgren F, Axelsson A (1988) The influence of physical exercise on susceptibility to noise-induced temporary threshold shift. Scand Audiol 17: 11-17.
- Arpornchayanon W, Canis M, Suckfuell M, Ihler F, Olzowy B, et al. (2011) Modeling the measurements of cochlear microcirculation and hearing function after loud noise. Otolaryngol Head Neck Surg 145: 463-469.
- Axelsson A, Vertes D, Miller J (1981) Immediate noise effects on cochlear vasculature in the guinea pig. Acta Otolaryngol 91: 237-246.
- Hawkins JE (1971) The role of vasoconstriction in noise-induced hearing loss. Ann Otol Rhinol Laryngol 80: 903-913.
- Utidjian HM (1974) Criteria for a recommended standard--occupational exposure to noise. I. Recommendations for a noise standard. J Occup Med 16: 33-37.
Citation: Kaul VF, Kidwai S, Lupicki A, Cosetti M (2019) An Unusual Case of Sudden Sensorineural Hearing Loss after Cycling Class. J Otolaryng Head Neck Surg 5: 026.
Copyright: © 2019 Vivian F Kau, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.