
Effects of Verapamil on Bone Cancer Cells in vitro
*Corresponding Author(s):
Rosemary DziakDepartment Of Oral Biology, State University Of New York, Buffalo University, New York, United States
Tel:+1 7168293827,
Fax:+1 7168293842
Email:rdziak@buffalo.edu
Abstract
Verapamil is a calcium channel blocker drug that inhibits the transmembrane flux of calcium ions and is used clinically in the management of cardiac arrhythmias. Several studies have proposed its role as an adjuvant in multi-drug chemotherapy due to its potential to decrease calmodulin activity in cancer cells and thus diminish drug resistance. However, newer studies have described the role of verapamil as an anti-cancer agent in the growth inhibition of several types of cancer cells. It is postulated that calcium signaling is a vital part of the survival of osteoblastic cells with alteration in the intracellular calcium levels leading to imbalances between several mechanisms ultimately resulting in inhibition of cell growth. The effects of verapamil (12.5-100 µg/ml) on the inhibition of cell growth of G292 osteosarcoma cells and normal HOB osteoblastic cells were studied in vitro. Moreover, the effects of this drug on growth factor induced effects in the G292 cells were also assessed. In order to evaluate potential verapamil releasing vehicles, we tested its release and effectiveness when incorporated in a nano Calcium Sulfate (nCS) delivery system. In higher doses, verapamil (50-100 µg/ml) significantly inhibited the activity of G292 osteosarcoma cells; however, it had no significant effect on normal HOB osteoblasts at these doses. Verapamil was also shown here to significantly inhibit Human Platelet Lysate (HPL) and Platelet Derived Growth Factor (PDGF) induced G292 cell activity. Additionally, it was found that nCS could release verapamil in a time-dependent manner and the released verapamil could inhibit G292 cell activity. In appropriately administered concentrations, verapamil may possess the potential to inhibit the activity of osteosarcomas and act as an anti-tumor agent down-regulating the effects of naturally occurring growth factors.
Keywords
INTRODUCTION
Osteosarcoma (OS) is a rare but devastating bone tumour that mainly affects children and adolescents [1]. The 5-year survival rates for patients with localized disease are between 50 and 70%, but in patients with metastases, the prognosis remains poor (∼20%). Today’s standard therapy of OS includes neoadjuvant chemotherapy with doxorubicin, methotrexate and cisplatin followed by surgical resection of the primary tumour and adjuvant chemotherapy [2-5]. Patients who relapse show increased chemoresistance against currently used chemotherapy approaches [6-8]. With about three cases per million people and year, OS is a rare disease, which makes the evaluation of new potential drugs difficult [9]. This potentiates a need for exploring existing or newer drugs in the treatment of osteosarcoma.
Until recently, verapamil was employed as a drug in cancer therapy in order to overcome multi drug resistance [10]. Some studies have suggested a possible role for its use in the suppression of bone cell activity. As an example of the effects of verapamil on bone cellular activity the direct inhibitory action of calcium channel blockers on rat calvarial skeletal collagen synthesis was previously reported [11]. Likewise, Breslau et al., found that calcium channel blockers such as verapamil have an effect on calcium metabolism through the alteration of bone turnover rate and the inhibition of bone formation in hypertensive patients [12]. Although osteosarcoma cells such as the G292 cell line studied here have been shown to possess voltage activated calcium channels sensitive to verapamil [13,14], there does not appear to be studies reported on the effects of this drug on the viability and growth responses of these cells.
Since in vivo the microenvironment of an osteosarcoma is a complex niche with numerous growth factors interacting as both autocrine and paracrine factors [15], it must be recognized that a calcium inhibitor such as verapamil can be acting on many levels to affect the overall activity of osteosarcoma cells. Acknowledging that verapamil has been shown in other cells to affect a number of different mechanisms involving postreceptor signaling pathways such as those with protein kinase C, calmodulin and phosphodiesterase in addition to calcium channels depending upon its concentration [16], we studied several in vitro incubation conditions and drug concentrations.
We report here some of the possible interactions of growth factors and verapamil with G292 osteosaroma cells with respect to their cell activity. Co-culture of G292 osteosarcoma cells with Human Platelet Lysate (HPL) and Platelet Derived Growth Factor (PDGF) enhanced their activity. However, when treated with verapamil at relatively high doses, this cellular activity was significantly decreased. Particularly, since verapamil at high in vivo concentrations can induce significant side effects, including high mortality [17] we present data on a possible local delivery system for verapamil that could potentially decrease its absorption into the blood stream and at the same time target an osteosarcoma site.
MATERIALS AND METHODS
G292 osteosarcoma and HOB osteoblasts cell culture
HOB osteoblastic cells: Human calvarial osteoblast cells, obtained from ScienCell, (Carlsbad, CA, USA, Catalog #4600) were incubated in alpha MEM culture medium (Gibco, USA) supplemented with 10% FBS and 1% antimycotic (Gibco, USA) and cultured in a similar manner as the G292 cells described above. For the experiments presented here, passages 5 & 6 were used.
CELL VIABILITY AND APOPTOSIS ASSAY
Cell viability and apoptosis were assayed using an ApoLive-Glo™ Multiplex Assay (Promega) after treatment with various concentrations of verapamil (Sigma-Aldrich, Inc.). For the studies with the G292 cells, there were five groups: 1. untreated cells, 2. cells treated with 100 µg/ml (220 µM) verapamil, 3. cells treated with 50 µg/ml (110 µM) verapamil, 4. cells treated with 25 µg/ml (55 µM) verapamil and 5. cells treated with 12.5 µg/ml (27.5 µM) verapamil.
The protocol used for the cell viability and apoptosis combines assay chemistries so that viability and caspase activation (associated with apoptosis) can be assessed within a single assay well. In the first part of the assay the peptide substrate (glycylphenylalanyl-aminofluorocoumarin; GF-AFC) was added. It enters intact cells where it can be cleaved by the live-cell protease activity to generate a fluorescent signal proportional to the number of living cells. In the second part of the assay, the Caspase-Glo® 3/7 Reagent was added in an “add-mix-measure” format described in the manufacturer’s protocol; this results in cell lysis, followed by caspase cleavage of the substrate and generation of a “glow-type” luminescent signal produced by luciferase.
G292 cells of approximately 5000/well were seeded in a flat 96-well micro-plate as triplicates with the five groups mentioned above. Cells were verapamil for 7, 12, or 17 days with media changes every 3 days. At the end of the incubation period, the medium present was removed from the wells and replaced with 100 µl of medium. For the first part, 20 µl of viability reagent containing the GF-AFC substrate was added to each well, and briefly mixed by orbital shaking at 300-500 rpm for 30 seconds and then incubated at 37°C for 30-180 minutes. Fluorescence was measured at 485Ex/520Em (in order to determine viability) by using a fluorescent plate reader (Biotek). For the second part of the assay, 100 µl of Caspase-Glo 3/7 reagent was added to each well and briefly mixed by orbital shaking at 300-500 rpm for 30 seconds and then incubated at room temperature for 30-180 minutes. Luminescence that is proportional to the amount of caspase activity present as an assessment of apoptosis was measured using a Perkin Elmer Victor3TMV Wallac plate reader.
ACTIVITY ASSESSMENTS WITH VERAPAMIL ADDED DIRECTLY TO CULTURE MEDIA
Effect of verapamil on G292 osteosarcoma cell line and HOB normal cells
For the studies with either the G292 or HOB cells, there were five groups: 1. cells alone, 2. cells treated with 100 ug/ml verapamil, 3. cells with 50 µg/ml verapamil, 4. cells treated with 25 µg/ml verapamil and 5. cells treated with 12.5 µg/ml verapamil. The cells were seeded in complete media (alpha MEM, 10% FBS and 1% anti-mycotic) and after 80% confluence, the cells were treated with the varying concentrations of verapamil for the indicated time. The cells µ clear then (without incubated phenol with red) 200 MEM µl MTT and 20 assay reagent (Sigma-Aldrich Inc.) for an additional 3 hrs and with 200 µl Dimethyl Sulfoxide (DMSO) at the end of the incubation period. Finally, the supernatants were transferred to a new 96 well plate for recording the absorbance at 540 nm using a 96-well plate reader.
Effect of calcium on G292 osteosarcoma cell line
Effect of PDGF on G292 osteosarcoma cell line
STUDIES WITH VERAPAMIL LOADED IN THE NCS SCAFFOLD
Preparation of the nCS/verapamil scaffold and determination of standard calibration curves
Standard calibration curves for verapamil were produced from common guidelines published by the National Measurement System Valid Analytical Measurement (VAM) program (United States Department of Trade and Industry) using a UV visible spectrophotometer. A set of standards containing a known amount of verapamil concentrations was prepared and the spectrophotometer response was measured for each standard to develop a calibration curve. The calibration curve was then used to determine the unknown drug concentration that was released in the test sample aliquots. Briefly, six standard calibration solutions with concentrations of (100, 50, 25, 12.5, 6.25 and 3.125 µg/ml) were prepared with Phosphate Buffered Saline (PBS) to similarly resemble the test sample aliquots. PBS media was used as a control blank. The standard calibration curves were then used to determine the unknown concentration of the verapamil in the sample aliquots.
Drug release detection from nCS samples
Preparation of drug loaded nCS samples for cell activity studies
Statistical Analyses
RESULTS
Verapamil can inhibit the activity of G292 cells
The graph in figure 1B shows the results of an ApoLive-Glo™ Multiplex assay assessing the viability of G292 cells treated with verapamil. Viability levels increased in the control group as the incubation periods were extended to 17 days but the verapamil treatment group of 50 µg/ml showed significantly lower viability levels at all time periods as compared to the other groups as represented by *(p < 0.05) (Figure 1B). Accordingly, apoptosis levels were low in the controls and did not change over the 17 day period of incubation while the 50 µg/ml verapamil groups exhibited the highest level of apoptosis over the 17 day period showing a statistically significant greater increase (p < 0.05) compared to the other groups including the 100 µg/ml group that produced a lower level of apoptosis, although still significantly greater than controls (Figure 1C).
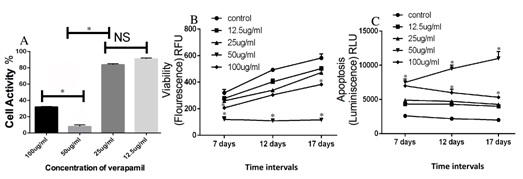
Figure 1: Inhibition of G292 cell activity by verapamil.
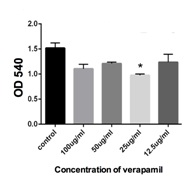
Low and high concentration of verapamil has no effect on HOB osteoblasts cells
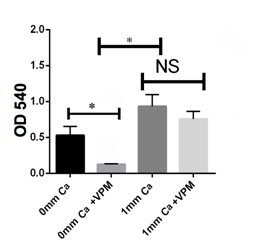
Calcium-free conditions can inhibit G292 osteosarcoma cell activity
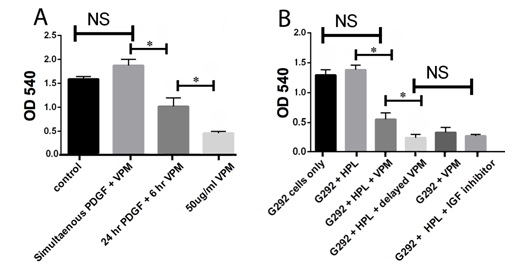
Verapamil can inhibit PDGF and HPL-induced G292 osteosarcoma cell activity
Nanocalcium sulfate (nCS) permits the slow release of verapamil and maintain efficacy of the drug
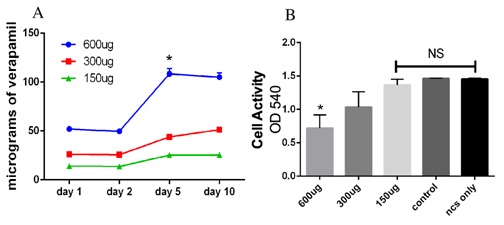
DISCUSSION
Several studies in the literature have suggested the role of verapamil, a Ca2+ channel antagonist, as an anti-tumor agent [20,21]. In this report, we provide, for the first time, evidence demonstrating that verapamil has an inhibitory effect on G292 osteosarcoma cells. We found that verapamil could inhibit the activity of G292 osteosarcoma significantly in the absence of calcium. Furthermore, verapamil could also inhibit activity induced by PDGF and IGF on G292 osteosarcoma cells. We also found that nanocalcium sulfate could efficiently release verapamil in a consistent manner, which could help in the local delivery of verapamil to the affected site.
It has been shown that verapamil can reverse the resistance of cancer cells to some chemotherapeutic agents [20,21]. However, the mechanisms of verapamil anticancer activity are not fully understood. Due to the success of its use in the treatment of osteosarcoma in patients [22] as well as its ability to lead to apoptosis as a result of an increase in intracellular calcium in human colon cancer cells [23], we investigated the role of verapamil in growth suppression parameters of G292 osteosarcoma cells. In our study, it was observed that high doses of verapamil (50-100 µg/ml) significantly inhibited cellular activity whereas; lower doses (12.5- 25 µg/ml) had no apparent inhibitory effects on the osteosarcoma cells. In figure 1, the MTT assay results correlate well with the viability as well as apoptosis aspects of the ApoLive-Glo™ Multiplex assay. This suggests that a decrease in the MTT results (which are reflective of mitochondrial enzymatic activity) can be reflective of both decreases in cell viability (measured by membrane integrity) and increases in cell apoptosis (measured by caspase activity). This consistency of responses is interesting considering that with all three assays 50 µg/ml verapamil produced the greatest response suggesting that the effect is not an assay procedural error and also that there can be a series of cellular events evoked by the drug that lead to a decrease in cell viability and cell death.
The lack of a linear dose response with verapamil in our studies can be due to the most probable complexity of mechanism of action of the drug involved in producing the effects on cellular responses. It has been reported that verapamil in other cell types can modulate intracellular calcium levels not only via ionic channels but also via intracellular calcium pools with subsequent effects on postreceptor signaling pathways involving several different mechanisms [24]. This multitude of effects each of which can be dose dependent can yield to end results on cellular viability that might be reflected in a nonlinear dose-response.
The complex nature of intracellular calcium regulation as well as potential actions of verapamil independent of a Ca2+ channel inhibitor can be responsible for its effects observed here in the absence of extracellularly added calcium. Since intracellular calcium is regulated not only by calcium channels but also by calcium pumps and ion exchangers in the plasma membrane as well as intracellular organelles, inhibition by verapamil can result even in the absence of added extracellular calcium since under this condition, the dynamics of calcium release by other mechanisms might lead to a net decrease in cytosolic calcium that results in changes in cell activity leading to decreased viability or increased apoptosis. Moreover, although verapamil is a potent and selective voltage dependent calcium channel antagonist at concentrations below 1 µM (0.45 µg/ml) it has been shown to have inhibitory effects on sodium or potassium conductances at higher concentrations in the 10-100 µM (4.5-45 µg/ml) range [25] where in the higher range we have seen significant effects in this present study. Verapamil has also been shown to act as a non-selective inhibitor, with a low binding affinity, of P-glycoprotein, a member of the ATP-Binding Cassette (ABC) transporter that functions as a physiological barrier to extrude toxins and xenobiotics out of cells [26]. These effects can lead to actions of verapamil not dependent upon extracellular calcium. Since in this present study we were interested in the effects of verapamil as a possible therapeutic agent, we did not compare its results to those of another regulator of calcium such as a chelator like BAPTA.
We chose to study the G292 cell line because it is a human osteosarcoma cell line that has been fairly well studied with respect to its hormonal and growth factor responses as well as its calcium regulatory mechanisms. Since we were studying verapamil, an agent that might function via calcium channels, we surveyed previous literature on calcium channels in human osteosarcoma cell lines and found that there are apparently no major differences in the types of voltage-activated calcium channels in a number of cell lines studies, but normal human cells do differ from the osteosarcomas due to a absence of the alpha 1S isoform of the L-type channel [14]. Since to our knowledge there are not major differences in regulation of calcium among the osteosarcoma cell lines that are presently available, we did not repeat our studies in other cells lines for this present paper. We acknowledge, however, that studies with a variety of osteosarcoma cell lines might reveal some interesting differences in response to verapamil and should be pursued in future work. In particular osteosarcoma cells with differences in telomere maintenance such as by activation of telomerase and an Alternative mechanism of Telomere Lengthening (ALT) characterized by elongated, heterogeneous telomeres without the presence of telomerase activity should be further studied with respect to the effects of verapamil. G292 cells have been reported to be ALT+ so further studies in cell lines lacking ALT might reveal important information regarding possible differences in the mechanism of action of this agent in different osteosarcoma cell types [27].
Furthermore, we investigated if verapamil could affect normal osteoblastic cells and hence we treated the HOB osteoblasts cells with varying doses of verapamil (12.5 µg/ml - 100 µg/ml). We found that high and low concentrations of verapamil failed to demonstrate a significant effect on the cellular growth activity of these normal human calvarial osteoblasts. Kim et al., demonstrated in their studies that in the absence of calcium in the media, verapamil could significantly inhibit cDNA synthesis in murine osteoblastic cells [28]. However, this phenomenon was reversed in the presence of calcium in the media.
Several studies have explored the role of platelet-derived growth factors in the proliferation and metastasis of osteosarcoma owing to their potential to induce platelet aggregation [29]. Additionally, there is positive correlation between the expression levels of platelet aggregation-inducing factors [30]. Apart from cell stimulation, these growth factors can also modulate cell migration and modulate matrix metalloprotease production. The interactions of tumor cells with platelets play a critical role in the progression of tumor malignancy [31]. Tumor cell-induced platelet aggregation enhances the rate of tumor embolization in the microvasculature and protects tumor cells from immunological assault and blood-shear stress [32]. Moreover, several factors such as transforming growth factor-β, insulin like growth factor, and Platelet -Derived Growth Factor (PDGF) are stored in platelet granules and are released during platelet aggregation. Such platelet-derived factors promote the epithelial-mesenchymal transition, tumor vascular angiogenesis and vascular permeability [33]. In our study, we observed increased growth of G292 upon incubation with PDGF and HPL. However, upon treatment with verapamil, a significant decrease in their activity was noticed. This could be attributed to the fact that verapamil at high doses can inhibit platelet aggregation, which is in agreement with studies carried out by Kreiskott et al., [34]. The effect of verapamil on the HPL stimulation of G292 cells demonstrated similar results as compared to the IGF-inhibitor treatment on the HPL stimulation of these cells. However, verapamil was unable to inhibit cellular activity when administered concurrently with HPL and PDGF to the G292 cells; instead, the cells, when treated with verapamil, 24 hours after stimulating the cells with HPL and PDGF showed a remarkable decline in activity. This could be attributed to the fact that verapamil may not inhibit initial growth factor events such as PDGF and IGF receptor binding but can inhibit downstream PDGF and IGF effects.
More than 90% of verapamil is absorbed when given orally, but due to high first-pass metabolism, bioavailability is much lower (10-35%). Additionally, it takes 1 to 2 hours to reach peak plasma concentration after oral administration [35]. Acute overdose is often manifested by nausea, asthenia, bradycardia, dizziness, hypotension and cardiac arrhythmia [36]. Furthermore, verapamil, in a dose dependent manner, has been associated with an increased risk of cancer in the elderly, as reported in the Rotterdam study [37]. These statements provide important implications for a need to deliver this drug in a local manner. This may be particularly true for osteosarcomas since in our study, anti-growth activity of verapamil on G292 cells was noted at a relatively high concentration of 50 µg/ml (110 µM). Concentrations in this range may lead to undesirable reactions if administered clinically, which might make it imperative to deliver this drug to the affected site directly while trying to bypass the blood stream.
Nano-Calcium Sulfate (nCS) is a biocompatible, bioactive and biodegradable material with a compressive strength that is greater than that of cancellous bone and has been shown to release added growth factors in a sustained manner [19,38]. In our previous studies, we have successfully demonstrated how this nanomaterial can promote bone regeneration as well as release growth factors in a consistent manner [19,38]. Our data support the timely release of verapamil as well. In addition, the slow degradation rate of nCS can aid in delaying the release of the drug as well as prolong its efficacy. The potential efficacy of this delivery approach is evident from our data showing the release of verapamil in a sustained manner. This novel local delivery system could eliminate in vivo the first pass effect (hepatic effect) and prevented the drug from being absorbed into the blood stream, although studies will be required to substantiate this in vivo. Within the limitations of this in vitro study, verapamil at high concentrations appears to be a potent anti-growth agent for osteosarcomas having an inhibitory effect on basal as well as PDGF and HPL-induced cell activity in the G292 cell line.
Acknowledgments/Research Support
This research was supported by funds provided as faculty mentor support to R Dziak administered by the University at Buffalo Foundation.
Conflicts of Interest
Dr. Rosemary Dziak is an inventor on a USA patent for nanocalcium particles use in bone regeneration. Dr. Gabriela Fernandes and Andrew Barone have no conflicts of interest with any aspects of the work described in this paper.
REFERENCES
- Botter SM, Neri D, Fuchs B (2014) Recent advances in osteosarcoma. Curr Opin Pharmacol 16: 15-23.
- Qiu Z, Liao Q (2010) [Progress of osteosarcoma therapy]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 24: 1469-1475.
- Nagarajan R, Clohisy D, Weigel B (2005) New paradigms for therapy for osteosarcoma. Curr Oncol Rep 7: 410-414.
- Lopez M, Vici P, Balice A, Carpano S, Papaldo P, et al. (1986) [Current status of osteosarcoma therapy]. Clin Ter 117: 409-425.
- Campanacci M (1984) [Therapy and prognosis in osteosarcoma: changing thoughts and numbers]. Chir Organi Mov 69: 7-9.
- Bacci G, Ferrari S, Longhi A, Perin S, Forni C, et al. (2001) Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer 37: 32-38.
- Ferrari S, Bacci G, Picci P, Mercuri M, Briccoli A, et al. (1997) Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann Oncol 8: 765-771.
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, et al. (1986) The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 314: 1600-1606.
- Mohseny AB, Hogendoorn PC, Cleton-Jansen AM (2012) Osteosarcoma models: from cell lines to zebrafish. Sarcoma 2012: 417271.
- Simpson WG (1985) The calcium channel blocker verapamil and cancer chemotherapy. Cell Calcium 6: 449-467.
- Dietrich JW, Duffield R (1979) Effects of the calcium antagonist verapamil on in vitro synthesis of skeletal collagen and noncollagen protein. Endocrinology 105: 1168-1172.
- Breslau NA, Kaplan NM, Pak CYC (1988) Effects of nifedipine on calcium metabolism in patients with essential hypertension. J Bone Miner Res 3: 112.
- Guggino SE, Wagner JA, Snowman AM, Hester, LD, Sacktor B, et al. (1988) Phenylalkylamine-sensitive calcium channels in osteoblast-like osteosarcoma cells. Characterization by ligand binding and single channel recordings. J Biol Chem 263: 10155-10161.
- Duncan RL, Akanbi KA, Farach-Carson MC (1998) Calcium signals and calcium channels in osteoblastic cells. Semin Nephrol 18: 178-190.
- Alfranca A, Martinez-Cruzado L, Tornin J, Abarrategi A, Amaral T, et al. (2015) Bone microenvironment signals in osteosarcoma development. Cell Mol Life Sci 72: 3097-3113.
- Zernig G (1990) Widening potential for Ca2+ antagonists: non-L-type Ca2+ channel interaction. Trends Pharmacol Sci 11: 38-44.
- Worthley LI (1984) Treating adverse effects of verapamil. JAMA 252: 1129.
- Jung D, Abu-Elheiga L, Ayuzawa R, Gu Z, Shirakawa T, et al. (2012) Mislocalization and inhibition of acetyl-CoA carboxylase 1 by a synthetic small molecule. Biochem J 448: 409-416.
- Park YB, Mohan K, Al-Sanousi A, Almaghrabi B, Genco RJ, et al. (2011) Synthesis and characterization of nanocrystalline calcium sulfate for use in osseous regeneration. Biomed Mater 6: 055007.
- Presant CA, Kennedy PS, Wiseman C, Gala K, Bouzaglou A, et al. (1986) Verapamil reversal of clinical doxorubicin resistance in human cancer. A Wilshire Oncology Medical Group pilot phase I-II study. Am J Clin Oncol 9: 355-357.
- Simpson WG, Tseng MT, Anderson KC, Harty JI (1984) Verapamil enhancement of chemotherapeutic efficacy in human bladder cancer cells. J Urol 132: 574-576.
- Brach Del Prever A, Massara FM, Besenzon L, Bianchi M, Picci P, et al. (1995) [Treatment of metastatic osteosarcoma with verapamil, cyclosporine and chemotherapy. A case report]. Minerva Pediatr 47: 147-151.
- Shchepotin IB, Soldatenkov V, Wroblewski JT, Surin A, Shabahang M, et al. (1997) Apoptosis induced by hyperthermia and verapamil in vitro in a human colon cancer cell line. Int J Hyperthermia 13: 547-557.
- Hoffman S, Gopalakrishna R, Gundimeda U, Murata T, Spee C, et al. (1998) Verapamil inhibits proliferation, migration and protein kinase C activity in human retinal pigment epithelial cells. Exp Eye Res 67: 45-52.
- Waelbroeck M, Robberecht P, De Neef P, Christophe J (1984) Effects of verapamil on the binding properties of rat heart muscarinic receptors: evidence for an allosteric site. Biochem Biophys Res Commun 121: 340-345.
- Srivalli KMR, Lakshmi PK (2012) Overview of P-glycoprotein inhibitors: a rational outlook. Braz J Pharm Sci 48: 353-367.
- Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR (1997) Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med 3: 1271-1274.
- Kim YS, Yang IM, Kim SW, Kim JW, Kim KW, et al. (1991) Responses of osteoblastic cell line MC3T3-E1 cell to the calcium channel blocker diltiazem and verapamil. Contrib Nephrol 91: 43-49.
- Takagi S, Takemoto A, Takami M, Oh-Hara T, Fujita N (2014) Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci 105: 983-988.
- Mehta P, Lawson D, Ward MB, Kimura A, Gee A (1987) Effect of human tumor cells on platelet aggregation: potential relevance to pattern of metastasis. Cancer Res 47: 3115-3117.
- Clezardin P, Drouin J, Morel-Kopp MC, Hanss M, Kehrel B, et al. (1993) Role of platelet membrane glycoproteins Ib/IX and IIb/IIIa, and of platelet alpha-granule proteins in platelet aggregation induced by human osteosarcoma cells. Cancer Res 53: 4695-4700.
- Voland C, Serre CM, Delmas P, Clézardin P (2000) Platelet-osteosarcoma cell interaction is mediated through a specific fibrinogen-binding sequence located within the N-terminal domain of thrombospondin 1. J Bone Miner Res 15: 361-368.
- Yamada K, Yoshitake Y, Norimatsu H, Nishikawa K (1992) Roles of various growth factors in growth of human osteosarcoma cells which can grow in protein-free medium. Cell Struct Funct 17: 9-17.
- Kreiskott H, Hofmann HP (1973) [Animal experiment studies on the inhibition of platelet aggregation by verapamil in vitro and in vivo]. Arzneimittelforschung 23: 1555-1560.
- Singh BN, Ellrodt G, Peter CT (1978) Verapamil: a review of its pharmacological properties and therapeutic use. Drugs 15: 169-197.
- Hofer CA, Smith JK, Tenholder MF (1993) Verapamil intoxication: a literature review of overdoses and discussion of therapeutic options. Am J Med 95: 431-438.
- Beiderbeck-Noll AB, Sturkenboom MC, van der Linden PD, Herings RM, Hofman A, et al. (2003) Verapamil is associated with an increased risk of cancer in the elderly: the Rotterdam study. Eur J Cancer 39: 98-105.
- He X, Dziak R, Mao K, Genco R, Swihart M, et al. (2013) Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2 gene-modified mesenchymal stem cells for bone regeneration. Tissue Eng Part A 19: 508-518.
Citation: Fernandes G, Barone A, Dziak R (2016) Effects of Verapamil on Bone Cancer Cells in vitro J Cell Biol Cell Metab 3: 013.
Copyright: © 2016 Gabriela Fernandes, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

