ABSTRACT
A steroid responsive autoimmune-like hepatitis triggered by the anti-androgen agent bicalutamide has not been reported. We describe a biopsy-proven instance of bicalutamide-induced autoimmune-like hepatitis against a background of prostatic carcinoma, without antecedent liver disease, in a 69 year old male. Abnormalities in serum transaminase activities worsened despite withdrawal of bicalutamide but with corticosteroid therapy resolved over 8 weeks. His clinical course and findings at liver biopsy expand the range of known bicalutamide-associated disease.
KEYWORDS
Autoimmune hepatitis; Bicalutamide; Biopsy; Corticosteroid; Liver; Prostate cancer
Introduction
A steroid responsive autoimmune-like hepatitis triggered by the anti-androgen agent bicalutamide has not been reported. We described a biopsy-proven instance.
Presentation
A 69-year-old was referred for evaluation of clinical-biochemistry evidence of hepatobiliary injury. He had undergone radical prostatectomy, with blood transfusion, for prostatic carcinoma 6 years before (T2, M0, N0: Gleason score 6). Regular medications included atenolol, movicol, losartan and aspirin. He had no personal or family history of liver disease, drank no alcohol, denied any foreign travel, unwell contacts and had no tattoos. Examination was unremarkable, with no peripheral stigmata of chronic liver disease, hepatosplenomegaly or encephalopathy.
Bicalutamide (150mg once daily) had been commenced 5 months earlier when serum prostate-specific antigen values rose after 5 years of disease remission. Serum transaminase activity rose and bicalutamide was stopped, but AST and ALT values continued to rise, peaking respectively at 734 IU/L (20x maximum normal) and 1273 IU/L (30x maximum normal; Table 1).
Investigation
Clinical laboratory studies found no abnormalities in coagulation and no evidence of hepatotropic-virus infection or autoimmunity; serum immunoglobulin values were within expected ranges, as were alpha-1-antitrypsin, alpha-foetoprotein and caeruloplasmin levels. The only abnormalities were raised LDH and ferritin values (749 U/L and 950 ng/L respectively). Abdominal sonography found several hepatic cysts up to 3cm in diameter, interpreted as incidental; hepatic and portal venous flow was normal. Computerized tomography found no evidence of parenchymal infiltrative disease.
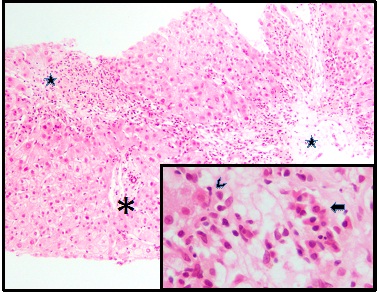
Core needle biopsy of liver was performed. Microscopy found portal-tract and lobular mixed inflammation disproportionately affecting the centrilobular liver, with parenchymal rarefaction and pigment-laden scavenger macrophages, both reflecting hepatocyte loss. The centrilobular inflammatory infiltrate at sites of hepatocyte loss contained macrophages laden with both lipofuscin and stainable iron. Necrotic hepatocytes were seen with a scattering of plasma cells and a large population of active lymphocytes. Portal tracts were oedematous but generally not inflamed. No viral inclusions, granulomata, cholestasis, deposits of copper-binding protein or evidence of storage disorder were found. These findings were interpreted as consonant with seronegative autoimmune hepatitis (Figure 1).
Outcome
Corticosteroids were commenced (prednisolone 40mg once daily) in addition to prophylactic bone protection and proton pump inhibitor therapy.
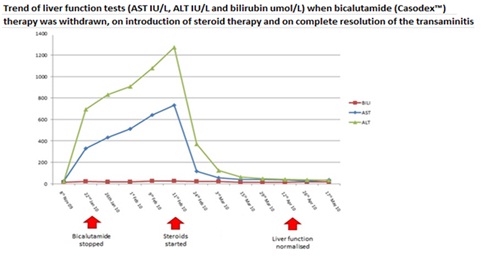
A marked fall in transaminase activities ensued, with AST and ALT at 116 IU/L and 370 IU/L by week 2 of corticosteroid treatment (Figure 2). AST and ALT levels had normalized by week 8 (AST 35 IU/L, ALT 39 IU/L) and have remained normal since.
Discussion
Autoimmune hepatitis
Autoimmune hepatitis is a chronic inflammatory disease with an incidence of 1-2 per 100,000 and female predominance. It is characterized by auto antibodies in circulation, hypergammaglobulinaemia and necroinflammatory changes on liver biopsy. Its prognosis is favorable when immunosuppressive treatment is given [1]. Seronegative autoimmune hepatitis also is a recognized entity [2].
Based on Autoimmune Hepatitis Group (IAHG) scoring our patient has a probable autoimmune hepatitis: Score 16 (>17 likely diagnoses) [2].
Bicalutamide and drug-Induced hepatitis
Bicalutamide is a non-steroidal anti-androgen agent, often used in conjunction with a luteinizing-hormone releasing agonist to reduce tumour flare in metastatic prostate cancer [3]. Other non-steroidal anti-androgens include flutamide and nilutamide.
Bicalutamide and its enantiomers impair liver function by unknown mechanisms. That flutamide induces liver injury via oxidative stress on mitochondria has been proposed [4,5]. In our patient the histopathologic pattern of injury suggested autoimmune disease, although no wider evidence of autoimmunity was found.
Five descriptions of acute liver failure ascribed to bicalutamide exist. Confusion, jaundice and encephalopathy were reported in a 60-year-old following 2 doses of bicalutamide for adenocarcinoma of the prostate [6]. Auto antibodies were not sought and immunoglobulin values were not determined. Bicalutamide was withdrawn; clinical-biochemistry abnormalities resolved after 8 weeks, with supportive treatment only.
Another case report described a 59-year-old who after 4 days of bicalutamide treatment presented with acute liver failure manifesting as abdominal pain and distension, with ALT activity 40x maximum normal, and who died 4 days thereafter [7]. Transaminitis and acute liver failure have also been described [8]. Hepatotoxicity has been described in a patient with metastatic prostate cancer and previous resolved hepatitis B infection (surface antibody and core antibody demonstrable) [9]. The authors proposed this was a drug induced liver injury. These four patients did not receive corticosteroid therapy or undergo a liver biopsy.
Our case and outcome is most similar to a fourth case report, describing a patient who underwent liver biopsy with histological findings consistent with a drug-induced liver injury.
Conclusion
We have attended a 69-year-old with biopsy-proven bicalutamide-induced autoimmune-like hepatitis against a background of prostatic carcinoma without antecedent liver disease. Abnormalities in serum transaminase activities worsened despite withdrawal of bicalutamide, but with corticosteroid therapy resolved over 8 weeks. Liver-biopsy and serologic findings, with clinical course, were consonant with seronegative autoimmune hepatitis. Events in our patient expand the range of known bicalutamide-associated disease.
Figures
Figure 1: Liver, predominantly centrilobular inflammation and injury (stars), with central-central bridging. A portal tract is spared (asterisk). Necroinflammatory change, inset with emperipolesis (lymphocyte invading hepatocyte, arrowhead) and a cluster of plasma cells (arrow) in region of hepatocyte loss. Haematoxylin / eosin; original magnifications 100x, principal image, and 400x, inset.
Figure 2: Trend of AST IU/L, ALT IU/L and bilirubin µmol/L.
Tables
| Analyte (normal range/units) | 8th Nov 2009 | 22nd Jan 2010 | 26th Jan 2010 | 9th Feb 2010 | 11th Feb 2010 (corticosteroids commenced) |
| Bilirubin (0-20) umol/L | 14 | 20 | 19 | 24 | 26 |
| AST (<37) IU/L | 24 | 328 | 433 | 640 | 734 |
| ALT (<41) IU/L | 20 | 695 | 830 | 1079 | 1273 |
| ALP (40-129) IU/L | 54 | 63 | 71 | 80 | 88 |
| Gamma GT (8-61) IU/L | 24 | 72 | 86 | 145 | 153 |
Table 1: Hepatobiliary-injury biomarker values before during and after bicalutamide administration and before corticosteroid therapy; bicalutamide started (09.2009), withdrawn (22.01.2010). Abnormal values underscored.