
Acupuncture and Herbal Moxibustion for the Treatment of ‘BiQiu’ (Allergic Rhinitis Symptoms) in a Hong Kong Chinese Medicine Clinic: Protocol for a Randomized Controlled Trial
*Corresponding Author(s):
Yung Ting YiuNethersole Chinese Medicine Service Cum The Chinese University Of Hong Kong Chinese Medicine Clinica, G/F, Block J, Staff Centre, Alice HoMiu Ling Nethersole Hospital, 11 Chuen On Road, Tai Po, Hong Kong
Tel:+852 26630004,
Fax:+ 852 26630345
Email:benny.yung@ucn.org.hk
Abstract
Introduction
‘BiQiu’ is a Traditional Chinese Medicine name for the disease with allergic rhinitis symptoms, which is one of the most common health complaints worldwide. Acupuncture has been widely used to treat patients with ‘BiQiu’ (allergic rhinitis symptoms) in East Asia, but the relevant evidence is insufficient. The study aims to evaluate the clinical effectiveness of acupuncture and/or with herbal moxibustion in Hong Kong population.
Methods
This study is a single-centre, randomized; assessor blinded controlled trial with three parallel arms. The acupuncture groups will receive acupuncture treatment three times per week for a total of 12 sessions in four weeks. Acupuncture combined with herbal moxibustion treatment group will receive herbal moxibustion once per week for a total of 4 sessions over four weeks in addition to the same acupuncture treatment. Participants in the waitlist group will not receive any acupuncture or herbal moxibustion treatments during this period. All patients will receive advice on life style, diet and exercise. The primary outcome measures the change in the total nasal symptom score; secondary outcome measures the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) score and the total IgE in serum.
Results
This study will demonstrate an evidence-based of acupuncture for BiQiu and will be published in a peer-reviewed journal.
Discussion
This trial will provide evidence for the effectiveness of either acupuncture combined with herbal moxibustion or acupuncture alone when compared to waitlist group as a treatment for allergic rhinitis.
Trial registration
ChiCTR-INR-16010047registered on November 25, 2016.
Keywords
Acupuncture; Allergic rhinitis; BiQiu; Randomized controlled trial; Study protocol
ABBREVIATIONS
TCM: Traditional Chinese Medicine
TNSS: Total Nasal Symptom Score
G6PD: Glucose-6-Phosphate Dehydrogenase
PASS: Power Analysis and Sample Size
SD: Standard Deviation
ANCOVA: Analysis of Co-Variance
RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire
IL-4: Interleukin-4
IL-5: Interleukin-5
ECP: Eosinophil Cationic Protein
EOS: Eosinophils
CMCTRs: Chinese Medicine Centres for Training and Research
HA: Hospital Authority
INTRODUCTION
Currently, the main prevention and treatment approaches for AR include allergen prevention, medication or immunisation. Surgical treatment can also be conducted if necessary. Medication is one of the most commonly used means to mitigate the symptoms. Clinically the medicinal treatments of AR include corticosteroids, antihistamines, mast cell stabilizer, anticholinergics and nasal decongestant drugs, etc., however, the effect generally lasts for a short time and there are often many side effects (such as sedation, nose bleeds, rebound nasal congestion and septal perforation or the risk of anaphylaxis); The symptoms are highly likely to recur. In addition, these medications have to be taken for prolonged periods, thus also prolonging the side effects experienced by the patients [6,7]. Immunisation can change the natural progress of AR by immunity regulation mechanism, but the treatment cycle is long and expensive [8,9]. There is also the potential risk to trigger serious allergic reaction [10]. Surgery is only applicable for some certain complications and comorbidities of rhinitis. It is also traumatic, costly and not easily accepted by patients [11]. Therefore, it is an essential task to identify an effective treatment for AR with low relapse rate and few side effects.
Acupuncture and herbal moxibustion are commonly used for treatment of AR in China. Some clinical studies have shown that acupuncture for treating AR can improve its symptoms, reduce the recurrence rate and improve the quality of life [12-14]. It has been shown that acupuncture could decrease serum IgE, IL-4 and IL-5 levels [15].
Moxibustion generally refers to burning moxa made from dried mugwort on acupoints. But herbal moxibustion here refers to applying the mixture of some specific herbs to paste on acupoints, which is a traditional treatment modality in Traditional Chinese Medicine (TCM). According to the theory of TCM, the basic pathogenesis of ‘BiQiu’ is mainly inadequate functioning of lung ‘Qi’, often companied by the ‘Qi’ insufficiency of spleen and kidney. Therefore, the general treatment principle for ‘BiQiu’ is regulating the function of lung ‘Qi’, unblocking the meridians and replenishing the ‘Qi’ of spleen and kidney. Because herbal moxibustion used herbs with properties of warming Yang and stimulating meridians, the channel of ‘Qi’ flowing inside body, they may exert the treatment effect similar to moxibustion. Such an effect of herbal moxibustion can complement acupuncture, and therefore improve the clinical effectiveness. Studies demonstrated that herbal moxibustion may improve general health, social life and vitality in quality of life [16].
The recently published systematic reviews on the effect of treating AR with acupuncture and herbal moxibustion have also demonstrated that these two methods may have promising treatment effect for AR [17-19]. However, the ratings on the quality of clinical studies were generally low. Some limitations with previous studies include inappropriate sample size, inappropriate outcome assessment measures, a lack of information on random allocation and blinding, and a lack of the standardization of acupuncture treatment procedure, etc [20-22].
To establish the reliable evidence about the effect of acupuncture or herbal moxibustion for AR, we propose to conduct a scientifically rigorous randomized controlled trial to assess the clinical effect of acupuncture or herbal moxibustion on treating the AR symptoms, namely ‘BiQiu’ in TCM.
METHODS
Aims and hypotheses
This study is designed to test three hypotheses: (1) Acupuncture combined with herbal moxibustion is clinically effective on treating AR symptoms compared to waiting list; (2) Acupuncture is clinically effective on treating AR symptoms compared to waiting list; (3) Acupuncture combined with herbal moxibustion is more clinically effectively than acupuncture alone on the treatment of AR symptoms.
Design
Trial participant
Inclusion criteria: Based on the clinical diagnosis of AR in Western medicine, subjects were included upon meeting the following criteria [23]:
• Medical history: The subject has a history of AR, which is related to change in temperature and allergens such as pollen, dust mites, etc.,
• Clinical symptoms: The subject has the primary symptoms of nasal congestion, runny nose, sneezing and nasal itchiness. Subjects must have two or more of these symptoms
• Aged between 18 - 65
• A Total Nasal Symptom Score (TNSS) ≥ 4
• The subject agrees to take part in this study and is fully capacitated, can express personal will and accurately describe symptoms
Exclusion criteria: The subjects will be excluded when they meet one of the following criteria:
• Has undertaken Chinese medicine treatment related to AR over the past month
• Has contracted respiratory infection or acute paranasal sinusitis within the past 14 days
• Has a history of chronic paranasal sinusitis
• Has organic nasal pathological changes or has had nasal surgeries
• Has asthma and other respiratory attack conditions
• Has taken antihistamines, steroids, decongestants (used on the nose, the mouth and the eyes), antibiotics, anti-coagulant and anti-platelet agents in the past month
• Has undertaken specific immune treatment or systemic hormone therapy in the past year
• Has serious cardiac, liver, renal, cerebrovascular conditions, or neural or mental disorders, etc.,
• Pregnant or nursing, or planning to conceive during the course of treatment
• Has Glucose-6-Phosphate Dehydrogenase Deficiency (G6PDD)
• Has wounds at the punctured locations or diabetes mellitus
RANDOMIZATION AND BLINDING
After being evaluated against the inclusion and exclusion criteria, the eligible participant will be asked to sign an informed consent form, and thereafter allocated with a serial number. The envelope with corresponding serial number will be given to the Chinese medicine practitioner. The Chinese medicine practitioner will provide treatment protocols as indicated on the card inside the envelope. The individual treatment protocols will be blinded to the data collector.
INTERVENTIONS
Acupuncture treatment
The following acupuncture points are used for the treatment of ‘BiQiu’ (AR symptoms): bilateral LI20 (Yingxiang), GB20 (Fengchi), LI4 (Hegu), ST36 (Zusanli) and EX-HN3 (Yintang) (Table 1).
|
Acupoints |
Location |
|
Yingxiang (LI20) |
At the midpoint laterals to the border of the ala nasi, in the nasolabial groove. |
|
Fengchi (GB20) |
Below the occiput, approximately midway between Fengfu (DU16) and Wangu (GB12), in the hollow between the origins of the sternomastoid and trapezius muscles. |
|
Hegu (LI4) |
On the dorsum of the hand, between the 1st and 2nd metacarpal bones, in the middle of the 2nd metacarpal bone on the radial side. |
|
Zusanli (ST36) |
Below the knee, 3 cun inferior to Dubi(ST35), one fingerbreadth lateral to the anterior crest of the tibia. |
|
Yintang(EX-HN3) |
At the forehead, at the midpoint between the two medial ends of the eyebrow. |
|
Dazhui (GV14) |
On the midline at the base of the neck, in the depression below the spinous process of the seventh cervical vertebra. |
|
Feishu (BL13) |
On the back, 1.5 cun lateral to the lower border of the spinous process of the 3rd thoracic vertebra. |
|
Shenshu (BL23) |
On the back, 1.5 cun lateral to the lower border of the spinous process of the 2nd lumbar vertebra. |
‘Hua Tuo brand’ disposable sterile acupuncture filiform needles of the 0.25x25mm or 0.25x40mm specification will be used. After all sites of acupuncture points are carefully sterilised with 75% alcohol pad, the needles are inserted using the lifting-thrusting and twirling-rotating techniques, until ‘deqi’ (arrival of ‘Qi’) sensation is achieved at all acupuncture points. In Chinese medicine, Deqi means the acupuncture needle are modulating the movement of qi along the meridian. It can be felt by the person receiving acupuncture through perception of a complex sensations including heaviness, numbness, soreness, or distension (with The acupuncture practitioner can feel deqi through the feeling of tenseness around the needle. The needle stays inserted for 20 minutes for each session. The acupuncture treatment will be conducted 3 times per week for continuous 4 weeks. There is a total of 12 acupuncture treatment sessions during one month.
Herbal moxibustion treatment
For preparation of the pasted herbal medicines, 240 ml of ginger juice and 200 g of powdered medicine (‘Tianjiu’ No.1) are mixed into a paste and cut into circular pellets weighing 2-3 g each; these will have a surface area with a diameter of 10mm and about 4-5 mm thick. A 4 cm x 4 cm plaster will be used to fix the medicine onto the abovementioned acupuncture points. After the medicine is patched, the skin will experience mild itchiness and a burning sensation. The patch should not be taken awayuntil 1 hour later, and it will be applied once a week for 4 weeks during the study. Notes on caring for the medicine application areas will be given to the patients.
Every 200 g of powdered medicine (‘Tianjiu’ No.1) contains Sinapis semen (Jiezi) 44.4g; Corydalis rhizome(treated Yanhusuo) 22.2g; Kansui radix (treated Gansui) 22.2g; Asarum sieboldii miq (Xixin) 22.2g; Ephedrae herba (Mahuang) 22.2g; Aconm lateralis radix praeparaia (treated Fuzi) 22.2g; Cinnamomi cortex(Rougui) 22.2g; Caryophylli flos (Dingxiang) 22.2g.
Health advice
Intervention assignment
Follow-up visit
OUTCOME MEASURES
Primary outcome
Secondary outcomes
All the outcome measurement will be collected before the initiation of treatment, four weeks after treatment beginning and one month after the end of treatment.
The demographic data of subjects, such as age, gender, type’s classification of AR and medical history, etc., will be collected at baseline.
Adverse events will be assessed by the registered Chinese medicine practitioner after each treatment, who will examine any possible adverse conditions such as pain, haemorrhage, needle faintness, needle sticking, etc. The redness, heatedness, itchiness, pain or even blisters appearing on the skin after herbal moxibustion will also be recorded. The occurrence and severity of adverse events, the time and handling method, etc., will be noted down in the adverse events (reaction) report (Table 2).
|
|
Treatment phase (weeks) |
Follow-up phase (weeks) |
||||
|
|
Baseline |
W1 |
W2 |
W3 |
W4 |
W8 |
|
Demographic data |
X |
|
|
|
|
|
|
Sign informed consent |
X |
|
|
|
|
|
|
Randomisation |
|
X |
|
|
|
|
|
TNSS |
X |
|
|
|
X |
X |
|
RQLQ |
X |
|
|
|
X |
X |
|
Total IgE |
X |
|
|
|
X |
X |
|
AEs |
|
X |
X |
X |
X |
|
STATISTICAL ANALYSIS
SAMPLING SIZE CALCULATION
TERMINATION OR DISCONTINUATION CRITERIA
• The subject has sudden or serious complications over the course of treatment
• The subject runs into serious adverse events or allergic reactions or requests to discontinue with the trial because of adverse events; or that a trial operator considers it necessary to terminate the trial because of adverse events
• The subject has low compliance and fails to execute as required, or fails to persist with the treatment and requests to be withdrawn
• The timely treatment become necessary because the rhinitis worsens; or the subject is considered unsuitable to continue with the trial according to the judgement of the research investigator
Although the patients in the waiting list group are encouraged to not undertake other therapies throughout the trial, in the situation when these patients feel they cannot tolerate the symptoms, for ethical reason, they are allowed to take drugs. All patients will be monitored throughout the trial and the protocol will follow an intention-to-treat approach. However, from our previous observations in the clinic, patients with AR may feel troubled by the symptoms but they are capable of tolerating it. Most of these patients have suffered AR for a long time and show exceptional tolerance to AR symptoms. The registered TCM practitioner will ask the subject if she or he has ever taken any prohibited drugs or received other therapies during each treatment (3 times per week) and record it. If yes, the patient will be dropped out, but we will continue to collect the pre-specified outcome data. The reasons for discontinuance will be recorded. The impact on the overall study will be considered and noted in the study report.
QUALITY CONTROL
• Assessor-blinding is strictly implemented. The designated investigator will conduct clinical rating over the assessment. During the assessment process, the assessor must be kept from knowing the treatment assignment, and the assessor will not involve any treatment during the study
• The researchers will be actively involved in pre-treatment promotion of the study intention and significance, therefore help subjects to stay with the study to reduce drop off cases
• All investigators who have completed good clinical practice training will independently collect the data and assess the effects of the treatments
• A remote data capture system will be used to store data on a password-protected computer that will store recorded data in a secure environment. In principle, clinical information will not be released without the permission of the principal investigator (ZHANG Hongwei), with the exception of an emergency or as necessary for monitoring and auditing by the data monitoring committee
DISCUSSION
In Hong Kong, a predominantly urban environment, persistent AR with intermittent aggravation is the most common presentation in clinic. In Hong Kong, where the climate is warm and humid, and with crowded living conditions, it has been identified that house dust mite and cockroach were the common aetiological agents of AR [30-32]. On the other hand, in central China, the majority of AR patients had perennial AR with or without seasonal aggravation [33].
This trial is designed as patients not being blinded. Since patients of the acupuncture group and the acupuncture plus moxibustion group will receive additional therapies than the patients in the waiting list group, the effectiveness will not be necessary caused by the specific treatment effects. Although many placebo-controlled RCTs of acupuncture have demonstrated the existence of placebo effect during acupuncture treatment, the current study aimed to investigate the total clinical effectiveness of acupuncture or/with herbal moxibustion, not the specific treatment effect. The existence and magnitude of specific treatment effect of acupuncture or herbal moxibustion are related to the acupoint selection and the technique of needling manipulation, which is not the research question in this study.
Total IgE reflects the allergenic response in human body. From previous mechanism study, it is known that acupuncture and herbal moxibustion may adjust the immune function of the human body so as to lower the total IgE level [34,35]. Total IgE test is used to provide an objective indicator besides those subjective assessments.
All subjects will receive health education on lifestyle, encouragement to carry out proper physical activities, adequate sleep, refrain from cold food and avoid contacting allergens, which is a part of ordinary TCM clinical practice. From the view of Chinese medicine, the onset of ‘BiQiu’ is highly related to lifestyle, whichwould affect the treatment efficacy if the modification of living style is neglected. So, the Chinese medicine practitioner always makesadvices on healthy lifestyle in order to facilitate the treatment. In addition, it would foster the compliance of the participants in the waiting list. The health education in this study is common advice given to patients by Chinese medicine doctors in the clinic. Some general allergens encountered in everyday living, such as those from dusty living environment, contact with cats or dogs, etc., can be delineated from everyday habit and via health education patients may improve their conditions by adjusting their life style accordingly. Although some patients may have eliminated or reduced their symptoms without acupuncture and/or moxibustion treatments, the fluctuation of symptoms in these patients will not pose bias to the study since patients will be properly randomized into the three study groups. The symptoms may be influenced after patients follow health education. But such fluctuation of symptoms is generally in a balanced distribution among three groups after randomization.
COMPETING INTERESTS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
AUTHORS’ CONTRIBUTIONS
FUNDING
AVAILABILITY OF DATA AND MATERIALS
ACKNOWLEDGEMENT
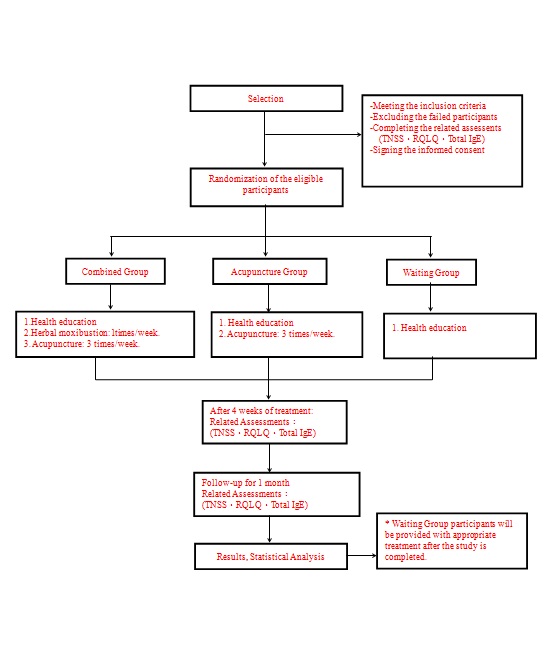
CHART OF STUDY FLOW
REFERENCES
- Goh YL, Liu J, Zhao B (2014) Use of the Layer Analysis Method of the Yellow Emperor's Inner Classic in modern society. J Acupunct Meridian Stud 7: 331-336.
- Dykewicz MS, Hamilos DL (2010) Rhinitis and sinusitis. J Allergy Clin Immunol 125: 103-115.
- Scadding GK (2015) Optimal management of allergic rhinitis. Arch Dis Child 100: 576-582.
- Buslau A, Voss S, Herrmann E, Schubert R, Zielen S, et al. (2014) Can we predict allergen-induced asthma in patients with allergic rhinitis? Clin Exp Allergy 44: 1494-1502.
- Zendejas Cervantes LH, Martinez Perez A, Castrejon Vazquez MI, Miranda Feria AJ (2003) [Quality of life evaluation in the patient with allergic rhinits]. Revista alergia Mexico 50: 91-95.
- Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356: 1255-1259.
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334: 1-100.
- Moingeon P, Batard T, Fadel R, Frati F, Sieber J, et al. (2006) Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy 61: 151-165.
- Meltzer EO, Bukstein DA (2011) The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol 106: 12-16.
- Bozek A, Kolodziejczyk K, Bednarski P (2015) The relationship between autoimmunity and specific immunotherapy for allergic diseases. Hum Vaccin Immunother 11: 2764-2768.
- Chhabra N, Houser SM (2014) Surgery for allergic rhinitis. International forum of allergy & rhinology 4: 79-83.
- Xue CC, An X, Cheung TP, Da Costa C, Lenon GB, et al. (2007) Acupuncture for persistent allergic rhinitis: a randomised, sham-controlled trial. Med J Aust 187: 337-341.
- Ng DK, Chow PY, Ming SP, Hong SH, Lau S, et al. (2004) A double-blind, randomized, placebo-controlled trial of acupuncture for the treatment of childhood persistent allergic rhinitis. Pediatrics 114: 1242-1247.
- Xue CC, English R, Zhang JJ, Da Costa C, Li CG (2002) Effect of acupuncture in the treatment of seasonal allergic rhinitis: a randomized controlled clinical trial. Am J Chin Med 30: 1-11.
- Park MB, Ko E, Ahn C, Choi H, Rho S, et al. (2004) Suppression of IgE production and modulation of Th1/Th2 cell response by electroacupuncture in DNP-KLH immunized mice. J Neuroimmunol 151: 40-44.
- Hsu WH, Ho TJ, Huang CY, Ho HC, Liu YL, et al. (2010) Chinese medicine acupoint herbal patching for allergic rhinitis: a randomized controlled clinical trial. Am J Chin Med 38: 661-673.
- Zhou F, Yan LJ, Yang GY, Liu JP (2015) Acupoint herbal patching for allergic rhinitis: a systematic review and meta-analysis of randomised controlled trials. Clin Otolaryngol 40: 551-568.
- Lee MS, Pittler MH, Shin BC, Kim JI, Ernst E (2009) Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol 102: 269-279.
- Roberts J, Huissoon A, Dretzke J, Wang D, Hyde C (2008) A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med 8: 13.
- Schnyer RN, Allen JJ (2002) Bridging the gap in complementary and alternative medicine research: manualization as a means of promoting standardization and flexibility of treatment in clinical trials of acupuncture. J Altern Complement Med 8: 623-634.
- Paterson C, Dieppe P (2005) Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. Bmj 330: 1202-1205.
- Zhang CS, Tan HY, Zhang GS, Zhang AL, Xue CC, et al. (2015) Placebo Devices as Effective Control Methods in Acupuncture Clinical Trials: A Systematic Review. PloS one 10: 0140825.
- Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association (2016) [Chinese guidelines for diagnosis and treatment of allergic rhinitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 51: 6-24.
- Saghaei M (2004) Random allocation software for parallel group randomized trials. BMC Med Res Methodol 4: 26.
- Choi SM, Park JE, Li SS, Jung H, Zi M, et al. (2013) A multicenter, randomized, controlled trial testing the effects of acupuncture on allergic rhinitis. Allergy 68: 365-374.
- Juniper EF, Guyatt GH (1991) Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy 21: 77-83.
- LS (2010) A randomized controlled study on acupuncture therapy for persistent allergic rhinitis. Beijing University of Chinese Medicine. China.
- Kim JI, Lee MS, Jung SY, Choi JY, Lee S, et al. (2009) Acupuncture for persistent allergic rhinitis: a multi-centre, randomised, controlled trial protocol. Trials 10: 54.
- Borm GF, Fransen J, Lemmens WA (2007) A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol 60: 1234-1238.
- Lee SL, Wong W, Lau YL (2004) Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood). Pediatr Allergy Immunol 15: 72-78.
- Teng B, He P, Huang L, Liang Y, Wang Y, et al. (2015) Overview of relation between air pollution and allergic rhinitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 50: 683-685.
- Hwang BF, Jaakkola JJ, Lee YL, Lin YC, Guo YL (2006) Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir Res 7: 23.
- Chen J, Zhao Y, Li B, Zhang Q, Wan L, et al. (2014) A multicenter study of the clinical features of allergic rhinitis in central China. Am J Rhinol Allergy 28: 392-396.
- Lau BH, Wong DS, Slater JM (1975) Effect of acupuncture on allergic rhinitis: clinical and laboratory evaluations. Am J Chin Med (Gard City NY) 3: 263-270.
- Jianli C (2006) The effect of acupuncture on serum IgE level in patients with chronic urticaria. J Tradit Chin Med 26: 189-190.
Citation: Yiu YT, Hongwei Z, Che TL, Lang Z, Ping LN, et al. (2019) Acupuncture and Herbal Moxibustion for the Treatment of ‘BiQiu’ (Allergic Rhinitis Symptoms) in a Hong Kong Chinese Medicine Clinic: Protocol for a Randomized Controlled Trial. J Altern Complement Integr Med 5: 065.
Copyright: © 2019 Yung Ting Yiu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

