
Quality Control of Botanical Tinctures: Endogenous Bacterial Flora Present in Botanical Extractions
*Corresponding Author(s):
Langland JODepartment Of Research, Southwest College Of Naturopathic Medicine, 2140 E, Broadway Road, Tempe, Arizona, United States
Tel:+1 4805184268,
Email:j.langland@scnm.edu
Abstract
Botanical-based therapeutics are often prepared as either water, ethanol or glycerin-based extractions of the harvested plant material. The raw botanical material is not grown in a sterile environment and as such, may contain a variety of microbial flora. This research evaluated the level of microbial flora present in common botanical extractions prepared under different methodologies and did basic characterization to determine if these microbial populations may include potential human pathogens. The results indicated that significant bacterial flora is commonly present in botanical extracts, including potential human pathogens and that the extraction process utilized will alter the level of microbes present. This research is encouraged help physicians be aware of potential microbial contaminants present in herbal preparations and to use proper care and follow-up when treating patients.
Keywords
INTRODUCTION
As worldwide demand for botanical medicine rises, it becomes increasingly paramount to understand the aspects of quality control used during the production of herbal therapeutics [2]. Oftentimes the only processing done during the agricultural phase of medicinal plant production is the drying of the raw plant material after harvest [3]. After desiccation, the herbal components are typically added to an extraction agent, such as hot water, alcohol or glycerin depending on whether the medicine is to become a tea, tincture or glycerite respectively [3,4]. Tinctures are defined as simple grain alcohol infusion [4,5]. Depending on the type of active constituent that is being extracted from the plant material, optimal ethanol by volume percentages of tinctures can typically range from 20%-70%. Glycerites are a glycerol (70-75%) infusion that is most often used when the active constituent is a water-soluble compound [5].
Although multiple studies have demonstrated the therapeutic value of many botanical preparations and the active constituents extracted [5-9], only limited research has profiled the microbiological populations present in the raw plant preparations. Some previous research has addressed concerns of fungal contamination in raw plant materials used for medicinal extractions [10,11], however potential bacterial contaminants remain largely uninvestigated.
Research exposing the presence of molds and fungi in medicinal plant preparations highlights the need for further investigation of other microbial contaminants in order to maintain therapeutic safety. It therefore becomes necessary to determine the likelihood and abundance of bacterial contaminants in medicinal plants and whether these contaminants could be found in the end products that are administered to patients. The goal of this study was to explore the safety of botanical supplements, specifically teas, tinctures and glycerites, by examining the abundance of bacteria in commonly used medicinal plants with different extraction agents. This data may provide a better understanding and awareness of which extraction process may be effective in reducing bacterial populations and whether or not patients could receive a therapeutic contaminated with potentially pathogenic bacteria. The outcomes of this research may help physicians to take appropriate precautions when treating patients with herbal preparations.
METHODS
Botanical sources
Extraction preparation
Bacterial assays
RESULTS
Total bacterial concentration related to extraction process
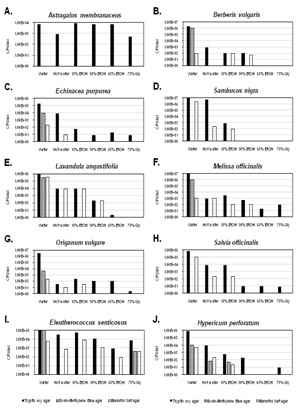
Botanicals used in medicinal teas often go through minimal processing and therefore may contain endogenous bacteria contaminants. A hot water extraction was used to simulate making of a botanical tea and to test if bacterial populations are reduced in the process. When comparing the hot water extractions to the cold water extraction, there was a substantial reduction in the total bacterial concentration in the majority of botanical samples with the exception being E senticosus (Figure 1, Tryptic Soy agar). There was an approximate 100-fold reduction in the number of CFU/ml in the hot water extractions of E purpurea, L angustifolia and H perforatum when compared with their water controls (Figure 1, Tryptic Soy agar). An approximate 1,000-fold reduction of CFU/ml was seen in the hot water extraction of S officinalisand S nigra when compared with their water controls (Figure 1, Tryptic Soy agar). In two of the ten hot water extracts (B vulgaris and M officinalis), an approximate 10,000-fold reduction in the number of CFU/ml was observed when compared to their water controls (Figure 1, Tryptic Soy agar). The botanical with the greatest reduction in CFU/ml (approximately 100,000-fold) from hot water extraction when compared to its control was O vulgare (Figure 1, Tryptic Soy agar). These results suggest that either the bacterial populations between the different herbs vary in sensitivity to heat or that the heating process may release different components from the herbs, some of which may have direct anti-bacterial properties.
Ethanol is known to have antibacterial properties. While not the most common extraction solution, 15% ethanol is used in some tinctures to extract certain constituents, however, because of the low alcohol content, anti-bacterial properties of the ethanol may be minimal. As shown, the majority of 15% ethanol extractions contained less CFU/ml than the coldwater samples and were typically similar to or lower than the hot water extraction (Figure 1, Tryptic Soy agar). In the 15% ethanol extractions of E senticosus and A membranaceus, no reduction in the number of CFU/ml was observed (Figure 1, Tryptic Soy agar). An approximate 100-fold reduction in the number of CFU/ml in L angustifolia and a 1,000-fold reduction in the number of CFU/ml in S officinalis, H perforatum and M officinalis were observed compared to their respective controls (Figure 1, Tryptic Soy agar). The largest reduction was an approximate10,000 fold reduction seen in the B vulgaris, E purpurea, S nigra and O vulgare samples when compared to their respective controls (Figure 1, Tryptic Soy agar). Due to the low ethanol concentrations in these extracts, the reductions in bacterial titers may be related to the extraction of antibacterial constituents from the herbs, especially since similar trends were observed with hot water and 15% ethanol for several herbs.
The most common extraction solution for tinctures is 30-40% alcohol since many active constituents can be extracted at in this concentration range [5]. In six of the samples (A membranaceus, B vulgaris, E purpurea, O vulgare, E senticosus and H perforatum) there was very little difference in the concentration of CFU/ml in the 30% ethanol and 15% ethanol extractions (Figure 1, Tryptic Soy agar). In M officinalis, S officinalis and L angustifolia there was an approximate 10-fold reduction in the concentration of CFU/ml from the 30% and 15% ethanol extractions (Figure 1, Tryptic Soy agar). Whereas in S nigra there was an approximate 100-fold reduction in the number of CFU/ml between the 30% and 15% ethanol extractions (Figure 1, Tryptic Soy agar). Overall, the 30% ethanol extraction reduced the average number of bacteria for all the herbs approximately 1,000-fold compared to the cold water extraction. This supports that the increase in ethanol to 30% likely produced a safer therapeutic comparatively, but one that still contained a substantial amount of bacterial contaminants.
Tinctures containing higher ethanol content are not uncommon but are typically focused on the extraction of specific components, including resins and oleoresins [5]. When compared to the hot water, 15% ethanol and 30% ethanol, 65% ethanol extraction had the greatest reduction in the number of CFU/ml when compared with the water control. Three of the ten botanicals contained no detectable bacteria (B vulgaris, S nigra and H perforatum). Two botanicals (L angustifolia and S officinalis) contained very low (<1x101) levels of bacteria (Figure 1, Tryptic Soy agar). This resulted in a 100,000 to 1,000,000-fold reduction in the number of CFU/ml in these samples. In four of the samples (A membranaceus, E purpurea, O vulgare, S officinalis) there was no significant reduction of the CFU/ml between the 30% and 65% ethanol extractions (Figure 1, Tryptic Soy agar). This result may suggest variations in ethanol sensitivity between endogenous bacteria or the extraction of different antimicrobial constituents at the higher ethanol concentration in some, but not all botanicals tested.
Tinctures containing glycerin have grown in popularity in recent years because of their palatability as well as their ability to extract water-soluble constituents from botanicals. The 70% glycerin extraction solution was very effective at reducing the number of CFU/ml when compared with the other extraction solutions. In two of the samples (B vulgaris and S nigra), both the 70% glycerin extraction and 65% ethanol extractions reduced the number of the bacteria to non-detectable levels in the sample (Figure 1, Tryptic Soy agar). This resulted in a 1,000,000-fold reduction of CFU/ml when compared to the control. O vulgare also produced an approximate 1,000,000-fold reduction in the number of CFU/ml (Figure 1, Tryptic Soy agar). In three samples (L angustifolia, M officinalis and S officinalis), 70% glycerin reduced the number of CFU/ml by an approximate 100,000-fold when compared to their respective cold water controls. There was an approximate 10,000-fold reduction in the CFU/ ml in both the H perforatum and E purpurea samples (Figure 1, Tryptic Soy agar). Compared to 65% ethanol, there was an approximate 10-fold reduction in the CFU/ml in the A membranaceus. Across all samples, both the 70% glycerin and 65% ethanol were the most effective at reducing the number of CFU/ml when compared to the water control and other extraction solutions.
Partial identification of bacteria present in botanical extractions
As shown in figure 1, ethanol or glycerin extractions from several herbs had no or minimal bacterial growth on EMB and MSA media. However, bacterial growth on EMB could still be detected with E senticosus extracted with 70% glycerin or H perforatum extracted with hot water or 15% ethanol. Growth on MSA was detected in most herbs extracted with hot water or 15% ethanol at levels up to 4x103CFU/mL. For some herbs, including B vulgaris, L angustifolia, M officinalis and E senticosus, growth on MSA was observed even at 30% ethanol extractions. Sixty-five percent ethanol or glycerin extraction reduced MSA growth to non-detectable levels for all herbs, except E senticosus. These results suggest that, although typically minimal, potential human pathogens may be occasionally present in herbal extraction preparations.
To further evaluate these potentially pathogenic bacteria, additional selective/differential media (including Salmonella-Shigella agar, hardy-chrom Staph aureus agar, KF Streptococcus agar) and Gram-staining procedures were utilized. Since hot water or ethanol extractions often had no detectable bacteria on EMB or MSA, these additional characterizations were done with cold water extracted botanicals only. Based on the growth characteristics (growth detected and colony color) and Gram-staining results, potential genera of the bacteria could be determined and the results are summarized in table 1. Although typically at minimal or low levels (1-10 CFU/ml of extract), potentially pathogenic bacteria were present in many of the botanical samples tested. Although definitive identification of these microbes was beyond the scope of this study, potential genera of these microbes including Escherichia, Proteus, Salmonella, Shigella, Staphylococcus, Streptococcus and Enterococci as shown in table 1, Streptococcus spp and Enterococcus spp. Were present in 10% and 20% of the herbs tested, respectfully. Similarly, potential Gram-negative bacteria including Escherichia spp (30% of herbs), Proteus spp (10% of herbs), Salmonella spp (30% of herbs) and Shigella spp (10% of herbs). Although the detection of potentially pathogenic bacteria was reasonably uncommon and when detected, was at minimal levels, these results do support the need for care when preparing and administering therapeutic extractions.
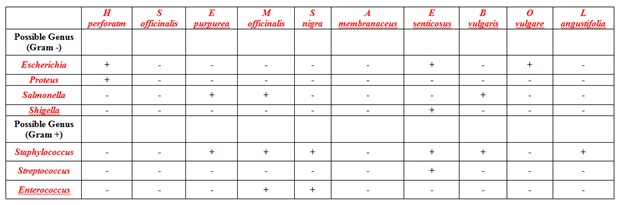
Cold water extracted botanicals (identified above each column) were serially diluted and grown on selective and differential media (Eosin-Methylene Blue agar (EMB), Salmonella-Shigella agar (SS), Mannitol Salt Agar (MSA) agar, HardyCHROM™ Staphylococcus aureus agar, and KF Streptococcus agar). Colonies present were subsequently analyzed by Gram stain. Potential genera of the bacteria were identified based on colony growth and color, Gram stain reaction, and cell shape. (-) indicated no detectable bacterial growth. (+) indicates 1-10 CFU/ml of extract.
DISCUSSION
Expanding upon the safety concerns raised by fungal contaminants, this study sought to investigate the level of bacterial contaminants present in raw medicinal plant extractions and how extraction methods may alter the level of these microbial contaminants. The goal of this study was not meant to identify specific bacteria present in specific botanicals since this could and will likely vary between different geographical sources and seasons. Instead, the overall goal was to give a screening of several medicinal herbs and different extraction processes and measure the level of total and potentially pathogenic bacterial contaminants present.
The overall results verified that medicinal plant material is by no means sterile and agrees with previous findings regarding microbial contaminants [10,11]. It should be noted that there seemed to be no correlation between the part of the plant harvested (roots, leaves and flowers) and the concentration of endogenous bacteria present. This is important to consider since roots and subterranean portions are often washed after harvest while aerial parts are harvested and dried without significant processing other than to separate out usable/desirable parts of the herb by removing all impurities and adulterations.
For medicinal use, botanicals are extracted in various solutions, such as hot water, alcohol or glycerin, depending on whether the medicine is to become a tea, tincture or glycerite, respectively. Most tinctures are prepared using ethanol by volume percentages typically ranging from 30-60% [5]. This concentration range is often optimal for extraction of the active constituents, but is often assumed to provide bactericidal activity as well. This is of concern since literature has established a threshold of 60-90% ethanol by volume for optimal bactericidal activity, also noting that bactericidal activity sharply declines at concentrations less than 50% [12,13]. Interestingly with most herbs tested, the bacterial populations decreased considerably when extracted using 15% ethanol and even further with 30% ethanol. These results may suggest that anti-microbial constituents are likely being extracted from the plant material at these low ethanolic extractions leading to the reduction in bacterial cell numbers. In support of this, a 2010 study established the antiseptic qualities of multiple phytochemicals present in the elderflower and illustrated the ability of S nigra elderflowers to inhibit a wide range of bacteria [9]. The absence of bacteria in the 35% ethanolic extraction of S nigra is consistent with previous research and supports the extraction of bactericidal active constituents in low ethanolic concentrations.
Like ethanol and glycerol has been shown to possess bactericidal activity [12,13]. A previous study examined the use of glycerol and demonstrated increased mortality against Gram-negative bacteria compared to Gram-positive bacteria [14]. In our glycerol-based tinctures (70% glycerol), bacterial titers were greatly reduced in 80% of the herbs tested (~10,000 fold) and substantially reduced in the other herbs 10-100 fold. Even if non-pathogenic, the substantial number of bacteria in many of the extraction processes may lead to additional and possibly undesirable effects when treating patients, including immunological, inflammatory or even toxin-related effects.
This study also sought to partially identify the presence of potential human pathogens in herbal preparations. Although specific and definitive identification of bacteria was not done, the presence of bacteria belonging to potentially pathogenic genera was determined. Although these genera were relatively uncommon, and when present, were at low to minimal levels, the mere presence of these organisms warrants concern as levels may vary between crops, seasons and geographical locations. Ultimately, without the sterilization of botanical medicines, administration of a botanical-based therapy, either topically or orally to a patient will likely introduce bacteria, including potentially pathogenic bacteria which may lead to deleterious responses. Currently, many nutraceutical companies that manufacture tinctures and herbal products do not take extra precautions for sterilization of their products. This practice is not mandated, regulated or a common standard of procedure in the field. Every treatment has a degree of risk to the patient and it falls to the discretion of the practitioner to determine if the risks outweigh the benefits. In many cases with botanicals, the therapeutic value has been established making them an essential part of treatment therapy. Previous studies have demonstrated a significant presence of fungal contaminants in herbal preparations and this study supports the presence of additional microbial contaminants, including bacteria. This cumulative data is not meant to discourage therapeutic botanical use but rather to educate prudence amongst those who use botanicals. In summary, when using raw plant materials or unsterilized extracts, special care and observation should be taken to limit possible secondary infections especially during the treatment of immunocompromised patients, or other unexpected responses, including inflammation, in other patients.
ACKNOWLEDGEMENT
AUTHOR DISCLOSURE STATEMENT
REFERENCES
- Calixto JB (2000) Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res 33: 179-189.
- Fu PP, Chiang HM, Xia Q, Chen T, Chen BH, et al. (2009) Quality assurance and safety of herbal dietary supplements. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27: 91-119.
- Falk CL, van Voorthuizen H, Wall MM, Kleitz KM, Guldan SJ, et al. (1999) Costs and returns of growing selected medicinal herbs in New Mexico indicate positive return to land and risk likely. HortTechnology 9: 681-686.
- Brevoort P (1998) The booming US botanical market: a new overview, Herbal Gram. The Journal of the American Botanical Council 44: 33- 48.
- Bone K (2003) A clinical guide to blending liquid herbs: Herbal Formulations for the Individual Patient. Churchill Livingstone, Edinburgh, UK.
- Denzler KL, Waters R, Jacobs BL, Rochon Y, Langland JO (2010) Regulation of inflammatory gene expression in PBMCs by immunostimulatory botanicals. PLoS One 5: 12561.
- Block KI, Mead MN (2003) Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther 2: 247-267.
- Tan BK, Vanitha J (2004) Immunomodulatory and antimicrobial effects of some traditional chinese medicinal herbs: a review. Curr Med Chem 11: 1423-1430.
- Hearst C, McCollum G, Nelson D, Ballard LM, Millar BC, et al. (2010) Antibacterial activity of elder (SambucuS nigra L) flower or berry against hospital pathogens. J Med Plant Res 4: 1805-1809.
- Halt M (1998) Moulds and mycotoxins in herb tea and medicinal plants. Eur J Epidemiol 14: 269-274.
- Ahmad B, Ashiq S, Hussain A, Bashir S, Hussain M (2014) Evaluation of mycotoxins, mycobiota and toxigenic fungi in selected medicinal plants of Khyber Pakhtunkhwa, Pakistan. Fungal Biol 118: 776-784.
- Morton He (1950) The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann N Y Acad Sci 53: 191-196.
- Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee (HICPAC) (2008) Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
- Saegeman VS, Ectors NL, Lismont D, Verduyckt B, Verhaegen J (2008) Short- and long-term bacterial inhibiting effect of high concentrations of glycerol used in the preservation of skin allografts. Burns 34: 205-211.
Citation: Nelson E, Kozin A, Ruiz G, Turner T, Langland JO (2016) Quality Control of Botanical Tinctures: Endogenous Bacterial Flora Present in Botanical Extractions. J Altern Complement Integr Med 2: 012.
Copyright: © 2016 Nelson E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

