
Spatholobus Suberectus Column Extract Suppresses Dendritic Cell Maturation and has Therapeutic Potential for Psoriasis
*Corresponding Author(s):
Ping LiBeijing Key Laboratory Of Clinic And Basic Research With Traditional Chinese Medicine On Psoriasis, Beijing Institute Of Traditional Chinese Medicine, Beijing Hospital Of Traditional Chinese Medicine, Affiliated With Capital Medical University, Beijing, China
Tel:+86 01052176679,
Fax:+86 02164085875
Email:Liping411@126.com
Abstract
Spatholobus Suberectus Column Extract (SSCE) is a natural product from the plant Spatholobus Suberectus Dunn, a kind of Traditional Chinese Medicine, which is widely used to invigorate the circulation of blood and replenish blood, has antitumor and anticoagulant properties and improves hematopoiesis. This study evaluated the clinical effects of Spatholobus Suberectus Column Extract (SSCE) in an Imiquimod (IMQ) induced psoriasis mouse model and investigated its role in regulating the differentiation and maturation of Dendritic Cells (DCs). BALB/c mice were used to establish the animal model for psoriasis-like skin lesion; SSCE at 12 mg/kg (high), 6 mg/kg (medium) and 3 mg/kg (low) respectively, were intragastrically administered. Psoriasis Area and Severity Index (PASI) was used to evaluate the skin lesions. Histological changes, the thickness of epidermis and the quantity of CD11c+ DCs in skin lesion and spleens were measured. In vitro experiments, bone marrow cells of mice were obtained, and CD11c+ cells were isolated. DCs with a mature state in differentiation and function were identified by flow cytometry. The influence of DCs on proliferation of allogenic lymphocytes was analyzed with CCK-8. SSCE treatment alleviated psoriasis-like skin with the decreased Psoriasis Area and Severity Index (PASI) score and obviously reduced the vertical thickness of epidermis. Besides, SSCE treatment decreased the quantity of CD11c+ DCs in skin lesions and spleens. Furthermore, SSCE reduced R848-induced murine bone marrow-derived DC maturation, characterized by reduced expression of CD80/86 and inhibited the alloproliferation of T cells. SSCE inhibited DC function and had potential as a therapeutic agent for psoriasis.
Keywords
INTRODUCTION
Psoriasis is a chronic inflammatory disorder of the skin affecting approximately 3% of the world’s population. It is the most common chronic inflammatory skin disease characterized by epidermal hyperplasia, scaling and erythematous plaque formation [1]. Although the pathogenesis of psoriasis is not fully elucidated, it is widely accepted that cellular infiltration of T cells, Dendritic Cells (DCs) and neutrophils is important in the pathogenesis of psoriasis by provoking inflammation [2].
DCs are key players in the immune mechanisms surrounding psoriasis as well as other autoimmune diseases [3]. DC-induced cytokine production subsequently stimulated T-cell activity [4]. A cross-talk between DCs and T cells is thought to be responsible for the disease development [5]. Recently, the clearance of psoriasis using targeted immunotherapies demonstrates the important role of DCs in the pathogenesis of psoriasis.
Spatholobus Suberectus Column Extract (SSCE) is a natural product from the plant Suberect spatholobus Stem, a kind of Traditional Chinese Medicine, which is widely used to invigorate the circulation of blood and replenish blood, has antitumor and anticoagulant properties, and improves hematopoiesis [6-8]. Current research has shown its potential value in immunomodulation and the management of autoimmune diseases. Considering the pharmacological effects of Suberect spatholobus stem, it was speculated that these substances could block the pathological changes brought about by psoriasis in various aspects [9,10].
This study determined whether SSCE exhibited antipsoriatic activity in a mouse model. It was the first study to evaluate the effects of SSCE on DCs In vitro and in vivo.
MATERIALS AND METHODS
Extraction and isolation of Spatholobus Suberectus Dunn
Animals and grouping
Scoring of skin inflammation severity
Histology
Immunohistochemistry
Cell culture
Cell treatment
Flow cytometric analysis
Mixed homogenous lymphocyte reaction
Statistical analysis
RESULTS
SSCE effectively attenuated psoriatic lesions and alleviated the histopathologic changes in IMQ-induced psoriasis-like mouse model
Similar to the MTX group, SSCE effectively ameliorated the histological appearance and the infiltrated lymphocytes of the psoriatic lesions compared with the IMQ control (Figure 1C). In addition, it was also found that SSCE effectively reduced the average epidermal thickness compared with the IMQ group (Figure 1D). These data showed that SSCE could effectively alleviate the histopathologic changes in IMQ-induced psoriasis-like mouse lesions.
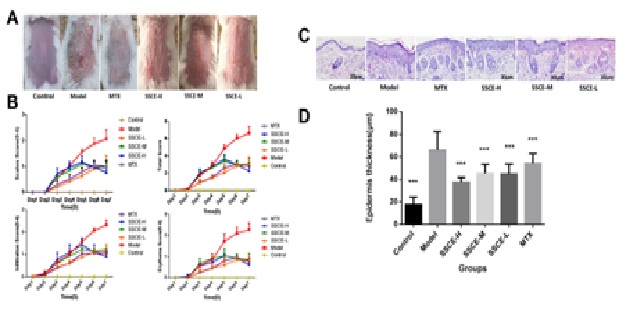 Figure 1: SSCE improved the morphological features and alleviated the histopathologic changes of IMQ-induced psoriasis in mice.
Figure 1: SSCE improved the morphological features and alleviated the histopathologic changes of IMQ-induced psoriasis in mice.(B) Erythema, scaling and thickness of the back skin were scored daily on a scale from 0 to 4. The cumulative score (erythema + scaling + thickness) is depicted. Symbols indicate the mean ± SD (n = 5).
(C) Histological changes in mice skin lesions on day 6 of imiquimod treatment and H&E staining of the back skin of mice (200×) (n = 5).
(D) Epidermis thickness changes of skin lesions in mice (μm, mean ± SD, n = 5).
***P < 0.001 vs model. H&E-Hematoxylin and eosin; IMQ-imiquimod; SD-standard deviation; SSCE-Spatholobus suberectus column extract.
SSCE reduced the infiltration of T cells and neutrophils in IMQ-induced psoriasis-like mouse lesions
SSCE decreased the number of CD11c+ DCs in IMQ-induced psoriasis-like mouse lesions and spleens
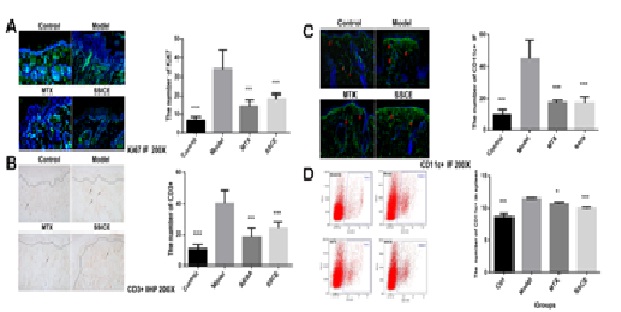
(B) CD3 expression in mice lesions on day 6 (n = 4).
(C) CD11c+ expression in mice lesions on day 6 (n = 4).
(D) CD11c+ expression in mice spleens on day 6 (n = 4).
SSCE: Mice were treated with 12 mg/(kg • day). *P < 0.05 vs model, ***P < 0.001 vs model. DC-dendritic cell.
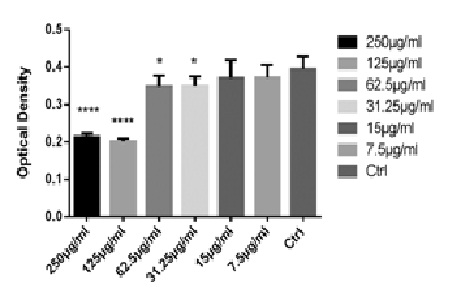
Effect of SSCE on cell viability in vitro
Effects of SSCE on the phenotypic and functional maturation of DCs
A main characteristic of mature DCs is their capacity of inducing allogeneic T-cell proliferation. To further investigate the effect of SSCE on the maturation of DCs, the allogeneic mixed lymphocyte reaction was examined. It was found that R848 increased CD4+ T-cell proliferation, while SSCE inhibited the capacity of allogeneic T-cell proliferation (Figure 4C).
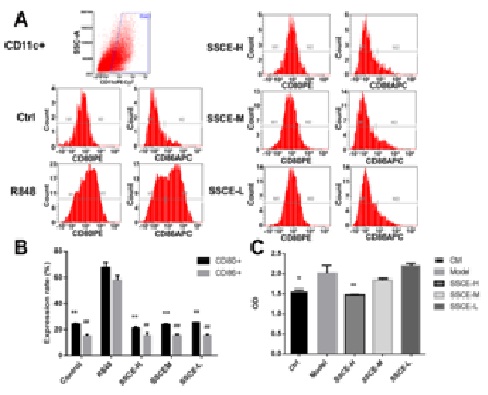
(C) After treatment with 10 ng/mL R848 (model) or 10 ng/mL R848+ SSCE (15, 7.5 and 3.75 µg/mL) for 24h, 4×105 allograft lymphocytes were co-cultured with DCs of each group, and the proliferation rate of lymphocytes was measured using the CCK-8 kit. *P
DISCUSSION
This study demonstrated that SSCE alleviated psoriasis-like skin with the decreased PASI score and obviously reduced the vertical thickness of epidermis. Furthermore, SSCE prevented the maturation of murine Bone Marrow Dendritic Cells (BMDCs), characterized by reduced levels of CD80/86, and reduced all oproliferation of T cells. These results suggested that SSCE regulated DC maturation, and that it might serve as a novel therapeutic agent for psoriasis.
Although SSCE has been reported to be an anticancer agent [13,14], the effect of SSCE in psoriasis and DCs has not yet been determined. This study found that SSCE treatment alleviated IMQ-induced psoriasis mice model, inhibited BMDC maturation and suppressed proliferation of allogeneic T cells. These results suggested that SSCE could inhibit the maturation and activation of DCs and might be of potential value in treating some autoimmune diseases.
Imiquimod (IMQ) is a TLR7/8 agonist, and IMQ-induced mouse model is a classic model of psoriasis [14]. To investigate the effect of SSCE on psoriasis, the effect of SSCE on IMQ-induced psoriasis mice model was evaluated. It was observed that SSCE effectively attenuated psoriatic lesions, alleviated the histopathologic changes, reduced the infiltration of T cells and neutrophils in psoriasis-like mouse lesions, and reduced the number of CD11c+ DCs in mouse lesions and spleens.
DCs are thought to be the initiators of some autoimmune diseases such as psoriasis. Maturation and activation of DCs are key steps in triggering the priming of autoreactive peripheral T cells, which then drive the development of inflammatory responses [16-19]. The effect of SSCE on DCs maturation was evaluated in R848-induced BMDCs. It was observed that SSCE treatment in R848-induced BMDCs resulted in a significant reduction in the expression of DC maturity markers and inhibited the capacity of allogeneic T-cell proliferation.
CONCLUSION
In summary, the present study showed that SSCE inhibited DCs maturation by reducing the expression of maturity markers, suppressed the ability of allogeneic T-cell proliferation, and effectively improved the disease condition of mice with psoriasis. The present data not only clarified a new cellular mechanism for the anti-inflammatory and immunosuppressive effects of SSCE, but also indicated the therapeutic potential for psoriasis.
DISCLOSURES
The authors have no financial conflicts of interest.
ACKNOWLEDGMENT
The study was supported by National Natural Science Foundation of China (No. 81403410).
REFERENCES
- Boehncke WH (2015) Etiology and Pathogenesis of Psoriasis. Rheum Dis Clin North Am 41: 665-675.
- Rahmani F, Rezaei N (2016) Therapeutic targeting of Toll-like receptors: a review of Toll-like receptors and their signaling pathways in psoriasis. Expert Rev Clin Immunol 12: 1289-1298.
- Liu J, Cao X (2015) Regulatory dendritic cells in autoimmunity: A comprehensive review. J Autoimmun 63: 1-12.
- Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W (2014) Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol 5: 7.
- Cao LY, Chung JS, Teshima T, Feigenbaum L, Cruz PD, et al. (2016) Myeloid-Derived Suppressor Cells in Psoriasis Are an Expanded Population Exhibiting Diverse T-Cell-Suppressor Mechanisms. J Invest Dermatol 136: 1801-1810.
- Yan L, Ping L, Yan W, Xin-ran X, Lei Z, et al. (2012) Effect of Active Part of Spatholobus Suberectus Flavonoid on Oxidative Stress of Human Pulmonary Adenocarcinoma Cell A549. Chinese Journal of Experimental Traditional Medical Formulae 4: 190-193.
- Guo-wang Y, Shan G, Qi F, Ping L, Xiao-qin L, et al. (2012) Molecular mechanism of cell cycle regulation of Spatholobus Suberectus flavonoids effective part on the A549 human lung adenocarcinoma cell based on CKI. China Journal of Traditional Chinese Medicine and Pharmacy: 3217-3220.
- Yan W, Ping L, Yan L, Jing-xia Z, Xin L, et al. (2011) Effect of Spatholobus Suberectus column extract on apoptosis and mitochondrial transmembrane potential of non-small cell lung cancer A549 in vitro. Journal of Capital Medical University 32: 811-815.
- J n m college (1997) Chinese medicine dictionary. Shanghai: Shanghai science and technology press, Shanghai, China.
- Bo Y, Zhi-yu L, Qi-dong Y (2008) The development in the research of anti-tumor mechanism of flavonoids. Progress in Pharmaceutical Sciences 32: 391-397.
- Fu Q, Tang Y, Luo X, Yang G, He W, et al. (2009) [Anti-tumor activity and mechanism with SSCE of Spatholobus suberctus]. Zhongguo Zhong Yao Za Zhi 34: 1570-1573.
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, et al., (2009) imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 Axis. J Immunol 182: 5836-5845.
- Shan G, Guo-wang Y, Qi F, Ping L, Xiao-qin L, et al. (2010) Molecular mechanism of cell cycle regulation of Spatholobus Suberectus flavonoids Effective part (SSCE) on the A549 human lung adenocarcinoma cell. Chinese Journal of Basic Medicine in Traditional Chinese Medicine 247-250.
- Wang G-C (1999) The family of CIP-KIP, Foreign Medical Sciences. Section of Molecular Biology. 31: 17-21.
- Singh M, Khong H, Dai Z, Huang XF, Wargo JA, et al. (2014) Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immuno 193: 4722-4731..
- Saha C, Das M, Stephen-Victor E, Friboulet A, Bayry J, et al. (2016) Differential Effects of Viscum album Preparations on the Maturation and Activation of Human Dendritic Cells and CD4? T Cell Responses. Molecules 21: 912.
- Said A, Weindl G (2015) Regulation of Dendritic Cell Function in Inflammation. J Immunol Res 2015: 743169.
- Kim TG, Kim DS, Kim HP, Lee MG (2014) The pathophysiological role of dendritic cell subsets in psoriasis. BMB Rep 47: 60-68.
- Batal I, Azzi J, Mounayar M, Abdoli R, Moore R, et al. (2014) The mechanisms of up-regulation of dendritic cell activity by oxidative stress. J Leukoc Biol 96: 283-293.
Citation: Wang Y, Zhao J, Di T, Xu X, Wang M, et al. (2017) Spatholobus Suberectus Column Extract Suppresses Dendritic Cell Maturation and has Therapeutic Potential for Psoriasis. J Altern Complement Integr Med 3: 017.
Copyright: © 2017 Yang Wang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

