
Stem Bark’s Extracts of Tieghemella heckelii (Sapotaceae) Against Imipenem-Resistant Pseudomonas aeruginosa: Identification of a Prospective Antibacterial Agent
*Corresponding Author(s):
Guede Kipre BertinDepartment Of Bacteriology Or Virology, Natural Sciences Training And Research Unit, Nangui Abrogoua University, Cote D'Ivoire
Tel:+225 89264112,
Email:bgkipre070@gmail.com, bkgp63@missouri.edu
Abstract
Background: Stem bark of Tieghemella heckelii (Sapotaceae) used as traditional medicine for its therapeutic effect on infectious diseases in Ivory Coast, was tested against imipenem-resistant bacteria for efficacy assessment.
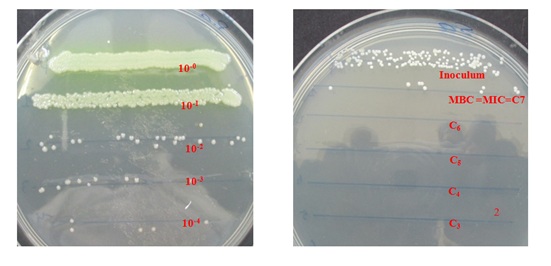
Methods: Six extracts (hexane, chloroform, ethyl acetate, ethanol, methanol and sterile distilled water) were prepared and tested on Imipenem-Resistant Pseudomonas aeruginosa (IRPA), using broth microdilution for activity evaluation. From this experiment, the Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) of the plant extracts were determined in 96-well micro-plates in order to search for both bacteriostatic and bactericidal effects. Thereafter, data analysis was performed using GraphPad Prism 5 software (One-way ANOVA, Bartlett test for equal variance and Turkey Multiple Comparison test). The results were then presented as Mean ± SD for experiment repeated three times.
Results: Four extracts (ethyl acetate, methanol, ethanol and sterile distilled water) showed credible potency, with strong, significant and moderate growth inhibition of the IRPA tested. The MIC values varied depending on microbial phenotype, and were within the range of 0.048 mg/mL to 12.5 mg/mL. As for the MBC values, also associated to bacteria strain type, they demonstrated both bacteriostatic and bactericidal effects of the active extracts towards Imipenem-resistant P. aeruginosa.
Conclusion: Stem bark extracts of Tieghemella heckelii showed an antibacterial effect towards imipenem-resistant P. aeruginosa. They could therefore be used to deplete the prevalence rate of the named resistant strains.
Keywords
ABBREVIATIONS
ATCC: American Type Collection Culture
DMSO: Dimethyl Sulfoxide
MBC: Minimum Bactericidal Concentration
MIC: Minimum Inhibitory Concentration
IRPA: Imipenem-Resistant Pseudomonas aeruginosa
IAI: Intra-Abdominal Infection
SMART: Study for Monitoring Antimicrobial Resistance Trends
INTRODUCTION
Background
MATERIALS AND METHODS
Plant material
Bacteria strains
METHODS
Preparation of extracts
Antimicrobial assays

Statistical analysis
RESULTS
The Ethanol extract, which showed moderate growth inhibition for bacterium 1076 C/11, exhibited weak activity towards bacteria 489C/11 and 891C/11. The potency of the extract was demonstrated at a minimum inhibition concentration value in the range 3.125 mg/mL< MIC < 12.5 mg/mL. This was displayed by a bacteriostatic effect against 489C/11, and a bactericidal activity over the remaining tested bacteria (Table 1). Additionally, the one-way analysis of variance did not show significant activity difference (P = 0.9865, R2 = 0.001506) of the extract on the whole bacteria tested. This trend was confirmed by the Bartlett’s test for equal variances of the efficacy within the range of 0.390 mg/ml to 12.5 mg/mL.
| Strain | Code | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | Effect |
| P. aeruginosa | 1810C/10 | 12.5 ± 0.001 | 12.5 ± 0.001 | 1 | Bactericidal |
| 1060C/11 | 12.5 ± 0.001 | 25 ± 0.001 | 2 | Bactericidal | |
| 1780C14 | 12.5 ± 0.001 | 12.5 ± 0.001 | 1 | Bactericidal | |
| 891C/11 | 6.25± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal | |
| 489C/11 | 6.25 ± 0.001 | 25 ± 0.001 | 4 | Bacteriostatic | |
| 1076C/11 | 3.125 ± 0.005 | 6.25 ± 0.001 | 2 | Bactericidal | |
| ATCC 27853 | 0.39 ± 0.001 | 0.78 ± 0.001 | 2 | Bactericidal |
Evaluation of the ethyl acetate extract’s activity showed growth inhibition of all IRPA tested, with a MIC figure of 6.25 mg/mL for strains 489C/11, 1076C/11, 1780C/14, which constitute the first set of bacteria tested and 12.5 mg/mL for 891C/11, 1060C/11 and 1810C/10 another bacteria set (Table 2). Using the one-way analysis of variance, the MIC means difference over each group of strains was not significant (P = 0.1134, R2 = 0.2149), which trend was also ascertained by the Bartlett’s test (P = 0.1913) for both strains groups.
| Strain | Code | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | Effect |
| P. aeruginosa | 1810C/10 | 12.5 ± 0.001 | 25 ± 0.001 | 2 | Bactericidal |
| 1060C/11 | 12.5 ± 0.001 | 25 ± 0.001 | 2 | Bactericidal | |
| 1780C/14 | 6.25 ± 0.001 | 12.5± 0.001 | 2 | Bactericidal | |
| 891C/11 | 12.5 ± 0.001 | 25 ± 0.001 | 2 | Bactericidal | |
| 489C/11 | 6.25 ± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal | |
| 1076C/11 | 6.25 ± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal | |
| ATCC 27853 | 6.25 ± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal |
Methanol extract mixed with different IRPA inoculum strongly prevented the bacteria growth with MIC highest value of 0.048 mg/ml for bacterium 891C/11 and 0.097 mg/ml for strains 489C/11, 1060C/11 and 1810C/10. For 1076C/11, the extract displayed moderate anti-IRPA potency at MIC value of 3.125 mg/ml and weak activity at 6.25 mg/ml for 1780C/14. Additionally, apart from bacterium 1060C/11, which the extract showed bacteriostatic effect for, a bactericidal effect was demonstrated for all strains assayed (Table 3). The statistical analysis also showed significant variation of MIC means difference within the extract concentrations range of 0.048 to 12.5 mg/mL (Turkey Multiple Comparison test), which was confirmed by the one-way analysis of variance (P < 0.0003; R2 = 0.7244).
| Strain | Code | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | Effect |
| P. aeruginosa | 1810C/10 | 0.097 ± 0.003 | 0.097 ± 0.003 | 1 | Bactericidal |
| 1060C/11 | 0.097 ± 0.003 | 0.39 ± 0.001 | 4 | Bacteriostatic | |
| 1780C/14 | 6.25 ± 0.001 | 6.25 ± 0.001 | 1 | Bactericidal | |
| 891C/11 | 0.048± 0.001 | 0.097 ± 0.003 | 2 | Bactericidal | |
| 489C/11 | 0.097 ± 0.003 | 0.195 ± 0.005 | 2 | Bactericidal | |
| 1076C/11 | 3.125 ± 0.005 | 6.25 ± 0.001 | 2 | Bactericidal | |
| ATCC 27853 | 0.097 ± 0.003 | 0.195 ± 0.005 | 2 | Bactericidal |
Aqueous extract tested on IRPA in the screening experiment, revealed growth inhibition towards strains 489C/11, 891C/11 and 1076C/11 with a MIC value of 3.125 mg/mL, activity against 1060C/11, 1780C/11 and 1810C/10 with MIC value of 6.25 mg/mL (Table 4). Another important result is the bactericidal effect displayed by the extract against all IRPA tested. Ultimately, the Turkey Multiple Comparison test showed significant variation of the extract efficacy within the concentrations range. This was confirmed by the one-way analysis of variance (P = 0.0253; R2 = 0.3353).
| Strain | Code | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | Effect |
| P. aeruginosa | 1810C/10 | 6.25 ± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal |
| 1060C/11 | 6.25 ± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal | |
| 1780C/14 | 6.25 ± 0.001 | 12.5 ± 0.001 | 2 | Bactericidal | |
| 891C/11 | 3.125 ± 0.005 | 6.25 ± 0.001 | 2 | Bactericidal | |
| 489C/11 | 3.125 ± 0.005 | 6.25 ± 0.001 | 2 | Bactericidal | |
| 1076C/11 | 3.125 ± 0.005 | 6.25 ± 0.001 | 2 | Bactericidal | |
| ATCC 27853 | 3.125 ± 0.005 | 6.25 ± 0.001 | 2 | Bactericidal |
DISCUSSION
The present study aimed at assessing the antibacterial effect of stem barks’ extracts of Tieghemella heckelii against imipenem-resistant P. aeruginosa. Results obtained from experiments carried out, showed plant extract’s efficacy against the tested strains. Thus, with the MIC ranging from 0.048 mg/mL to 12.5 mg/mL, they opposed bactericidal effect to most of the tested bacteria. When considering the cutoff point set up in early studies, methanol extract displayed significant antibacterial activity with MIC lowest values of 97 µg/mL and 48 µg/mL against imipenem-resistant P. aeruginosa [11]. In evaluating the activity of all plant extracts towards the reference material ATCC 27853, a bactericidal effect was noticed. Moreover, the MIC value of each extract put into contact with the ATCC isolate showed the significance of this trend. Consequently, the methanol extract not only displayed bactericidal effect, but also revealed the lowest MIC value of 0.097 mg/mL. This, followed by the ethanol extract, which showed MIC value of 0.390 mg/mL, and aqueous extract (3.125 mg/mL) along with ethyl acetate extract (6.25 mg/mL). The MIC difference of these extracts may be due to the lipophilic outer membrane of this negative gram bacterium, which is easily penetrated by alcoholic driven secondary metabolites, as compared to aqueous and ethyl acetate extracts. Some of the metabolites probably acted as chelating organic ligands for bacteria metallic-enzyme proteins. These in vitro proven bactericidal effects could explain the use of the plant part in traditional medicine to treat ulcers and reinfections caused by P. aeruginosa. This result ascertains the plant to display higher antibacterial activity when compared to work done by Ulloa-Urizar et al., [12]. For this group of researchers, which investigated the potency of five Peruvian medicinal plant extracts towards P. aeruginosa, the MIC value for the most active, was achieved at 25 mg/mL. The extracts aforementioned were from M. macrocarpa, D. loretense, U. tomentosa and E. Camaldulensis. Another comparison made with the faith plant (T. impetiginosa) studied by the same research team, showed the bark’s extracts of Tieghemella heckelii to be highly active. Carex pumila extracts also inhibited P. aeruginosa according to Hyun et al., but the MIC value (200 µg/mL) proved the plant stem barks used to conduct the current experiment to be more active [13]. Finally, when compared to the study performed by Datta et al., the stem bark’s extracts of Tieghemella heckelii were active on P. aeruginosa, whereas Piper betle leaf extract at different concentrations did not show any decrease in bacteria growth [14]. As far as the MBC is concerned, the methanol extract of the stem barks of Tieghemella heckelii showed a lower value 97 µg/mL, which is roughly of the same order of magnitude as that obtained by Jahani et al., [15]. Nevertheless, the stem bark’s extracts which showed antibacterial activity against these metallic-enzymes producing micro-organisms (Carbapenemases), did not show inhibition against Enterobacteriaceae tested in a complementary experiment.
Additional experiment conducted to screen out the prospective compounds responsible for antibacterial activity of the stem barks’ extracts revealed saponins, coumarins and flavonoids [15]. These natural products could therefore encompass the activity evaluated.
CONCLUSION
To conclude, the present study has shown the antibacterial potential of the tested plant extracts against imipenem-resistant P. aeruginosa. Provided this characteristic of the stem bark of Tieghemella heckelii, it could be used as a good candidate for drug resistance modulation.
ACKNOWLEDGEMENT
The authors of this manuscript are grateful to Institute Pasteur Ivory Coast, which provided necessary laboratory equipment.
AVAILABILITY OF DATA AND MATERIALS
Institute Pasteur Ivory Coast/Université Nangui Abrogoua/Centre Suisse de Recherches Scientifiques Ivory Coast.
AUTHORS CONTRIBUTIONS
BGK conceived, designed, carried out the extraction experiments, performed the bioassays, conducted the statistical analysis and wrote the draft of the manuscript.
NKG designed the bioassays, validated the results of the experiments and participated in the drafted manuscript.
MWK participated in the experiment’s design and results validation, in the drafted manuscript and statistical analysis.
VG participated in bioassays design and results interpretation. In addition, VG received the patients who consented for routine sample collection and diagnosis.
JKC contributed in plant extracts preparation and fractionation.
MD designed the bioassays, validated the results of the experiments and participated in the draft manuscript.
All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- Morrissey I, Hackel M, Badel R, Bouchillon S, Hawser S, et al. (2013) A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 6: 1335-1346.
- Koné M (2001) Evaluation in vitro de l’activité antibactérienne de Chromolaena odorata L. sur des genres de surinfection de l’ulcere de Burili. Mémoire DEA, Université d’Abobo-Adjamé, Abidjan, Ivory Coast.
- Ruppé É, Woerther P-L, Barbier F (2015) Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care 5: 21.
- Kumari H, Balasubramanian D, Zincke D, Mathee K (2014) Role of Pseudomonas aeruginosa AmpR on β-lactam and non-β-lactam transient cross-resistance upon pre-exposure to subinhibitory concentrations of antibiotics. J Med Microbiol 63: 544-555.
- Guédé-Guina F, Kra AM, Bonga GM, De Souza C (1995) Activate antimicrobienne d’un extra it vegetal centre les germs opportunists au course du SIDA. Rev Pharm Afr 9: 13-19.
- Fankam AG, Kuiate JR, Kuete V (2017) Antibacterial and antibiotic resistance modulatory activities of leaves and bark extracts of Recinodindron heudelotii (Euphorbiaceae) against multidrug-resistant Gram-negative bacteria. BMC Complement Altern Med 17: 168.
- Institute Pasteur Ivory Coast (IPCI) (2015) Rapport Scientifiques. Institute Pasteur Ivory Coast, Ivory Coast.
- Guédé BK, Guessennd-Kouadio AN, Kouadio JN, Koné MW (2017) Cytotoxicity assessment of the stem bark of Tieghemella heckelii Pierre ex. A Chev. (Sapotaceae) towards Vero and RD human cancer Cell Lines. J Phytopharmacol 6: 11-19.
- Koné WM, Atindehou KK, Terreaux C, Hostettmann K, Traoré D, et al. (2004) Traditional medicine in north Côte-d'Ivoire: screening of 50 medicinal plants for antibacterial activity. J Ethnopharmacol 93: 43-49.
- Clinical and laboratory standards institute (2015) Performance standards for antimicrobial susceptibility testing; Twenty-Fifth informational supplement. Clinical and laboratory standards institute, Pennsylvania, USA.
- Kipre BG, Guessennd NK, Koné MW, Gbonon V, Coulibaly JK, et al. (2017) Antibacterial activity of the stem bark of Tieghemella heckelii Pierre ex. A Chev against methicillin-resistant Staphylococcus aureus. BMC Complement Altern Med 17: 170.
- Ulloa-Urizar G, Aguilar-Luis MA, Lama-Odria MCD, Camarena-Lizarzaburu J, Mendoza JV (2015) Antibacterial activity of five Peruvian medicinal plants against Pseudomonas aeruginosa. Asian Pacific J Trop Biomed 5: 928-931.
- Cho HS, Lee JH, Ryu SY, Joo SW, Cho MH, et al. (2013) Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 biofilm formation by plant metabolite ε-viniferin. J Agric Food Chem 61: 7120-7126.
- Datta S, Jana D, Maity TR, Samanta A, Banerjee R (2016) Piper betle leaf extract affects the quorum sensing and hence virulence of Pseudomonas aeruginosa PAO1. 3 Biotech 6: 18.
- Jahani S, Saeide S, Javadian F, Akbarizadeh Z, Sobhanizade A (2016) Investigating the Antibacterial effects of plant extracts on Pseudomonas aeruginosa and Escherichia coli. Int J Infect 3: 34081.
Citation: Bertin GK, Nathalie GK, Witabouna KM, Carole MGV, Julien CK, et al. (2017) Stem Bark’s Extracts of Tieghemella heckelii (Sapotaceae) Against Imipenem-Resistant Pseudomonas aeruginosa: Identification of a Prospective Antibacterial Agent. J Altern Complement Integr Med 3: 032.
Copyright: © 2017 Guede Kipre Bertin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

