
The Procaine-Base-Infusion: 20 Years of Experience of an Alternative Use with Several Therapeutical Effects
*Corresponding Author(s):
Ralf OettmeierAlpstein Clinic, Dorfplatz 5, CH-9056 Gais, Switzerland
Tel:+41 717918100,
Email:ralf.oettmeier@icloud.com
Abstract
SUMMARY
Keywords
Inflammation; Infusion; Pain; Procaine, Rheumatism; Sodium bicarbonate
INTRODUCTION
In 1925, HUNEKE brothers were the first to administer procaine intravenously. Further, these German authors investigated several effects of procaine finding that it could be useful in the treatment of numerous pathologies via subcutaneous, intradermal, intramuscular and neural infiltrations. For this reason, they firstly titled this therapy as “therapeutic anesthesia”. Later, they also reported not only changes in pathologies when it was applied segmentally, but also noticed immediate changes distant to the segment (the so-called “lighting reaction”). They later recommended this kind of therapy, referred today as neural therapy [1]. Nowadays, neural therapy is widely practiced by the medical community in Europe and Latin America, mainly [4-10]. In Russia, several authors were also investigating the therapeutic effects of Procaine. AD Speransky, a disciple of IP Pavlov, in 1936, published “Basis for a new theory of Medicine” in which he demonstrated the broad anti-dystrophic effect of procaine in numerous acute and chronic pathologies within which infectious diseases were included [3]. His observations were confirmed by AV Vishvensky and AA Vishvensky who explained procaine’s mechanism of action as having an eutrophic effect on the organism which, in turn, is based on conditioned reflexes theory of IP Pavlov [2,11]. The term “throphism” refers to a physiological process of metabolism which keeps a normal physicochemical state of the internal medium in the organism and which is regulated by the sum of all innervation systems [4].
In Romania, Ana Aslan, a disciple of the neurologist Marinescu, investigated together with Pharon the effect of procaine intravenous injections according to Leriche’s method and intramuscular injections. Later, she focused treatment on several geriatric disorders, using the concepts of Vishnevsky about procaine’s eutrophic action. She reported with statistical data a wide therapeutic effect of procaine on nervous, cardiovascular, locomotor, cutaneous and gastrointestinal diseases in elderly people [2].
The local anesthetic procaine is characterized by a sum of pharmaceutical features. With this in mind, Prof. Aslan, the founder of eponymous therapy, spoke of it as a vitamin-like action beside the anesthetic effects [1]. Contrary to all other anesthetic drugs, it causes vasodilatation of vessels and capillaries [1,12-17]. Therefore, and with this therapy, it is possible to reach and optimally influence very poorly circulated tissue [especially in case of inflammation and pain]. Further benefits of procaine are its good tractability and low-grade toxicity due to its short half-life and plasma degradation, the capillary impermeability effect, the inhibition of inflammation, anti-oxidative and fat-reducing action [18-25]. Krause has demonstrated that the anti-inflammatory effect of Procaine in rheumatic disease was especially high when combined with an alkali additive [7]. Beside the effect of blocking voltage-dependent sodium channels with the result of a short-term anesthesia [26], additional actions of procaine on cell membranes and the matrix, as well as sympatholytic actions, were also highlighted [27-32]. In the field of oncology, the effect of procaine’s reduction of radiotherapy side-effects or to improve the influence of chemotherapy is reported [33-38]. Furthermore, a wide epigenetic action of the procaine has been demonstrated. A growth-inhibition after incubation with human cancer cells due to the partial blockade of DNA-methylase in vitro was described in 2003 [39]. A diminishing effect of 5-methylcytosine into global genomic DNA and cell proliferation due to procaine was reported in a study of tumour suppressor genes [40]. In the same way, inhibition of DNA methylation in human hepatoma cells was found by Tada et al. [41]. In 2016, Sabit et al., showed that the use of procaine combined with carboplatin was the most effective treatment for diminishing the global level of DNA methylation in colon cancer cells [42]. Finally, procaine is used in the course of heart and coronary surgery as additive in cardioplegic solutions to block the axonal ion flow and to stabilize and conserve the membrane [43-47].
The first reference of combining procaine with alkaline salts appeared in 1930 [24]. With the aim of combining the well-known pure alkaline infusion and the pluripotent features of procaine, the first study was published as the so-called “neural infusion therapy” in 1997 [48,49]. After impressive positive results were demonstrated in chronic pain patients [50], the method gained popularity very fast in the German-speaking countries and was incorporated into textbooks of pain and neural therapy [51,52]. Glusa et al., were also able to confirm the vasodilation effect of procaine-base-mixture by using an animal model [53]. An increase of intra-cellular procaine concentration due to the addition of sodium bicarbonate and an accelerated initial effect were also observed in animal studies [54-56]. The continuous application of procaine-base via a medical pump demonstrated impressive results in many severe cases of pain and inflammation [57-59].
With the osteoarthritic model of rats the anti-rheumatic and joint-protective action of procaine-base after intra-articular injection was clearly superior compared to giving the drug Dexamethasone [60].
The primary aim by additionally adding the natural buffer-base sodium bicarbonate was its plasmatic degradation influence on procaine due to the action of serum esterase. All local anesthetics have the common characteristic of general build-up and ionization. These characteristics are essential for their action on the voltage-dependent sodium channels. The unloaded procaine molecule represents the transporting structure which is able to permeate. The loaded form, procaine-H+ (ionized form) binds the sodium channel receptor and thus blocks the propagation of an impulse. By changing the pH value of the solution and the terrain, the ionized and non-ionized forms of procaine can be influenced [61].
In figure 1 it can be observed that due to the pH value shift important features such as solubility and membrane penetration can be influenced. Therefore, by adding an alkaline additive the relationship between the form of transport and the form of action is changed. In the case of a low pH value (<6), only 0.1% of procaine was found in the lipid-soluble from [40]. Further, it is known that different sodium bicarbonate concentrations can influence the intracellular pH [62].
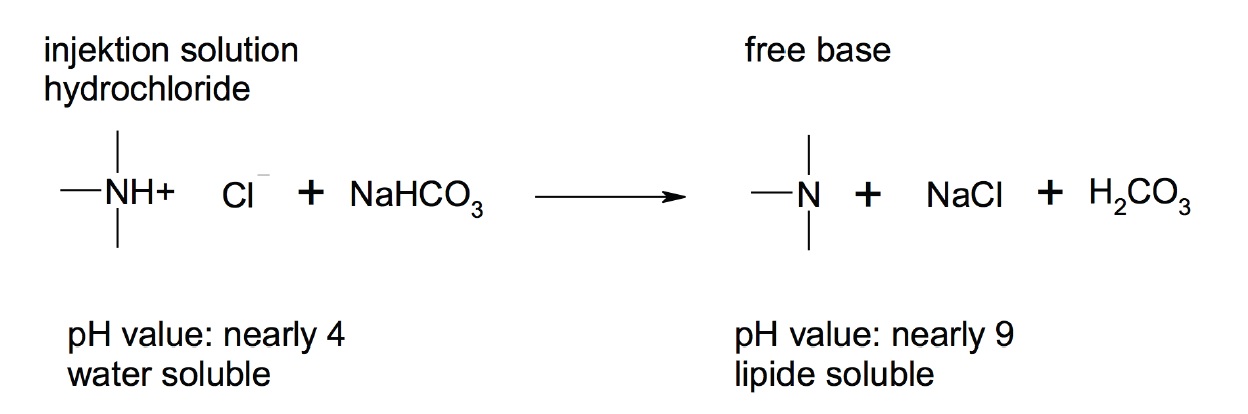
Figure 1: Principle of change of the solubility and ability to penetrate depending on the pH value.
Initially it was postulated that under an alkaline condition, that the conversion of Procaine to Para-Aminobenzoic Acid (PABA) and Diethylaminoethanol (DEAE) will be distinctly reduced. Contrary to this assumption, it is believed that after intravenously injecting procaine-base it is diluted in the blood of large vessels leading to a quick drop in pH, reaching normal physiological levels. In addition, the pulmonary circulation will cause a respiratory compensation of alkalosis. The actual retardation of procaine degradation can be explained as follows: The pH-dependent dissociation shift explained above will result in increased amounts of well-penetrating transport forms. This is generally typical for all local anesthetics and thus 3-40% of the liberated base is present depending on the pKa value of the anesthetic drug. Besides the distribution in a steady state, the speed of distribution is also important. The speed of distribution is the limiting factor meaning that the diffusion through the membrane is the rate determining step [63]. Accordingly, the distribution depends directly on the lipophilicity of the agent. By shifting the pH, the lipophilic features are changed. A higher amount of free base implies also a higher amount for permeation, which is immediately available to the surrounding tissue and cannot be metabolized so easily by the serum esterase [40].
The pure procaine infusion was firstly described by Seifen et al., and was mostly used as a continuous treatment in cases of acute pancreatitis and for epidural anesthesia in infants, children and risk patients, which underlines the low toxicity of the substance [64-76]. O’Donnel et al., reported about the use of procaine infusion to block the cardiac nerves [77].
Presently, it’s reported that long-term relaxing, anti-depressive and anxiolytic effects are often observed when IV applications or short-term infusions of Procaine are given [78,79]. It has been demonstrated that when procaine is administrated intravenously in humans, it increases blood flow to the anterior para-limbic zones and the amygdala cerebral, as well as improves hemodynamic effects of the heart [80,81]. Other areas of the limbic system have been studied after procaine administration in animal models, finding activity on many muscarinic cholinergic receptors of hippocampus. Several authors have reported procaine’s activity on many biochemical systems such as dopamine, norepinephrine, serotonin, glutamate, among others. For these reasons, procaine is considered as useful for studying limbic system and emotions [82,83].
Recent studies have pointed out that procaine injection into the ventral tegmental area is able to temporarily suppress the fear conditioned avoidance response in rats and also acts on hippocampal theta rhythms which are related to arousal and attention [84]. Apparently, the metabolites of procaine are responsible for the additional pharmacologic actions. DEAE is able to act as an anti-inflammatory due to the inhibition of the fatty acid amide hydrolase which causes an increase in endo-cannabinoid levels [85,86]. The second metabolite PABA operates as an antihistamine, capillary sealant and as a stabilizer for the membranes due to the ester binding with ceramide [87-89].
METHODOLOGY OF PROCAINE-BASE-INFUSIONS
We recommend to start with a dosage of 50-100 mg Procaine-HCl and 20 ml sodium hydrogen carbonate (8.4%) diluted in a 250 to 500 ml carrier solution. Meanwhile the isotonic sodium chloride solution, used routinely for many years can be exchanged by a similar electrolyte solution to prevent hypernatremia. The infusion takes place for approximately 45-60 minutes. By adding increments of 50 mg Procaine-HCl and 10 ml sodium bicarbonate (8.4%), the Procaine-Base infusion will be titrated until the desired therapeutic effect is reached. For a normal-weight person the maximal dosage of Procaine-HCl is 300 mg (Table 1a & 1b). In patients with cardiovascular risk factors, we recommend the use of a surveillance technique (EKG, oximetry) for dosages above 300 mg Procaine-HCl. It is advised to ensure an after-treatment observation period of 30 minutes. Furthermore, it is advised to avoid driving for about one hour after treatment. Because Procaine-Base-mixture stability, it should be used up within two hours because of Procaine’s progressing degradation.
Without any prior acid-base diagnostic, the procaine-base infusion should not be administered more than three times per week with a minimum of one day break between treatment days. A series of 6 to 10 infusions depending on the medical condition have been approved.
The classic blood parameters for inflammation such as blood sedimentation rate and C-Reactive Protein (CRP) should improve after a series of procaine-base infusions. Frequently, and after four to six infusions, patients report a much better mood and improved overall condition. If there is a positive reaction to the treatment (so-called “responders”, in approx. 80 % of patients) it is advised especially in chronic diseases, to continue with a long-term therapy using the helpful dosage for longer intervals, e.g., one to two times a monthly [34-36].
|
Procaine dosage |
sodiumhydrogencarbonate |
Sodium chloride |
Total volume |
|
100 mg = 10 ml |
20 ml |
500 ml |
530 ml |
|
200 mg = 20 ml |
40 ml |
500 ml |
560 ml |
|
300 mg = 30 ml |
60 ml |
500 ml |
590 ml |
|
400 mg = 40 ml |
80 ml |
500 ml |
620 ml |
|
500 mg = 50 ml |
100 ml |
500 ml |
650 ml |
|
Procaine dosage |
sodiumhydrogencarbonate |
Sodium chloride |
Total volume |
|
100 mg = 5 ml |
20 ml |
500 ml |
525 ml |
|
200 mg = 10 ml |
40 ml |
500 ml |
550 ml |
|
300 mg = 15 ml |
60 ml |
500 ml |
575 ml |
|
400 mg = 20 ml |
80 ml |
500 ml |
600 ml |
|
500 mg = 25 ml |
100 ml |
500 ml |
625 ml |
GENERAL EXPERIENCES WITH PROCAINE-BASE-INFUSION
|
Procaine-HCl + |
100 mg + |
200 mg + |
300 mg + |
400 mg + |
500 mg + |
over 500 mg |
|
Na-HCO3 8.4 % |
20 ml |
40 ml |
60 ml |
80 ml |
100 ml |
+ 100 ml |
|
analysed |
|
|
|
|
|
|
|
data (n) |
215 |
2241 |
3031 |
88 |
105 |
18 |
|
PULS rate per |
74.8 ± 13.1 |
74.2 ± 12.4 |
74.5 ± 12.0 |
74.4 ± 13.1 |
75.8 ± 11.7 |
73.1 ± 12.6 |
|
Minute (Min.) |
[44 - 121] |
[55 - 145] |
[48 - 164] |
[48 - 123] |
[59 - 105] |
[55 - 95] |
|
after 15 Min. |
|
|
|
|
|
|
|
02-saturation |
95.3 ± 4.6 |
95.4 ± 4.9 |
95.7 ± 5.9 |
95.1 ± 4.5 |
95.3 ± 5.8 |
95.3 ± 2.2 |
|
(%) |
[80 - 100] |
[79 - 100] |
[77 - 100] |
[81 - 100] |
[88 - 99] |
[85 - 98] |
|
after 15 Min. |
|
|
|
|
|
|
|
RR systolic |
145.6 ± 21.5 |
145.3 ± 21.0 |
145.7 ± 21.8 |
144.6 ± 20.5 |
139.2 ± 24.1 |
151.0 ± 14.1 |
|
after 15 Min. |
[90 - 220] |
[84 - 214] |
[74 - 221] |
[89 - 198] |
[83 - 210] |
[115 - 190] |
|
RR diastolic |
83.0 ± 10.9 |
83.9 ± 11.5 |
84.5 ± 11.1 |
86.4 ± 12.0 |
81.7 ± 15.3 |
87.6 ± 13.0 |
|
after 15 Min. |
[45 - 146] |
[47 - 137] |
[44 - 135] |
[54 - 133] |
[53 - 116] |
[70 - 107] |
|
RR systolic |
139.8 ± 19.7 |
140.1 ± 19.2 |
139.2 ± 19.1 |
134.5 ± 22.2 |
127.0 ± 22.1 |
140.6 ± 19.1 |
|
after 30 Min. |
[68 - 210] |
[80 - 205] |
[80 - 210] |
[97 - 206] |
[86 - 208] |
[105 - 198] |
|
RR diastolic |
80.4 ± 11.9 |
80.7 ± 11.2 |
80.9 ± 11.9 |
81.1 ± 12.8 |
81.7 ± 12.1 |
82.9 ± 11.8 |
|
after 30 Min. |
[48 - 122] |
[47 - 140] |
[44 - 155] |
[45 - 122] |
[55 - 127] |
[76 - 116] |
After over 400.000 applications of procaine therapy infusions according to the described regime in our clinic and outpatient department, we have not observed one case with long-term or severe side effects. No case was registered with a serious allergic emergency situation, which underlines the observations of Becke concerning the huge therapeutic safety of procaine [58]. Sometimes patients report of heart palpitation (6%) and profuse sweating (5%). According to our experience, patients who are using nitro compounds, calcium antagonists and beta-blockers, seem to have a higher disposition to these side effects. Apparently in such cases the reflective and over-segmental disinhibiting effect of the anesthesia dominates over the negative-ionotropic and negative-rhythmotropic potential of procaine. As expected, in approximately 6% of patients a short-time reduction in blood-pressure and vasovagal syncope situations can occur during the application. All these symptoms disappear within a few minutes, especially after reducing the infusion speed [34,63].
Some patients report sleep disorders (5%) and a general hyperactive feeling up to one day after finishing the infusion, which does not reduce the physical working capacity. Approximately 4.5% of treated persons complained of temporary headaches and slight vertigo. Especially during the first couple of infusions such reactions can occur in scope of a so-called “first reaction” (Hering’s effect) according to the holistic thinking in neural therapy and homeopathy [1,93].
INDICATIONS AND CONTRAINDICATIONS OF PROCAINE-BASE-INFUSIONS
The few contra-indications should receive attention in practice which is summarized in table 3b.
|
Acute situations |
Radicular syndrome, pseudo-radicular syndrome, acute infection, |
|
Chronic pain |
multiple arthralgia, chronic radicular-/pseudo-radicular syndrome, |
|
Chronic inflammations |
Lupus erythematosus, rheumatoid arthritis, psoriatic arthritis, |
|
Others |
periphery circulatory disorders, constipation, dysmenorrhea |
|
Patient |
Hypersensitivity towards Procaine, |
|
Therapist and Staff |
Lack of knowledge in handling local anesthetics, inadequate educated staff, |
CURRENT STATUS: PROCAINE-BASE-INFUSION ADAPTED TO THE ACID-BASE-BALANCE
The author Saha reported that after a total of seven procaine-base-infusions with daily increasing dosages up to a maximum of 300 mg procaine-HCl and 120 ml 8,4% sodium bicarbonate, 3 out of 13 patients showed clinical symptoms of metabolic alkalosis [94]. Before and after these series of infusions the Base Excess (BE) measured via arterial blood gas analysis was determined and in all cases amounted to over plus two, which indicates a verified metabolic alkalosis. It is important to emphasize that according to the above-described procedure of procaine-base-infusion, a daily application of such a high dose of sodium bicarbonate is unacceptable. This kind of daily treatment only applies to patients with an adequate acid-base homeostasis. Simply, the body intrinsic buffer system should not be overloaded.
However, patients having a metabolic alkalosis with a reduced compensatory ability in acid-base balance are increasing. Quite often, this is found in cases of over-proteinization, advanced stages of cancer, liver weakness and putrefaction dysbiosis of the large intestine. Furthermore, the use of antacids, alkaline powders, loop diuretics and excessive sodium intake enhances the shift in the acid-base-balance towards alkalosis [95,96]. For the practical analysis of the acid-base balance we prefer the venous blood titration system BUFFY® over the arterial Blood Gas Analysis (aBGA) because it is hematocrit-adapted and calibrated to 37°Celsius [97-100]. The test gives very good information about the buffer capacity of whole blood and plasma and indicates exactly the amount of base needed [101,102]. Metabolic alkalosis can also occur in hyponatremia, hypokalemia and in increased ammonia levels (in EDTA plasma).
In cases of inflammation, cardiac and renal dysfunctions, rheumatic and pain-related diseases, a metabolic acidosis are mostly likely detected [103]. These patients have an increased need of a buffer base and should receive sodium bicarbonate ranging from 60 - 120 ml (8.4% solution) in addition to procaine.
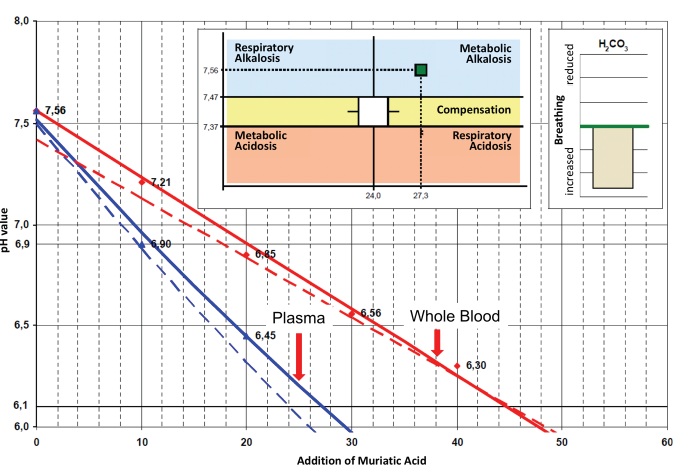
In contrast to cases of metabolic alkalosis, see figure 2 below, there is only a small or no need of additional base treatment. In this case, we only administer infusions of procaine-HCl together with a carrier solution. In addition, we suggest to give 3-5 ampules of L (+)-lactic acid. Alternatively, the newly developed ProcCluster® compound as a ready to use fast infusion (0.1-0.3%) is recommended [104].
Another possibility of a safe procaine-base therapy is the use of a moderate alkaline base solution (1.68%), which is used in the Paracelsus Clinic Lustmühle. In this case, the dosage of procaine-HCl is increased stepwise.
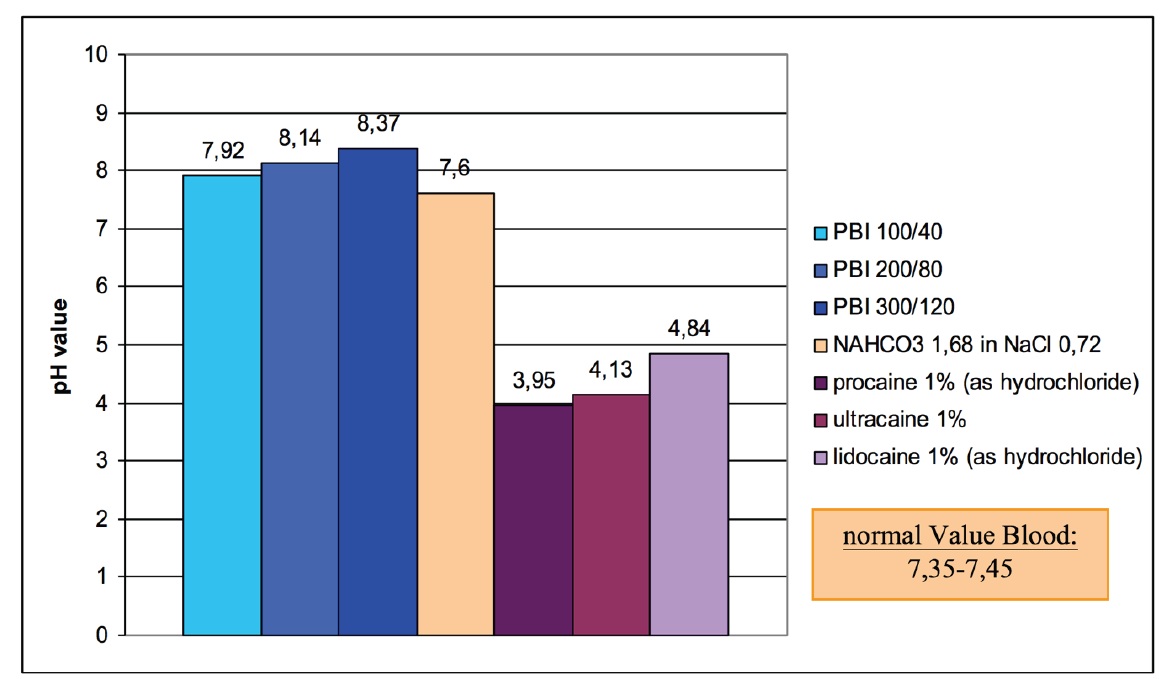
In figure 3 the pH-values of different concentrations of procaine-HCl, different alkaline mixtures and some of the most important local anesthetics are compared.
EXAMPLES FROM TREATED PATIENTS
Case 1
State before procaine treatment (11th November 2016): Diffuse muscle cramps and pain permanently between VAS 6-7 despite complex medication (Sirdalud®, Relaxane®, Ibuprofen, Prednisolon 12.5 mg, Novaminsulfon (2x500 mg), Otezla® 60 mg), awkward gait with two crutches, intensive pain and reduction of movement left shoulder, hip and metacarpophalangeal joints both sides, CRP 41 mg/l, aBGA: pH: 7.43, BE: 2.1 mmol/l.
Therapeutic procedure: first series (10x) procaine-base-infusion twice a week (titration till 400 mg procaine), due to much better general condition continuation of infusion weekly.
Follow up: Stepwise reduction of pain until VAS 1-2, successful reduction of pain medication, including Otezla®, much better general condition, mood distinctly brightened, energy feeling significantly better, swelling and restriction of movement in affected joints markedly improved, control CRP < 5 mg/l. After 20 procaine infusions, the patient is almost painless, happy, has stopped using NSAR and Otezla®.
Additional complementary treatment: start with alkaline-rich nutrition, supplementation with Vitamin E (800 IE) and antioxidant complex, frankincense (Bosvay®, 3x800 mg), Harpagophytum (3x480 mg).
Comment: The positive effect of neural therapy and intravenously injected procaine is mentioned in old literature [105].
Case 2
State before procaine treatment (17th April 2009): All together 12 (!) operations during four months stay at the university hospital, multiple rejection reactions after autologous skin transplantations, wound healing disorders on harvesting points, CRP 98.5 mg/l, highly inflammatory ulceration of the dorsum of foot, partly extensor tendons and bones are visible, buffy test with metabolic acidosis.
Therapeutic procedure: 2x per week procaine-base-infusion (titration until 500 mg procaine and 120 ml 8.4% sodium bi-carbonate).
Follow up: 25.5.09: CRP: 23.3 mg/l, wounds on forearm and upper leg completely healed, ulceration foot 80% closed, pain reduced from VAS 8 to 2-3, Opioid drug (Tilidin ret. 200 mg) discontinued, 17.6.09: CRP: 6.8 mg/l, foot wound also completely dry, increase of motion training, 4.10.09: maintenance dose 1 tablet ProcCluster® 50 mg, very beneficial, no recurrence.
Additional complementary treatment: exposure to ozone gas locally (two month), homeopathic remedies, anti-oxidative acting supplement complex, alkaline-rich and hypo-allergic food.
Comment: Together with the anti-inflammatory and vasodilating features of procaine, the known problem of metabolic alkalosis in patients with chronic kidney diseases and after transplantation can be solved by a combination with sodium bi-carbonate [106,107].
Case 3
State before procaine treatment (7th May 2013): He reports double vision, rotary vertigo, right side of face with intensive dysesthesia, general exhaustion, stress syndrome, MRI (25.4.2013) showed a clear demyelination in white matter periventricular right more than left.
Therapeutic procedure: 1st week every other day, 2nd to 3rd week procain-base-infusion in rising dosage (total 8 x).
Follow up: Rapid symptom improvement, after two weeks already free of any complaints, complete period of disability only 4 weeks, his impression is very well and energetic, last clinical control in November 2014 without any change, control MRI (28.7.2013).
Additional complementary treatment: Neural therapy of neuro-modulative foci (Tonsils, Sinuses), brain organ cell extract weekly (Curafaktur), homeopathy with conium D12 2x3 globuli over 10 days and Gelsemium C30 1x3 globuli (1 day).
Case 4
State before procaine treatment (14th October 2016): Increasing drug side effects (stomach, flatulence, weakness), weather dependent pain in the small joints, swelling of metacarpophalangeal joints with warmness, stiffness in the morning, CRP: 11.2 mg/l.
Therapeutic procedure: At the beginning, weekly procain-base-infusion, after two months, reduction to twice a month, currently ProcCluster® capsule (50 mg) every other day and one infusion per month.
Follow up: Better general condition within two weeks, all pharmaceutical drugs were stopped after 6 weeks, no more joint pain, still little morning stiffness, much better general mobility and no progress of deformation.
Additional complementary treatment: Change of diet (vegetarian), supplementation with anti-oxidants, removal of all irritating root-canal teeth, amalgam sanitation, probiotics for intestinal health.
Comment: Procaine was used as additive to penicillin for intraarticular treatment of pyogenic arthritis over two decades [108-110]. Perhaps the local anesthetic component had more influence to control the inflammation than expected before.
Case 5
State before procaine treatment (24th January 2015): Joint pain permanently (VAS between 4-5), coldness of fingers, dysaesthesia, dry eyes, beginning of radiologic changes on distal finger phalanges.
Therapeutic procedure: Two infusions procaine-base per week (titration to 300 mg procaine and 80 ml 8.4% sodium bi-carbonate), after one month reduction of infusion interval to twice per month.
Follow up: Very good response, almost free of symptoms over a period of two years.
Additional complementary treatment: Change of diet (vegetarian), supplementation with anti-oxidants, detox of Aluminum load, probiotics for intestinal health.
Comment: The case confirmed some hints from previous literature [111-113].
Case 6
State before procaine treatment (29th March 2012): Very weak and depressive mood, diffuse back pain (VAS 6-7), problems in climbing stairs, five kilogram loss of weight within last 6 weeks, laboratory: AP: 324 U/l, CA 15-3: 344 kU/l, CA 125: 76 kU/l, CEA: 7.5 mcg/l, pain medication with Tramadol ret. 100 mg (2x1), Novaminsulfon (2x500 mg) and Ibuprofen (2-3x400 mg), buffy with compensated metabolic alkalosis.
Therapeutic procedure: Series of 12 infusions with dosage titration over 6 weeks (optimal range 350 mg Procaine and 60 ml 8.4% sodium bi-carbonate), then weekly and meanwhile one to two per month.
Follow up: Within a scope of a holistic complementary concept completely free of pain, general better condition (appetite, energy, range of movement), distinct increase of weight, tumor markers significantly diminished, the pharmaceutical pain killers were stopped completely, last PET-CT (November 2016) has shown partial remission with complete disappearance of lung spots and reduced activity (SUV) into the skeleton.
Additional complementary treatment (stepwise made after first diagnosis): Change of diet (vegetarian), supplementation with anti-oxidants, intestinal support, removal of metal fillings in teeth and one root-canal tooth, mistletoe treatment over one year, whole body hyperthermia every three months, use of silver willow and aspen extract (Phytodolor®) for natural analgesic as needed.
Comment: The case underlines the importance to treat also the tumor-associated inflammation which is achievable with Procaine and other natural remedies [114,115].
CONCLUSION AND OUTLOOK
It is very important for the authors of this article to emphasize that the treatment method of procaine is conducted properly, especially to individualize the therapy according to the acid-base-homeostasis and clinical parameters of the patient. Finally, it is noticeable that the method is not a replacement for neural therapy injections, especially for treatment of neuro-modulative triggers.
REFERENCES
- Dosch P, Dosch M (2007) Manual of Neural Therapy according to Huneke (2ndedn). Thieme, Stuttgart, Germany.
- Aslan A (1960) Procaine therapy in old age and other disorders (Novocaine factor H3). Geront Clin 2: 148-176.
- Speransky AD (1943) A basis for the theory of medicine (2ndedn). International Publishers, New York, USA.
- Beltrán Molano ML, Pinilla Bonilla LB, Beltrán Dussan EH, Vásquez Londoño CA (2014) Anatomo-Functional Correlation between Head Zones and Acupuncture Channels and Points: A Comparative Analysis from the Perspective of Neural Therapy. Evid Based Complement Alternat Med 2014: 836392.
- Kidd RF (2005) Neural Therapy: Applied Neurophysiology and Other Topics. General Store Publishing House, Canada.
- Beltran E, Vega J (2015) Neuraltherapeutic Medicine: An approach based on complex medical systems. Editorial Universidad Nacional de Colombia. Bogotá, Colombia.
- Weinschenk S, Hollmann MW, Strowitzki (2016) New perineal injection technique for pudendal nerve infiltration in diagnostic and therapeutic procedures. Arch Gynecol Obstet 293: 805-813.
- Egli S, Pfister M, Ludin SM, Puente de la vega K, Busato A, et al. (2015) Long-term results of therapeutic local anesthesia (neural therapy) in 280 referred refractory chronic pain patients. BMC Complement Altern Med.
- Hui F, Boyle E, Vayda E, Glazier RH (2012) A randomized controlled trial of a multifaceted integrated complementary-alternative therapy for chronic herpes zoster-related pain. Altern Med Rev 17: 57-68.
- Henrard A (1957) [Impletol in neural therapy]. J Belge Med Phys Rhumatol 12: 245-254.
- Vishnevsky A (1958) El Bloqueo Novocaínico y los Antisépticos Oleo balsámicos como una forma Terapéutica Patógena. Buenos Aires, Cartago.
- Huang Y (1997) Studies on vasorelaxation by tetrapentylammonium ions in rat aortic rings. Life Sci 61: 1811-1817.
- Willatts DG, Reynolds F (1985) Comparison of the vasoactivity of amide and ester local anaesthetics. An intradermal study. Br J Anaesth 10: 1006-1011.
- Wills MH, Johns RA, Stone DJ, Moscicki JC, Difazio CA (1989) Vascular effects of 2-chloroprocaine and sodium metabisulfite on isolated rat aortic rings. Reg Anesth 14: 271-273.
- Fulton D, McGiff JC, Quilley J (1994) Role of K+ channels in the vasodilator response to bradykinin in the rat heart. Br J Pharmacol 113: 954-958.
- Adeagbo AS, Malik KU (1990) Endothelium-dependent and BRL 34915-induced vasodilatation in rat isolated perfused mesenteric arteries: role of G-proteins, K+ and calcium channels. Br J Pharmacol 100: 427-434.
- Willatts DG, Reynolds F (1985) Comparison of the vasoactivity of amide and ester local anaesthetics. An intradermal study. Br J Anaesth 57: 1006-1011.
- Hahn-Godeffroy JD (1993) Procaine in the neural therapy after Huneke. German Der Allgemeinarzt 14: 34-38.
- Donaldson LF, McQueen DS, Seckl JR (1994) Local anaesthesia prevents acute inflammatory changes in neuropeptide messenger RNA expression in rat dorsal root ganglia neurons. Neurosci Lett 175: 111-113.
- Levine JD, Moskowitz MA, Basbaum AI (1985) The contribution of neurogenic inflammation in experimental arthritis. J Immunol 135: 343-347.
- Krause W (2000) ID-Pharma: Research materials. Germany
- Fischer L, Ludin SM, Vega KP, Sturzenegger M (2015) Neuralgia of the Glossopharyngeal Nerve in a Patient with Posttonsillectomy Scarring: Recovery after Local Infiltration of Procaine-Case Report and Pathophysiologic Discussion. Case Rep Neurol Med 2015: 560546.
- Kasch H (2000) The scavanger effect of a definded procaine base mixture. Presentation german pain congress. Germany.
- Rusu C, Borsa C, Gradinaru D, Ionescu C (1996) Antioxidant and lipid-lowering effects of the original Procaine-based products. Rom J Geront Geriatr 3: 47-61.
- Dolganiuc A, Radu D, Olinescu A, Vr?biescu A (1998) Procain and diethylaminoethanol influence on the release of free oxygen radicals by polymorphonuclear leukocytes, in rabbits and humans. Roum Arch Microbiol Immunol 57: 23-32.
- Mutschler H (2001) Drugs effects (8thedn). Springer Publishing, USA.
- Becke M (1996) The effect of Procain on the cell membrane. German. Ärztezeitschrift f. Naturheilverfahren 37: 90- 97.
- Wander R (1999) Actions of Procaine in the ground substance. Germany.
- Hille B (1992) Ionic channels of excitable membranes (2ndedn). Sunderland.
- Jurius AR, Jarrush-Saadeh D, Nassar C (1988) Modulation of some human mononuclear cells activities by procaine. Middle East J Anaesthesiol 9: 417-428.
- Mrose HE, Ritchi JM (1978) Local anestetics: Do Benzocaine and Procaine act on the same single site? J Gen Physiol 70: 223-225.
- Jalili S, Saeedi M (2017) Study of Procaine and tetracaine in the lipid bilayer using molecular dynamics simulation. Eur Biophys J 46: 265-282.
- Yau TM, Kim SC (1980) Local anaesthetics as hypoxic radiosensitizers, oxic radioprotectors and potentiators of hyperthermic killing in mammalian cells. Br J Radiol 53: 687-692.
- Feinendegen LE, Mühlensiepen H, Lindberg C, Marx J, Porschen W, et al. (1984) Acute and temporary inhibition of thymidine kinase in mouse bone marrow cells after low-dose exposure. Int J Radiat Biol Relat Stud Phys Chem Med 45: 205-215.
- Chlebowski RT, Block JB, Cundiff D, Dietrich MF: Doxorubicin cytotoxicity enhanced by local anesthetics in a human melanoma cell line. Cancer Treat Rep 66: 121-125.
- Esposito M, Viale M, Vannozzi MO, Zicca A, Cadoni A, et al. (1996) Effect of the antiarrhythmic drug procainamide on the toxicity and antitumor activity of cis-diamminedichloroplatinum(II). Toxicol Appl Pharmacol 140: 370-377.
- Viale M, Pastrone I, Pellecchia C, Vannozzi MO, Cafaggi S, et al. (1998) Combination of cisplatin-Procaine complex DPR with anticancer drugs increases cytotoxicity against ovarian cancer cell lines. Anticancer Drugs 9: 457-463.
- Pastrone I, Viale M, Cafaggi S, Mariggiò MA, Parodi A, et al. (1999) Effect of the cisplatin-Procaine complex DPR in combination with several anticancer agents on murine P388 leukemic cells in vitroand in vivo. Invest New Drugs 16: 297-302.
- Villar-Garea A, Fraga MF, Espada J, Esteller M (2003) Procaine is a DNA-demethylating Agent with Growth-inhibitory Effects in Human Cancer Cells. Cancer Res 63: 4984-4989.
- Villar-Garea A (2005) Epigenetic transcriptional repression of tumour suppressor genes and its reversion by drugs. Doctoral thesis. Chemical Sciences. Department of Biochemist and Molecular Biology of Univesity of Valencia. Mayo, USA.
- Tada M, Imazeki F, Fukai K, Sakamoto A, Arai M, et al. (2007) Procaine inhibits the proliferation and DNA methylation in human hepatoma cells. Hepatol Int 1: 355-364.
- Sabit H, Samy MB, Said O, El-Zawahri MM (2016) Procaine Induces Epigenetic Changes in HCT116 Colon Cancer Cells. Genetics Research International 2016: 8348450.
- Bleese N, Döring V, Kalmar P, Krebber HJ, Pokar H, et al. (1979) Clinical application of cardioplegia in aortic cross-clamping periods longer than 150 minutes. Thorac Cardiovasc Surg 27: 390-392.
- Bleese N, Döring V, Kalmar P, Pokar H, Polonius MJ, et al. (1978) Intraoperative myocardial protection by cardioplegia in hypothermia. J Thorac Cardiovasc Surg 75: 405-413.
- Isselhard W, Schorn B, Hügel W, Uekermann U (1980) Comparison of three methods of myocardial protection. Thorac Cardiovasc Surg 28: 329-336.
- Epting WS, Hoffmeister HE, Stunkat R (1977) Aortic valve replacement utilizing induced ischemic arrest with magnesium-aspartate-procaine. J Cardiovasc Surg (Torino) 18: 421-426.
- Kalmár P, Bleese N, Döring V, Gercken G, Kirsch U, et al. (1975) Induced ischemic cardiac arrest. Clinical and experimental results with magnesium-aspartate-procaine solution (Cardioplegin). J Cardiovasc Surg (Torino) 16: 470-475.
- Worlitschek K (2012) Practice of acid base household. Haug Publisher, Germany.
- Reuter U, Oettmeier R (1997) Regulation and pain treatment with infusion neural therapy. German. Natura Med 12: 20-25.
- Reuter U, Oettmeier R (1999) The high-dosed Procaine-Base-Infusion. German Ärztezeitschrift f. Naturheilverfahren 11: 776-783.
- BERG Fvd.(Eds.): Applied Physiology, Volume 4. Understanding and influencing pain. German. Thieme Publisher Stuttgart, (2004).
- Weinschenk S (Eds) Handbook of Neural Therapy - Diagnostics and Treatment with local Anesthetics. German. Urban and Fischer (2010).
- Glusa E (1999) Spasmolytic action of Procaine-Base on the aorta of rats. German. ID-Pharma, Research material.
- Ibusuki S, Katsuki H, Takasaki M (1998) The effects of extracellular pH with and without bicarbonate on intracellular Procaine concentrations of anesthetic effects in crayfish giant cells. Anesthesiology 88: 1549-1557.
- Yung E, Lahoti T, Jafari S, Weinberg JD, Schianodicola JJ, at al. (2009) Bicarbonate plus epinephrine shortens the onset and prolongs the duration of sciatic block using chloro-Procaine followed by bupivacaine in sprague-dawley rats. Reg Anesth Pain Med 34: 196-200.
- Stevens RA, Chester WL, Grueter JA, Schubert A, Brandon D, et al. (1989) The effect of pH adjustment of 0.5% bupivacaine on the latency of epidural anesthesia. Reg Anesth 14: 236-239.
- Oettmeier R, Reuter U (2000) The continuous Procaine-Basen infusion/-perfusion: New ways for systemic influencing regulation, inflammation and pain. German Erfahrungsheilkunde 2: 75-84.
- Iudenkov N (1959) Intra-arterial infusion of penicillin and procaine in treatment of suppurative inflammations and open trauma. Voen Med Zh 86: 77-79.
- Fuzaylov G, Kelly TL, Bline C, Dunaev A, Dylewski M, et al. (2015) Post-operative pain control for burn reconstructive surgery in a resource-restricted country with subcutaneous infusion of local anesthetics through a soaker catheter to the surgical site: Preliminary results. Burns 41: 1811-1815.
- Bräuer et al. (2000) The anti-rheumatic and joint-protective effect of a defined Procaine-Base mixture. Presentation german pain congress, German.
- Kasch H, Engert B, Reuter U, Oettmeier R (2009) Internal research materials. Jen Cluster Gmb H Jena, German.
- Levraut J, Labib Y, Chave S, Payan P, Raucoules-Aime M, et al. (1996) Effect of sodium bicarbonate on intracellular pH under different buffering conditions. Kidney Int 49: 1262-1267.
- Langguth P, Fricker G, Wunderli-Allenspach H (2004) Biopharmacy, Weinheim, Wiley-VCH-Publisher, German.
- Seifen AB, Ferrari AA, Seifen EE, Thompson DS, Chapman (1979) Pharmacokinetics of intravenous procaine infusion in humans. Anesth Analg 58: 382-386.
- Smith RH, Hunt DH, Seifen AB, Ferrari A, Thompson DS (1979) Pharmacokinetic model for procaine in humans during and following intravenous infusion. J Pharm Sci 68: 1016-22.
- Layer P, Bronisch HJ, Henniges UM, Koop I, Kahl M, et al. (2011) Effects of systemic administration of a local anesthetic on pain in acute pancreatitis: a randomized clinical trial. Pancreas 40: 673-679.
- Meng W, Yuan J, Zhang C, Bai Z, Zhou W, et al. (2013) Parenteral analgesics for pain relief in acute pancreatitis: a systematic review. Pancreatology 13: 201-206.
- Lankisch PG (2012) Procaine Infusion in Pain Treatment of Acute Pancreatitis: yes or no, that is the Question. Z Gastroenterol 50: 323-324.
- Veneziano G, Tobias JD (2017) Chloroprocaine for epidural anesthesia in infants and children. Paediatr Anaesth 27: 581-590.
- Lee SC, Moll V (2017) Continuous Epidural Analgesia Using an Ester-Linked Local Anesthetic Agent, 2-Chloroprocaine, During Labor: A Case Report. A A Case Rep 8: 297-299.
- Veneziano G, Iliev P, Tripi J, Martin D, Aldrink J, et al. (2016) Continuous chloroprocaine infusion for thoracic and caudal epidurals as a postoperative analgesia modality in neonates, infants, and children. Paediatr Anaesth 26: 84-91.
- Muhly WT, Gurnaney HG, Kraemer FW, Ganesh A, Maxwell LG (2015) A retrospective comparison of ropivacaine and 2-chloroprocaine continuous thoracic epidural analgesia for management of postthoracotomy pain in infants. Paediatr Anaesth 25: 1162-1167.
- Kamata M, Corridore M, Tobias JD (2014) Thoracic epidural infusion with chloroprocaine for postoperative analgesia following epicardial pacemaker placement in an infant. J Pain Res 7: 609-613.
- Landriscina DM (1992) The effect of pH-adjusted 2-chloroprocaine on the duration and quality of pain relief with a subsequent continuous epidural bupivacaine infusion. AANA J 60: 174-180.
- Tobias JD, Rasmussen GE, Holcomb GW, Brock JW, Morgan WM (1996) Continuous caudal anaesthesia with chloroprocaine as an adjunct to general anaesthesia in neonates. Can J Anaesth 43: 69-72.
- Veneziano G, Tobias JD (2017) Chloroprocaine for epidural anesthesia in infants and children. Paediatr Anaesth 27: 581-590.
- O'Donnell CP, Scheuer DA, Keil LC, Thrasher TN (1991) Cardiac nerve blockade by infusion of procaine into the pericardial space of conscious dogs. Am J Physiol 260: 1176-1182.
- Hahn-Godeffroy JD (2011) Procain-Reset: Ein Therapiekonzept zur Behandlung chronischer Erkrankungen. Schweiz Z Ganzheitsmed 23: 291-296.
- Herdegen T, Mangold S, Hahn-Godeffroy JD (2016) Therapeutic effects of Procaine Infusions: Result of a multicentric observation study. German. Presentation medical week Baden-Baden
- Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, et al. (1996) Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry 53: 59-69.
- Waaben J, Sørensen O, Wiberg-Jørgensen F, Flachs H, Skovsted P (1984) Haemodynamic effects of intravenous procaine as a supplement to general anaesthesia in patients with valvular heart disease. Acta Anaesthesiol Scand 28: 34-36.
- Benson BE, Carson RE, Kiesewetter DO, Herscovitch P, Eckelman WC, et al. (2004) A Potential Cholinergic Mechanism of Procaine's Limbic Activation. Neuropsychopharmacology 29: 1239-1250.
- Butterworth JF, Cole LR (1990) Low concentrations of procaine and diethylaminoethanol reduce the excitability but not the action potential amplitude of hippocampal pyramidal cells. Anesth Analg 71: 404-410.
- Matulewicz P, Orze?-Gryglewska J, Braszka ?, Zawistowski P, Jurkowlaniec E (2015) Hippocampal theta rhythm after local administration of procaine or amphetamine into the ventral tegmental area in fear conditioned rats. Neurosci Lett 589: 132-137.
- Little JW, Ford A, Symons-Liguori AM, Chen Z, Janes K, et al. (2015) Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain 138: 28-35.
- Vandevoorde S, Lambert DM, Smart D, Jonsson KO, Fowler CJ (2003) N-Morpholino- and N-diethyl-analogues of palmitoylethanolamide increase the sensitivity of transfected human vanilloid receptors to activation by anandamide without affecting fatty acid amidohydrolase activity. Bioorg Med Chem 11: 817-825.
- Abounassif MA, El-Obeid HA, Gadkariem EA (2005) Stability studies on some benzocycloheptane antihistaminic agents. J Pharm Biomed Anal 36: 1011-1018.
- Uchiyama M, Oguri M, Mojumdar EH, Gooris GS, Bouwstra JA (2016) Free fatty acids chain length distribution affects the permeability of skin lipid model membranes. Biochim Biophys Acta 1858: 2050-2059.
- Paloncýová M, DeVane RH, Murch BP, Berka K, Otyepka M (2014) Rationalization of reduced penetration of drugs through ceramide gel phase membrane. Langmuir 30: 13942-13948.
- Becke M (1996) Procaine and the discussion concerning the allergy. German. Ärztezeitschrift f Naturheilverfahren 37: 908 -912.
- Hahn-Godeffroy JD (1993) Concerning the indispensability of Procaine in neural therapy. German. Ärztezeitschrift f. Naturheilverfahren 3: 722-730.
- Oettmeier R, Reuter U (200) High-dosed Procaine-Base-Infusions - is the risk calculable? Metaanalysis of surveillance data from important parameters. German. Company product information-ID-Pharma.
- Cleave E (2016) Cleave´s biographical cyclopaedia of Pennsylvania. (1874).
- Saha FJ (2016) Procaine-Infusions in neural therapy – with or without alkalic additive? Presentation medical week Baden-Baden.
- Oettmeier R, Reuter U (2017) Examinations concerning the importance of metabolic alkalosis in cancer patients. German. Umwelt, Medizin, Gesellschaft.; 30: 15-18.
- Luke RG, Galla JH (1989) Does chloride play an independent role in the pathogenesis of metabolic alkalosis? Semin Nephrol 9: 203-205.
- Lynch F (2009) Arterial blood gas analysis: mplications for nursing. Paediatr Nurs 21: 4-44.
- Mandy J (2008): Arterial blood gas analysis. 1: Understanding ABG reports. Nurs times 104: 28-29.
- Allen K (2005) Four-step method of interpreting arterial blood gas analysis. Nurs Times. 101: 42-45.
- Woodrow P (2004). Arterial blood gas analysis. Nurs Stand 18: 45-52.
- Van Limburg Stirum J (2008) Modern Acid-Base-Medicine. German. Hippokrates.
- Van Limburg Stirum J (2006) The Acid-Base Household-Diagnostics and Concepts for Treatment. German DHZ 3: 29-33.
- Shapiro JI (1997) Pathogenesis of cardiac dysfunction during metabolic acidosis: therapeutic implications. Kidney Int Suppl 61: 47-51.
- Expert information of Curafaktur GmbH (Heilbronn) and JenCluster GmbH (Jena). German (2016).
- Tello EE (1953) Treatment of psoriasis with novocaine injected intravenously; preliminary report. Spanish. Prensa Med Argent.;40: 3161-3163.
- Ortega LM, Arora S (2012) Metabolic acidosis and progression of chronic kidney disease: incidence, pathogenesis, and therapeutic options. Nefrologia 32: 724-730.
- Batlle DC, Mozes MF, Manaligod J, Arruda JA, Kurtzman NA (1981) The pathogenesis of hyperchloremic metabolic acidosis associated with kidney transplantation. Am J Med 70: 786-796.
- Gristina AG, Pace NA, Kantor TG, Thompson WA (1970) Intra-articular thio-tepa compared with depomedrol and procaine in the treatment of arthritis. J Bone Joint Surg Am 52:1603-1610.
- Trentham DE, Mccravey JW, Masi AT (1976) Low dose penicillin for gonococcal arthritis. A comparative therapy trial. JAMA.;236: 2410-2412.
- Goldstein WM, Gleason TF, Barmada R (1983) A comparison between arthrotomy and irrigation and multiple aspirations in the treatment of pyogenic arthritis: a histological study in a rabbit model. Orthopedics 6: 1309-1314.
- Turner JP, Schmidt FR (1950) Treatment of scleroderma with procaine; report of cases. J Am Med Assoc 144: 1560-151.
- Bennee J (1954) Scleroderma treated with 2% procaine intravenously. Can Med Assoc J 70: 71.
- Farrington J (1958) Intravenous procaine in the management of some cutaneous manifestations of collagen diseases. South Med J 51: 1426-1431.
- Lin Z, Nini Z, Guilin H, Xiaohua H, Jie Y, et al. (2015) Effects of anti-infection treatment on expressions of HLA-DR and CD86 in dendritic cells in rabbit buccal VX2 squamous cell carcinoma tissue with inflammation. Hua Xi Kou Qiang Yi Xue Za Zhi 33: 141-144.
- Orellana Alvarellos G, Ruiz De Viñaspre Alvear P, Kaszkin-Bettag M (2010) A series of case reports: clinical evaluation of a complex homeopathic injection therapy in the management of pain in patients after breast cancer treatment. Altern Ther Health Med 16: 54-59.
Citation: Oettmeier R, Reuter U, Bonilla LBP (2019) The Procaine-Base-Infusion: 20 Years of Experience of an Alternative Use with Several Therapeutical Effects. J Altern Complement Integr Med 5: 061.
Copyright: © 2019 Ralf Oettmeier, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

