Department Of Surgery, Vascular And Endovascular Surgery Division, Laboratory Of Medical Investigation, University Of São Paulo School Of Medicine, Brazil
Abstract
Introduction and Objectives: Intra-abdominal pressure (IAP) has been shown to have an important effect on homeostasis and might be influenced by numerous conditions. Recent case reports describe full collapse of the abdominal aorta due to IAP increase. The present study was designed to estimate, through simulation, how the intra-abdominal pressure increment would compromise the systemic arterial pressure and the flow through the abdominal aorta. We sought to simulate how the cardiovascular monitoring of a critical patient could be disturbed by IAP increment and could compromise the health professional team diagnosis of the real hemodynamic condition.
Materials and Methods: A pulsatile circulatory flow loop was designed, including a compliance-matching silicone aorta located inside an abdominal cavity phantom, whose pressure simulates IAP. Experiments started with five different initial hemodynamic conditions and simulated 24 different IAP levels. The flow through the tube as well as the pressures upstream and downstream the cavity were registered, representing the upper and lower limbs arterial pressures respectively. Additionally in order to simulate surgical approaches to the aorta, prosthetic conduits were set inside the silicone tube and complementary measurements were made.
Results: Intra-abdominal pressure, after it breaches a threshold, started to collapse the aorta resulting in measurable increase in upstream pressures, reduction in downstream pressures and reduction in aortic flow. Thus this happened earlier in hypotensive scenarios. In the vascular surgery context, endoprosthesis phantom was slightly the most resistant to IAP.
Conclusion: The present simulation suggests that IAP would mask a patient’s real cardiovascular status. This data also suggest that hemodynamic monitoring of critical patients based on upper limbs arterial pressures without the concomitant IAP measurement could lead to incorrect clinical judgment and consequently, to mistaken decision making. Within a simulation context, the IAP has influence over the aorta and also over prosthetic material.
Keywords
Aortic flow; Cardiovascular monitoring; Intra-abdominal pressure; Pulsatile circulatory flow loop; Simulation
BRIEF PARAGRAPH
Intra-Abdominal Pressure (IAP) has been shown to have an important effect on homeostasis and might be influenced by numerous conditions. Recent case reports describe patients who were admitted to the emergency department with a collapsed aorta but presenting hemodynamically stable condition. Due to this apparent contradiction between the two information an apparatus was constructed to simulate the behavior of the aorta and the consequent changes in upper and lower limbs pressures and aortic flow over a wide range of IAP variation. By analogy the test results pointed to a strong interference of the IAP values on the abovementioned cardiovascular parameters. The results obtained with the present simulation suggest that IAP could mask the actual hemodynamic conditions of critical patients. This fact could possibly impair their monitoring and consequently lead to the adoption of inadequate clinical decisions.
INTRODUCTION
Since the 19th century much has been discussed about the effects of Intra-Abdominal Pressure (IAP) on homeostasis. In 1876, Wendt apud Hunter published what would be the first record of the association between increased intra-abdominal pressure and oliguria [1,2]. Emerson apud Papavramidis Published the results of his study, reporting the effects of high PIA on the cardiovascular system and showed that Ascites fluid drainage led to the recovery of hemodynamic parameters such as blood pressure and heart rate [3,4]. Due to the growing interest in the physiological consequences of increasing the IAP, numerous publications have proposed various ways of measuring and monitoring this. Kron IL et al., University of Virginia, were the first to use intravesical pressure (obtained by the connection between the urinary catheter and a pressure transducer) as an instrument for measuring the IAP [5]. This was the first IAP measurement technique described in medical literature, being the most used technique to date.
The observations outlined above have drawn the attention of researchers from around the world, culminating in 2004 with the creation of the World Society of Abdominal Compartment Syndrome (WSACS) [6]. This entity was founded by an international group of clinicians and surgeons who recognized the need for a cohesive approach to promote research and continuing education with the goal of promoting the improvement of patients with elevated IAP. In healthy adults, the normal value of IAP is around 3 mmHg. The World Society of the abdominal compartment syndrome published a consensus in 2013, which defines Intra-Abdominal Hypertension (IAH) as IAP above 12 mmHg and Abdominal Compartment Syndrome (ACS) as IAP above 20 mmHg, associated with new dysfunction of organs or systems [7].
Considering the abovementioned, most reports or studies of IAP effects based on clinical observations and animal models describe IAP values only up to 30-35 mmHg [8-14]. Certainly these limits encompass most situations observed in clinical practice and within animal experimentation context. However, several case reports involving IAP increments, published in the last decade, describe massive distention of the abdomen due to varied causes and most of them present CT scan images which reveal full abdominal aorta collapse probably associated to high IAP [15-18].
Kim et al., published a case report of a 19 years old female patient who presented a massive abdominal distention due to a bulimic attack [15]. Although hemodynamically stable, the femoral pulses were absent and the CT scan images revealed the collapse of the abdominal aorta.
Similarly, Jambet et al., published in 2012 a case report of a 57 years old female patient who presented severe acute megacolon (Ogylve’s syndrome) due to amitriptyline, but 130/90 mmHg blood pressure at emergency admission [16]. CT scan images revealed abdominal aortic compression as well.
In 2013, Paschoald et al., reported full collapse of the abdominal aorta on a computed tomography scan in a 63 years old male patient, hemodynamically stable upon admission to the emergency department, who presented a massive pneumoperitoneum [17].
Eetvelde et al., published in 2014 a case report describing a 19 years old female patient whose CT scan images also revealed extrinsic compression of the aorta by extensive gastric distension [18].
A publication by Iqbal et al., informs that even in ordinary physiological situations such as coughing and vomiting, it is possible to register intrabladder pressures as high as 226 mmHg and 255 mmHg, respectively [19]. Obviously, the short duration nature of these events makes them irrelevant considering the deleterious effects of the PIA, but the high pressure values achieved can be considered important information.
In fact, it is intriguing that in various cases in medical literature the patients have achieved IAP values high enough to promote the abdominal aorta collapse. Many of them remained hemodynamically stable even under this severe condition. The reports on this topic suggest that a better understanding of how aortic hemodynamics is affected by intra-abdominal pressure can be valuable in terms of appreciating the need for IAP measurement in the daily clinic.
They also suggest that, despite the IAP values usually adopted in the studies on this field are lower than 30 mmHg; it is indispensable to investigate the effects of higher values of IAP over the abdominal aorta, and identify the whole spectrum of IAP values up to this vessel collapse.
In this context, through simulation, this study sought to address four questions: Considering that is possible to collapse the largest abdominal artery of human anatomy with the increase of intra-abdominal pressure, what is the IAP range in which is probable the occurrence of such a severe disturbance in the vascular physiology? How are arterial pressures proximal and distal to the abdomen and aortic flow affected by the entire plausible range of IAP in hypotensive, normotensive and hypertensive patients? If the upper limb arterial pressure could be affected by IAP increase, how the cardiovascular monitoring of a critical patient could be disturbed by IAP? And finally, within a vascular surgery context, how does the presence of an implanted aortic vascular graft affect these relationships?
Addressing these questions can lead to key insights on the role of IAP and novel methods for clinical management of critical care patients. We chose to perform an experimental laboratory investigation because they allow for reliable control of variables involved and permit the study of the effect of extremely high IAP values that are plausible, but difficult to spot and measure in the clinic. While an experimental study is indispensable for addressing the questions driving this study, we recognize that we are sacrificing the realism of clinical observations and therefore, have strived to incorporate many realistic aspects of the clinical situation in this study.
MATERIALS AND METHODS
A great number of studies about the IAP effects over organs and systems use animals of experimentation to fulfill their goals. Moreover the experimental increase of IAP could clearly cause important physiological changes on these healthy animals exposing them to distress and death risk. We perceive that the circulatory system, if considered as a hydraulic system could have many of its functions simulated in an experimental scenario. We chose to perform a laboratory investigation through the creation of a low cost, reproducible and pulsatile circulatory flow loop. It could avoid the usage of animals of experimentation, allowing for reliable control of variables involved.
An experimental apparatus was developed to simulate pulsatile infrarenal aorta hemodynamics and the abdominal pressure (Figures 1 and 2). Aortic hemodynamics was simulated through a pulsatile flow loop. Flow of a blood analog fluid is driven from a reservoir by a propulsion pump through a solenoid valve whose opening and closing phases are electronically controlled. Resistance valves are included in the main circuit and in a bypass loop. We sought to simulate the peripheral circulation of lower limbs including in our apparatus an extra register downstream the pressurized chamber. The adjustments on this register simulate the arterial peripheral resistance.
 Figure 1:
Figure 1: Photography of the experimental apparatus. 1: phantom chamber, 2: digital pressure gauges, 3: flowmeter, 4: electric circuit control box, 5: fluid reservoir, 6: pump.
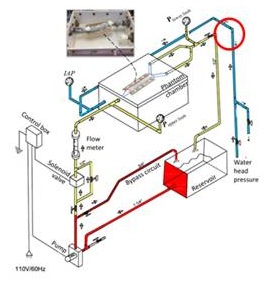 Figure 2:
Figure 2: Schematic of the experimental apparatus to simulate the effect of elevation in IAP on aortic hemodynamics.
The flow in the main circuit passes through an air-tight polyvinyl chloride phantom chamber with a compliance matching silicone aorta inside it. This aorta phantom is 2.4 cm in diameter, 1 mm in thickness made of XP-565 silicone that has a realistic compliance which approximates a native artery. These conduits have been developed especially for the present experiments and are a courtesy from Dr. Madhavan Lakshmi Raghavan, Professor at the Laboratory of Biomedical Engineering of Iowa State University Engineering College - USA.
Considering that the compliance of a vessel is defined as the percentage of change in its diameter between systolic and diastolic pressure, it was possible to evaluate this biomechanical property of the aforementioned silicone tube using the formula proposed by Tai et al., [20].
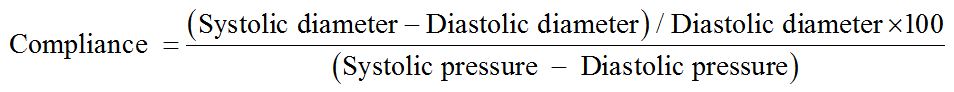
The calculated compliance of the silicone tube was 3.246%/mmHg × 10-2 under a blood pressure of approximately 90 mmHg. Tai et al., described the compliance of human arteries (in this case, samples of external iliac arteries collected from donors of organ transplants) of approximately 3.0%/mmHg x 10-2 under similar arterial pressure [20]. This information experimentally demonstrates the biomechanical similarity between the silicone tube phantom used as specimen and an actual artery validating it as an adequate aorta replacement for the present experiment. Is important to highlight that Tai et al. studied the compliance of organ donors iliac arteries. In general, these patients are younger and have a mild degree of atherosclerosis. Ninomiya et al., demonstrated experimentally that the aging process makes the infrarenal aorta tissue less elastic / more rigid [21]. The present silicone phantom has a biomechanical behavior similar to an artery with mild atherosclerosis.
By controlling the solenoid valve motion and the resistances in the main and bypass loops we were able to create realistic pulsatile aortic flow and pressure and control these parameters in the silicone aorta phantom. The phantom chamber is equipped with a transparent lid in order to visualize the specimen during experiments. To realistically simulate the perivascular environment, the silicone aorta rested on a vertebral column phantom on its posterior developed using DurepoxTM resin, based on the anatomical information of Gocmen-Mas et al., study and a flexible thin plastic film on its anterior, representing the periaortic peritoneum (Figures 3 A, B and C) [22]. The abdominal pressure was simulated using a static water head system equipped with a control valve. Measurements of experimental parameters were accomplished using a flow meter (Blaster controles, Inc. Veleiros, São Paulo, Brazil) in the main circuit (to measure aortic flow), two digital pressure gauges (SSI Technologies, Inc, Janesville, Wisconsin - USA) located proximal to and distal to the silicone aorta phantom (to measure the equivalent of upper and lower limb pressures respectively) and a third digital pressure gauge at the abdominal chamber (to measure IAP). A glycerin-water mixture (42:58) was used as the blood analog fluid owing to its bloodlike viscosity of 4.0 cP [23]. Preliminary experiments with this apparatus in which we video recorded and digitized the oscillations in the pressure gauges and flow meter demonstrated that we can simulate pulsatile (60 bpm) flow and pressure through the aorta phantom for hypotensive, normotensive and hypertensive patients. We were also able to simultaneously generate IAP in the abdomen chamber ranging from 0 to 230 mmHg with increments of 10 mmHg in order to study in a controlled manner, the effect that elevation in IAP has on aortic hemodynamics.
 Figure 3A:
Figure 3A: Vertebral column phantom developed using Durepox
TM resin, based on the anatomical information of Gocmen-Mas et al. [22].
 Figure 3B:
Figure 3B: Silicone aorta resting on the vertebral column phantom.
 Figure 3C:
Figure 3C: Flexible thin plastic film representing the periaortic peritoneum.
The experiments conducted to study the effect of gradually increasing IAP on infrarenal aorta hemodynamics involved five different types of initial conditions. The five conditions simulated subjects with severe hypotension (65/40 mmHg, 0.57 L/min), slight hypotension (105/55 mmHg, 0.67 L/min), normotension (120/85 mmHg, 0.8 L/min), slight hypertension (150/100 mmHg, 1 L/min), severe hypertension (195/155 mmHg, 1.1 L/min) [24]. In each case, after setting up the initial conditions, the IAP was gradually increased from 0 mmHg (baseline) to 230 mmHg (extreme IAP) at 10 mmHg increments (i.e., 24 IAP values were simulated) while documenting the behavior of six variables of interest: systolic and diastolic aortic flow and systolic and diastolic pressures proximal and distal to the abdomen phantom (simulation of measuring upper and lower limb pressures in the subject respectively).
After performing the above experiments with silicone aorta, we also repeated these experiments by deploying inside the silicone aorta phantom, a Maquet™ Dacron vascular graft - 20 mm diameter (simulating a subject with open aneurysm repair) and a Medtronic™ Endurant endoprosthesis - 28×13×145 mm (simulating a subject with endovascular aneurysm repair). The purpose of these additional experiments was to study how IAP affects aortic flow in patients who have had aortic repair surgically or endovascularly. We were particularly interested in the latter because endoprosthesis are known to exhibit increased radial rigidity and are likely more resistant to IAP than the native aorta. Overall therefore, with 24 IAP values for each of 5 subject initial conditions for each of 3 aorta phantoms, we performed 360 flow experiments.
Statistical methodology: In addition to the basic techniques of exploratory analysis of mean and standard deviation, two statistical methods were used to meet the objectives of this study: Analysis of Variance (ANOVA) and the Tukey test for comparison of means, which were performed with SPSS 19.0 for Windows (IBM, United States), with significant P values being <.05.
RESULTS
The statistical evaluation using ANOVA revealed that the IAP has a clear influence on the six variables of interest (systolic an diastolic pressures in upper and lower limbs and aortic systolic and diastolic flow) regardless of the experimentation scenario and specimen (P<.001).
The effect of IAP elevation on the Mean Arterial Pressure (MAP) proximal (upper limb) and distal (lower limb) to the abdomen is shown in figure 4 for severely hypotensive, normotensive and severely hypertensive subjects. For all cases, there was little measurable effect on limbs pressures until IAP starts to approach diastolic pressure after which it starts to partially collapse the aorta resulting in reduced flow, increased upper limb pressure and decreased lower limb pressures. After IAP breaches diastolic pressure, every 10 mmHg elevation in IAP resulted approximately in 10 mmHg elevation in upper limb MAP, 5 mmHg drop in lower limb MAP and 30 cc/min drop in mean aortic flow rate for normotensive initial conditions.
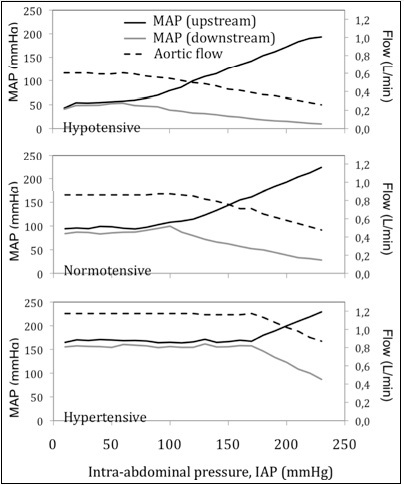 Figure 4:
Figure 4: Effect of IAP on aortic flow and mean arterial pressure upstream (upper limb pressure) and downstream (lower limb pressure) to the aorta, for three initial hemodynamic conditions: hypotensive, normotensive and hypertensive. As IAP breaches diastolic pressure and rises past it, the aortic flow starts to drop, the upper limb pressure rises and lower limb pressure drops.
For severely hypotensive and severely hypertensive initial conditions, every 10 mmHg elevation in IAP also resulted approximately in 10 mmHg elevation in upper limb MAP. But it was different for lower limb MAP and mean aortic flow. For severely hypotensive initial condition, after IAP breaches diastolic pressure, every 10 mmHg elevation in IAP resulted approximately in 2, 5 mmHg drop in lower limb MAP and 24 cc/min drop in mean aortic flow rate. For severely hypertensive initial condition, after IAP breaches diastolic pressure, every 10 mmHg elevation in IAP resulted approximately in 12 mmHg drop in lower limb MAP and 55 cc/min drop in mean aortic flow rate.
The opposing pressure responses in the upper and lower limbs can be explained as an understandable consequence of aortic collapsing effect. The effect of IAP elevation on the mean aortic flow rate is shown in figure 4 for severely hypotensive, normotensive and severely hypertensive subjects. Flow rate started to drop as IAP approached diastolic pressure and continued to drop when the aorta became almost fully collapsed. Again, the drop in flow rate also is a consequence of the pinching effect from the collapsing aorta due to elevating perivascular pressure.
And finally, the differences in IAP effect on pressure and flow between silicone aorta, Dacron and endoprosthesis inside silicone aorta are shown in figure 5. The Tukey test showed that, most of the times, there was a statistically significant difference (P<.05) in the behavior of all pressure and flow variables when specimens were compared two by two. In general, the endoprosthesis was more resistant to the perivascular effect from IAP. The endoprosthesis started showing a measurable effect at about 20 mmHg higher IAP than the silicone aorta and continued to be more resistant although it too collapsed almost fully eventually with elevating IAP. After IAP breaches diastolic pressure, 10 mmHg elevation in IAP caused 7 mmHg elevation in upper limb MAP within endoprosthesis scenario (10 mmHg in silicone aorta scenario) and 20 cc/min drop in mean aortic flow within endoprosthesis scenario (30 cc/min in silicone aorta scenario).
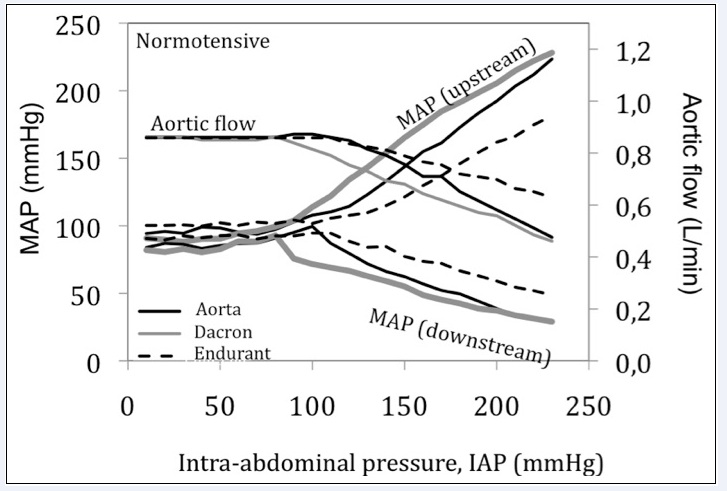 Figure 5:
Figure 5: Differences in hemodynamic response to IAP for silicone aorta, subject with a Dacron graft (open repair) and with an Endurant graft (endovascular repair) for normotensive initial conditions.
DISCUSSION
Elevated intra-abdominal pressure can have fatal consequences for the patient if not managed in a timely manner. Specially at the moment of admission in emergency room or intensive care unit, surgical / trauma patients are part of a high risk group for IAP increase either because they would had an abdominal procedure (causing swelling and ileus) and/or because they usually receive a large amount of fluids for volemic expansion [7]. Many case reports present evidences that high IAP can even collapse the aorta. But even considering this information, a significant percentage of physicians who deal with this high risk group of patients, still don’t measure IAP in all susceptible patients.
In a survey of UK intensivists, Ravishankar et al., determined that 24% of respondents have never measured IAP because they considered this information unnecessary [25]. De Laet et al., in a survey of Belgians surgeons, registered that only 41% of the participants have ever measured the IAP [26]. A multicentric survey among intensive care Chinese physicians registered that only 29,3% of this group of professionals, who deal specifically with severe patients, measure the IAP routinely [27].
Further, Malbrain et al., concluded that physicians have less than a 50% chance of correctly identifying the presence of increased IAP by abdominal palpation alone compared with intrabladder measurement results [28].
Considering that IAP is not routinely measured in the clinic and the results of the present experiment, if elevated IAP itself affects upper limb pressures, could monitoring them without concomitant IAP measurement lead to misdiagnosis of the underlying hemodynamic condition? How risky is the common method of just monitoring upper limb pressures without IAP mensuration? How do patients with endoprosthesis respond to IAP?
Such important questions remain unanswered within clinical practice. Elucidating in a controlled and quantitative manner, the effect that acute IAP increment have on aortic hemodynamics can help add quantitative insights to this serious condition and help in the management of patients in the intensive care unit. But given the lack of routine IAP measurement in the clinic, a careful bedside study is not easily performed.
In the present study, we performed a controlled laboratory investigation of the IAP effect where the hemodynamics was simulated under reasonably realistic conditions. Despite limitations inherent to any simulated experiments they do permit a high degree of control and if performed with care, this may contribute to the investigation of cause and effect from which we can gain key insights about the IAP phenomenon that are likely impossible to obtain from clinical studies.
The results from this study point to four key insights about the relationship between elevated IAP and aortic hemodynamics. First it confirms that, within a simulation scenario, there is indeed a cause and effect relationship between elevated IAP and aortic hemodynamics (Figure 4). In the present experiments, at low IAP, this effect was almost not measurable. But as IAP approaches and starts to exceed diastolic pressure in the aorta, three effects are apparent - increase in upper limb pressure, decrease in lower limb pressure and decrease in aortic flow. As IAP elevates to above systolic pressure and continues to rise, these effects start to magnify until the aorta collapses almost fully resulting in severe rise in upper limb pressure and severe drop in lower limb pressure with low flow. These have now been quantitatively documented within an experimental scenario.
The second insight is on the possible implications to patient management from the rise in upper limb pressures as IAP itself reaches aortic diastolic pressure. Consider specifically, the curves for the hypotensive subject in figure 4. It can be noted that elevated IAP can cause upper limb pressures to rise, making them seem normotensive. These experimental findings shed light on phenomena which could happen in real clinical practice: if patients monitoring is based mainly on upper limb arterial pressures, these patients can be misdiagnosed as normotensive when in reality they require urgent management of their IAP. Most of the abovementioned case reports describing full aortic collapse, present patients hemodynamically stable at emergency department admission. The caregiver team would be misinformed about the real cardiovascular status of a patient if basing the decision making on the upper limb arterial pressures without the concomitant IAP monitoring.
The patients more susceptible to IAP increments usually have a bladder catheter already in place for diuresis control. Thus, the tools for IAP measurement are in general available for the healthcare team to use them. The present assay has the important aim of reinforcing how harmful and silent IAP could be and how ease IAP monitoring is particularly in critical patients. For most critical patients, the lack of IAP monitoring is inexcusable, and our experiments had the intention to reinforce this idea.
As a third insight, is important to think about examples of real clinical scenarios common in the intensive care units in which IAP might have a crucial role:
A. Patients in hypovolemic shock: It is well known in the medical literature that vigorous fluid resuscitation, positive water balance or polytransfusion may be important causes of IAP increase (extravasation to the third space). Considering the scenario of the severely hypotensive patient, it is possible that the intensive care team that assists the patient prescribe large amounts of fluid in these cases, which may contribute to the increase of the IAP. Considering that blood pressure monitoring is routinely done in the upper limbs, a large infusion of fluids may increase arterial blood pressure in upper limbs not only by the obvious increase in intravascular volume, but also by the increase in the IAP. Although blood pressure in the upper limbs may be numerically adequate, a percentage of the volume infused can be extravasated to the third space, which would increase the IAP and consequently upper limb pressure. This is a practical example of how IAP could mask the real cardiovascular status of a critical patient;
B. Patients with large third degree burns in the abdominal wall: this is a high-risk situation for IAP increment because of the reduction on abdominal wall compliance observed in severe burns in this body area. These critical patients may also have their real cardiovascular status masked by IAP, especially when it is highlighted that they present a critical condition for which vigorous volume resuscitation is also a formal recommendation in the medical literature.
Considering the present experimental findings and the potential implications to critical patients management, two simple attitudes could be included in our daily clinical practice to improve critical patients monitoring which present high risk of IAP increase: 1) measurement of upper limbs blood pressure concomitant to IAP measurement. 2) the concomitant measurement of arterial pressures in upper and lower limbs and the palpation of femoral pulse. These could be simple, but strong tools for the fast assessment of hemodynamic status of these critical patients, with exception of cases with special conditions (lower limb amputations and occlusive arterial atherosclerosis for example). This could contribute to the management of critical patients, avoiding iatrogenic decisions.
The fourth insight is regarding our investigation of differences in how a subject with endoprosthesis would respond to IAP. As anticipated, given the known increased radial rigidity of the endoprosthesis from its stent frame, it is more resistant to IAP. However, this increased resistance is only about 10-15 mmHg. Thus, while the simulated patient with an endoprosthesis is slightly more resistant to IAP, eventually, he too succumbs to severe IAP.
Limitations: It is important to consider some limitations in our study. Even though our experimental apparatus was developed with rigor to maximize realism in our simulations, it is unlikely to capture all aspects of the clinical situation. One limitation was the fact that we did not simulate the effect from vena cava flow. Another limitation is that the simulator pump is not responsive as the Left Ventricle (LV). It means that the pump is not able to make automatic self adjustments on its operation along the IAP increase. The simulator pump only has possibilities of adjustment at the initial moment of each experiment.
The primary intention of the present experimental assay was to simulate and evaluate the impact of IAP increase over the physiology of abdominal aorta and its consequences to arterial pressure in upper and lower limbs and to aortic flow. Anatomical increments to this present model may be added as a future perspective.
We are conscious that different degrees of atherosclerosis and the aging process would make the aortic tissue present a varied biomechanical behavior. Specimens with more atherosclerotic plaques (harvested generally from elderly individuals) are more rigid and less elastic. The specimens with less atherosclerosis signs are more elastic. However, our goal in the present assay was to detect a tendency of behavior of the variables arterial pressure (in upper and lower limbs) and aortic flow along the IAP increment up to extreme values. As the experimental data showed that even the set of experiments involving an endoprosthesis with metallic skeleton succumbed to severe IAP evolving to aortic collapse, we believe that a more rigid and atherosclerotic wall would not be capable to avoid the aortic collapse.
CONCLUSION
Within an experimental context intra-abdominal pressure can interfere with aortic hemodynamics. The present simulation proposes that high IAP could reduce aortic flow increase upper limb pressures and reduce lower limb pressures. Our results suggest that hemodynamic monitoring of critical patients based on upper limbs arterial blood pressure without the concomitant IAP measurement could lead to incorrect clinical judgment and consequently, to mistaken decision making. The present simulation suggests that patients with endoprosthesis may be only slightly more resistant to elevated IAP. Overall, our assay highlights the importance of measuring and monitoring intra-abdominal pressure. Our results propose that, in critical patients, it would be even more important because IAP elevation can have potentially serious consequences to aortic hemodynamics and hence patient’s health.
ACKNOWLEDGMENT
We acknowledge Pedro Puech Leão and Nelson de Luccia from University of São Paulo (USP) School of Medicine, Department of Surgery -Vascular and Endovascular Surgery Division and José Pinhata Otoch, Junko Takano Osaka and all the collaborators from Surgical Technique Laboratory - USP School of Medicine.
CONFLICTS OF INTEREST / SOURCES OF FUNDING
The whole process of developing the present experiments took place without receiving any financial support from any institution or agency. All financial needs presented in this work were provided with private resources of the first author. The authors state that they have no commercial sponsorship and no conflicts of interest to declare.
REFERENCES
- Hunter JD, Damani Z (2004) Intra?abdominal hypertension and the abdominal compartment syndrome. Anaesthesia 59: 899-907.
- Wendt E (1876) Ueber den Einfluss des intraabdominalen Druckes auf die Absonderungs Geschwindigkeit des Harness. Walter Wigand’s Buchdruckerei.
- Papavramidis TS, Marinis AD, Pliakos I, Kesisoglou I, Papavramidou N (2011) Abdominal compartment syndrome – Intra-abdominal hypertension: Defining, diagnosing, and managing. J Emerg Trauma Shock 4: 279-291.
- Emerson H (1911) Intra-abdominal pressures. Arch Intern Med (Chic) 6: 754-784.
- Kron IL, Harman PK, Nolan SP (1984) The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg 199: 28-30.
- WSACS (2018) World Society of the Abdominal Compartment Syndrome. What is WSACS? VA, USA.
- Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, et al. (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39: 1190-206.
- De Waele JJ (2008) Intra-abdominal hypertension in patients with severe acute pancreatitis. Eur J Trauma Emerg Surg 34: 11-16.
- Staelens AS, Van Cauwelaert S, Tomsin K, Mesens T, Malbrain ML et al. (2014) Intra-abdominal pressure measurements in term pregnancy and postpartum: An observational study. PLOS One 9: 104782.
- Arabadzhiev GM, Tzaneva VG, Peeva KG (2015) Intra-abdominal hypertension in the ICU - A prospective epidemiological study. Clujul Med 88: 188-195.
- Correa-Martín L, Párraga E, Sánchez-Margallo FM, Latorre R, López-Albors O, et al. (2016) Mechanical intestinal obstruction in a porcine model: Effects of intra-abdominal hypertension. A Preliminary Study. PLoS One 5: 11.
- Elvevoll B, Husby P, Øvrebø K, Haugen O (2014) Acute elevation of intra-abdominal pressure contributes to extravascular shift of fluid and proteins in an experimental porcine model. BMC Research Notes 7: 738.
- Díaz F, Erranz B, Donoso A, Salomon T, Cruces P (2015) Influence of tidal volume on pulse pressure variation and stroke volume variation during experimental intra-abdominal hypertension. BMC Anesthesiol 15: 127.
- Regli A, Mahendran R, Fysh ET, Roberts B, Noffsinger B, et al. (2012) Matching positive end-expiratory pressure to intra-abdominal pressure improves oxygenation in a porcine sick lung model of intra-abdominal hypertension. Crit Care 16: 208.
- Kim BS, Kwon JW, Kim MJ, Ahn SE, Park HC, et al. (2011) Abdominal compartment syndrome caused by a bulimic attack in a bulimia nervosa patient. J Korean Surg Soc 81: 1-5.
- Jambet S, Guiu B, Olive-Abergel P, Grandvuillemin A, Yeguiayan JM, et al. (2012) Psychiatric drug-induced fatal abdominal compartment syndrome Am J Emerg Med 30: 513.
- Paschold M, Gockel I, Oberholzer K, Lang H, Düber C (2013) Pneumoabdomen with abdominal compartment and aortic collapse due to gastric bursting acute release by trocar insertion. Circulation 127: 417-418.
- Van Eetvelde E, Verfaillie L, Van De Winkel N, Hubloue I (2014) Acute gastric dilatation causing acute limb ischemia in an anorexia nervosa patient. J Emerg Med 46: 141-143.
- Iqbal A, Haider M, Stadlhuber RJ, Karu A, Corkill S, et al. (2008) A study of intragastric and intravesicular pressure changes during rest, coughing, weight lifting, retching, and vomiting. Surg Endosc 22: 2571-2575.
- Tai NR, Salacinski HJ, Edwards A, Hamilton G, Seifalian AM (2000) Compliance properties of conduits used in vascular reconstruction. Br J Surg 87: 1516-1524.
- Ninomiya OH, Tavares Monteiro JA, Higuchi ML, Puech-Leão P, de Luccia N, et al. (2015) Biomechanical properties and microstructural analysis of the human nonaneurysmal aorta as a function of age, gender and location: An autopsy study. J Vasc Res 52: 257-264.
- Gocmen-Mas N, Karabekir H, Ertekin T, Edizer M, Canan Y et al (2010) Evaluation of lumbar vertebral body and disc: A stereological morphometric study. Int J Morphol 28: 841-847.
- Hoskins PR, Anderson T, McDicken WN (1989) A computer controlled flow phantom for generation of physiological Doppler waveform. Phys Med Biol 34: 1709-1717.
- Cheng CP, Herfkens RJ, Taylor CA (2003) Comparison of abdominal aortic hemodynamics between men and women at rest and during lower limb exercise. J Vasc Surg 37: 118-123.
- Ravishankar N, Hunter J (2005) Measurement of intra-abdominal pressure in intensive care units in the United Kingdom: A national postal questionnaire study. Br J Anaesth 94: 763-766.
- De Laet IE, Hoste EA, De Waele JJ (2007) Survey on the perception and management of the abdominal compartment syndrome among Belgian surgeons. Acta Chir Belg 107: 648-652.
- Zhou JC, Zhao HC, Pan KH, Xu QP (2011) Current recognition and management of intra-abdominal hypertension and abdominal compartment syndrome among tertiary Chinese intensive care physicians. J Zhejiang Univ Sci B 12: 156-162.
- Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, et al. (2004) Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med 30: 822-829.

 Figure 1: Photography of the experimental apparatus. 1: phantom chamber, 2: digital pressure gauges, 3: flowmeter, 4: electric circuit control box, 5: fluid reservoir, 6: pump.
Figure 1: Photography of the experimental apparatus. 1: phantom chamber, 2: digital pressure gauges, 3: flowmeter, 4: electric circuit control box, 5: fluid reservoir, 6: pump. Figure 2: Schematic of the experimental apparatus to simulate the effect of elevation in IAP on aortic hemodynamics.
Figure 2: Schematic of the experimental apparatus to simulate the effect of elevation in IAP on aortic hemodynamics. 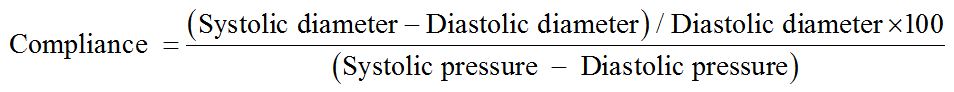
 Figure 3A: Vertebral column phantom developed using DurepoxTM resin, based on the anatomical information of Gocmen-Mas et al. [22].
Figure 3A: Vertebral column phantom developed using DurepoxTM resin, based on the anatomical information of Gocmen-Mas et al. [22]. Figure 3B: Silicone aorta resting on the vertebral column phantom.
Figure 3B: Silicone aorta resting on the vertebral column phantom. Figure 3C: Flexible thin plastic film representing the periaortic peritoneum.
Figure 3C: Flexible thin plastic film representing the periaortic peritoneum. Figure 4: Effect of IAP on aortic flow and mean arterial pressure upstream (upper limb pressure) and downstream (lower limb pressure) to the aorta, for three initial hemodynamic conditions: hypotensive, normotensive and hypertensive. As IAP breaches diastolic pressure and rises past it, the aortic flow starts to drop, the upper limb pressure rises and lower limb pressure drops.
Figure 4: Effect of IAP on aortic flow and mean arterial pressure upstream (upper limb pressure) and downstream (lower limb pressure) to the aorta, for three initial hemodynamic conditions: hypotensive, normotensive and hypertensive. As IAP breaches diastolic pressure and rises past it, the aortic flow starts to drop, the upper limb pressure rises and lower limb pressure drops. Figure 5: Differences in hemodynamic response to IAP for silicone aorta, subject with a Dacron graft (open repair) and with an Endurant graft (endovascular repair) for normotensive initial conditions.
Figure 5: Differences in hemodynamic response to IAP for silicone aorta, subject with a Dacron graft (open repair) and with an Endurant graft (endovascular repair) for normotensive initial conditions.
