
The Matter of Inflammatory Responses in Endovenous Radiofrequency Ablation and Conventional Venous Stripping
*Corresponding Author(s):
Nawaphan TaengsakulDepartment Of Surgery, Chulabhorn Hospital, Bangkok, Thailand
Tel:+66 839316688,
Email:ploynawa@hotmail.com
Abstract
Varicose veins surgery was evolved in the past decade. Many studies showed that Endovenous Radiofrequency Ablation (EV-RFA), a novel method for varicose veins treatment, produced complications fewer than conventional venous stripping, particularly postoperative pain and ecchymosis. There were a lot of studies reported about inflammatory response but there was few studies compare inflammatory response between procedures. This study purpose compare preoperative and postoperative level of serum Interleukin 6 (IL-6) and C-Reactive protein (CRP) to determine the inflammatory response of EV-RFA and conventional venous strip- ping. This study also compares Visual Analogue Scale (VAS) and ecchymoses between these two procedures.
Methods
A prospective cohort study measuring IL-6 and CRP level at before and 24-hour after surgery in symptomatic varicose vein patients who underwent either EV-RFA or conventional venous stripping.
Results
Fifty-nine patients were included, 27 patients were treated by conventional venous stripping and 32 patients were treated by EV-RFA. There was no different in demographic characteristics among two groups. Twenty-four hour postoperative level of IL-6 and CRP significantly increased (p < 0.001 and < 0.001, respectively) in both EV-RFA group (p < 0.001 and < 0.001, respectively) and venous stripping group (p = 0.043 and < 0.001, respectively) compare to pre- operative level. Increasing in 24-hour postoperative level of IL-6 and CRP in EV-RFA group was significantly lower than in venous stripping group (p < 0.001 and p = 0.045, respectively). Post- operative VAS and ecchymosis were also significantly different between EV-RFA group and ve- nous stripping group. (p < 0.001 and p < 0.001, respectively).
Conclusion
EV-RFA produced inflammatory response, VAS and ecchymosis significantly lower than conventional venous stripping.
Keywords
INTRODUCTION
Main pathophysiology mechanism of CVI is venous hypertension caused by shear stress and reflux from incompetent valves [3]. Venous hypertension cause venous dilatation, worsened valvular insufficiency and increased intravenous pressure. Changing in hemodynamics interfere microcirculation, Endothelial Cells (ECs) and vessel microenvironment, leading to venous microangiopathy and dilation and tortuosity of capillary beds [3]. The mechanosensors of endothelial cells are triggered by altered hemodynamics, transducer physical signals into harmful pathways resulting in ECs damages. Particularly these complex biological processes activated inflammatory and proteolytic cascades in vascular microenvironment including leukocyte adhesion, degranulation and releasing of cytoplasmic granules from neutrophils, macrophages, mastocytes, ECs and platelets [4]. All of these mechanisms lead into impairing of both microcirculatory and macro circulatory systems, cause remodeling of the venous walls and valves, venous hypertension, formation of varicosities, edema and leg ulceration [3,5].
Inflammations are an essential immune response to pathogens and damaged cells. In varicose veins, there are refluxes and incompetent valves and venous wall dilation resulting in increased venous pressure. Phenotypic modulation of Vascular Smooth Muscle Cells (VSMC) alters Extracellular Matrix (ECM) metabolisms. Angiogenesis are main mechanisms contributing morphological and functional modifications of varicose veins remodeling. Inflammatory cytokines and adhesion molecules included Transforming Growth Factor Beta (TGF-β), Interleukin 6 (IL-6), Interleukin 8 (IL-8) and Vascular Cell Adhesion Molecule 1 (VCAM-1) [6]. Increased ve- nous wall tension creates Matrix Metalloproteinases (MMPs) activities, which induce ECM degradation and affect structural integrity of the venous walls. ECs injury also triggers inflammation by leukocytes infiltration and activation, resulting in further venous wall damage and fibrosis, leading to progressive venous insufficiency and varicose veins formation. Monocytes and macrophages migrate into venous wall and valve turning patient into venous insufficiency. Venous stasis cause inflammatory cytokines releasing by monocyte and macrophage including Interleukin-1b (IL-1b), IL-6 and Tumor Necrosis Factor Alpha (TNF-α). Refluxed vein activate ECs of luminal vein and vasa vasorum, indicate by up regulation of Intercellular Adhesion Molecule 1 (ICAM-1), Interleukin 1a (IL-1a) and TNF-α [7].
Treatments of varicose veins are conventional surgery and endovenous treatment. Conventional surgical treatment is high ligation and stripping. Modern endovenous treatments are defined such as Endovenous Radiofrequency Ablation (EV-RFA), Endovenous Laser Ablation (EVLA), sclerotherapy, mechanochemical endovenous ablation, N-butyl-2-cyanoacrylate injection. High Ligation and Venous Stripping (HL/VS) is traditional treatment that cause unsatisfactory cosmetic outcome for concerning patients due to multiple incisions. However, HL/VS still indicate in some situations including superficial saphenous tributary adherent closely to skin less than 1 cm, tortuous superficial veins and aneurysmal venous segment larger than 2.5 cm, chronic thrombophlebitis and acute superficial thrombosis [8]. Endovenous treatments are the alternative treatment giving better cosmetic result and faster recovery. Rasmussen et al reported results of four-arm RCT and concluded that postoperative pain was higher in HL/VS and EVLA, although efficacy of four modalities was not significantly different [9]. In past decade, most of patients with varicose veins in our institute were treated by HL/VS and EV-RFA. We questioned in difference of inflammatory reaction between our both varicose veins treatment options. Study of inflammatory responses and clinical outcomes was designed. IL-6 secretes by T-cell and macrophage responds to tissue trauma. Smooth muscle cells produce IL-6 as a proinflammatory cytokine induced activities of B-lymphocyte and cytotoxic T-lymphocyte. C-Reactive Protein (CRP) is acute-phased reactant protein produced by inflammation and tissue trauma response of hepatocyte. CRP level start increasing in 6 to 10 hours and peaked at 36-50 hours after inflammation or trauma. It returned to normal level within 1-2 weeks [10].
This study was designed to compare the difference of preoperative and 24-hour postoperative inflammatory response from tissue trauma after HL/VS and EV-RFA, identified by serum level of IL-6 and CRP.
MATERIALS AND METHODS
Fifty-nine patients were enrolled, 32 patients were in EV-RFA group and 27 patients were in HL/ VS group. We were performed either EV-RFA or traditional venous stripping for these patient and selected operation by patient preference.
Data collection
Venous blood sampling
Diagnostic of Great Saphenous Vein (GSV) reflux
High ligation with venous stripping
Endovenous radiofrequency ablation
Postoperative evaluation and management
Statistical analysis
RESULT
|
Characteristics |
EV-RFA (32) n (%) |
HL/VS (27) n (%) |
P-value |
|
Age (years ) mean (SD) |
56.03 (14.46) |
51.89 (10.77) |
0.235 |
|
Sex 0.185 |
|||
|
male |
10 (31.3) |
13 (48.1) |
|
|
female |
22 (68.8) |
14 (51.9) |
|
|
Underlying disease |
|||
|
DM |
8 (25) |
2 (7.4) |
0.073 |
|
HT |
10 (31.3) |
8 (29.6) |
0.893 |
|
DLP |
3 (9.4) |
4 (14.8) |
0.520 |
|
Career |
|||
|
Farmer |
1 |
1 |
|
|
Government officer |
6 |
4 |
|
|
Housewife |
6 |
7 |
|
|
Teacher |
9 |
8 |
|
|
Soldier |
4 |
4 |
|
|
Shopkeeper |
4 |
3 |
|
|
Symptom |
|
|
|
|
Aching |
16 (50) |
14 (51.9) |
0.887 |
|
Heaviness |
17 (53.1) |
16 (59.3) |
0.636 |
|
Cramping |
6 (18.8) |
8 (29.6) |
0.328 |
|
Edema |
9 (28.1) |
6 (22.2) |
0.604 |
|
Hyper pigmentation |
5 (15.6) |
2 (7.4) |
0.331 |
|
Superficial vein 0.780 |
|||
|
1-3 mm |
7 (21.9) |
4 (14.8) |
|
|
3-5 mm |
21 (65.6) |
19 (70.4) |
|
|
>5 mm |
4 (12.5) |
4 (14.8) |
|
|
GSV 0.513 |
|||
|
<5 mm |
0 (0) |
1 (3.7) |
|
|
5-10 mm |
27 (84.4) |
21 (77.8) |
|
|
>10 mm |
5 (15.6) |
5 (18.5) |
|
|
Clinical |
EV-RFA (32) n (%) |
HL/VS (27) n (%) |
P-value |
|
Pain score < 0.001* |
|||
|
Mean±SD |
1.41±1.1 |
3.89±1.53 |
|
|
Median (min-max) |
1 (0-4) |
4 (1-7) |
|
|
Ecchymosis < 0.001* |
|||
|
no |
29 (90.6) |
11 (40.7) |
|
|
yes |
3 (9.4) |
16 (59.3) |
|
|
Numbness 0.456 |
|||
|
no |
31 (96.9) |
25 (92.6) |
|
|
yes |
1 (3.1) |
2 (7.4) |
|
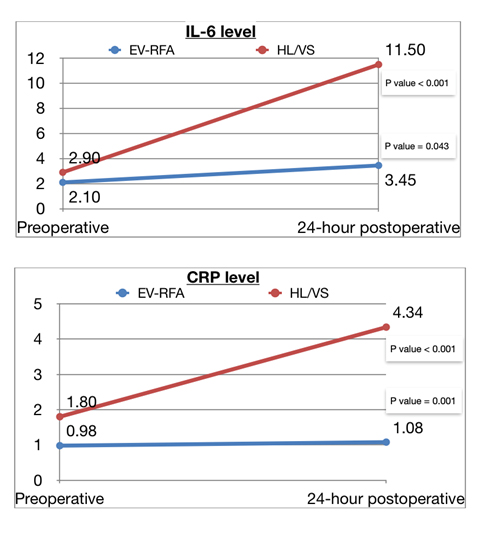
Mean VAS was significantly lower in EV-RFA group compare to HL/VS (1.41±1.1 vs 3.89±1.53, p < 0.001). Postoperative ecchymosis was also significantly lower in EV-RFA (9.4% vs 59.3%, p < 0.001). Numbness was not significantly among two groups (3.1% vs 7.4%, p = 0.456). There was no significant difference of median baseline level of IL-6 between EV-RFA and HL/VS group (p = 0.05), however, there was significant difference of median baseline level of CRP among two groups. (p = 0.045). Both IL-6 and CRP level increased significantly from preoperative to 24-hour postoperative in both EV-RFA (p < 0.001 and p = 0.043, respectively) and HL/VS (p < 0.001 and p =0.001, respectively) group. Rising of IL-6 and CRP level were lower in EV-RFA group compare to HL/VS group (p < 0.001 and p = 0.045, respectively) (Tables 3-5) (Figure 1).
|
|
EV-RFA |
Venous stripping |
P-value |
|
|
Median |
Median |
|
|
IL-6 |
2.10(1.5-9.39) |
2.90(1.5-23.5) |
0.05 |
|
CRP |
0.98(0.0-7.13) |
1.8(0.14-12.45) |
0.045 |
|
|
Median |
p-value |
|
IL-6 |
||
|
EV-RFA |
< 0.001 |
|
|
Preoperative |
2.10(1.5-9.39) |
|
|
Postoperative |
3.45(2.0-16.91) |
|
|
HL/VS |
0.043 |
|
|
Preoperative |
2.90(1.5-23.5) |
|
|
Postoperative |
11.50(3.5-179.6) |
|
|
CRP |
||
|
EV-RFA |
< 0.001 |
|
|
Preoperative |
0.98(0.0-7.13) |
|
|
Postoperative |
1.08(0.19-13.12) |
|
|
HL/VS |
0.001 |
|
|
Preoperative |
1.8(0.14-12.45) |
|
|
Postoperative |
4.34(0.17-93.46) |
|
|
|
EV-RFA |
HL/VS |
p-value |
|
|
Median |
Median |
|
|
IL-6 |
1.15(-3.44-12.2) |
8.61(-1.1-174.8) |
<0.001 |
|
CRP |
0.11(-1.11-11.25) |
0.7(-3.49-86.98) |
0.045 |
DISCUSSION
Rasmussen et al published four-arm RCT compared results of HL/VS, EVLA and EV-RFA and foam sclerotherapy. They found that postoperative pain was significantly lower and return to normal activity time was significantly shorter in EV-RFA and foam sclerotherapy group com- pared to HL/VS and EVLA group. However efficiency of treatment was not significantly difference among 4 modalities [9].
Kheirelseid et al reported meta-analysis compared results between EV-RFA, EVLA, foam sclerotherapy and conventional HL/VS and found that there was no significant difference in recurrent rate, reintervention, neovascularization, and GSV recanalization among 4 groups [12]. Rasmussen published a four arm RCT comparing HL/VS with EVLA, RFA, and foam sclerotherapy. The post-operative average pain scores at 10 days were significantly lower in the groups treated with EV-RFA and foam sclerotherapy compared with HL/VS and EVLA, with a shorter time to resumption of normal activities and work. The efficiency of the four modalities was not significantly different [9]. Common adverse effects including ecchymosis (5.8%), paresthesia (3.4%), hyperpigmentation (2.4%), erythema (2.0%), hematoma (1.4%) and phlebitis (1%). Most of complications subsided within the first week [13]. Recent studies reported incidence of thrombotic events after EV-RFA and EVLA, called Endovenous Heat-Induced Thrombosis (EHIT). Systematic review and meta-analysis reported by Healy at al, including 52 published studies, show that there was rate of DVT and Pulmonary Embolism (PE) was 0.3% and 0.1%, respectively [14]. Risk factors of EHIT were large vein size (11.0+/-4.3 mm) and concomitant sclerotherapy [15].
In this study, rate of numbness was comparable to other studies (3.1% VS 3.4%) al- though rate of ecchymosis was higher (9.4% VS 5.8%) [13]. However, rate of ecchymosis, and postoperative pain were lower in EV-RFA group compared to HL/VS group. There was no DVT and PE reported in our study.
CVI is a common condition with a wide spectrum of clinical presentations. Structural and functional alterations in healthy veins lead to symptoms and signs usually seen in CVI. There were published studies described pathophysiologies of CVI in last 2 decades, recently, many studies focused on the role of inflammation and subsequent localized endothelial activation and dysfunction. There was reducing in synthesis of anti-inflammatory agents, and enhancing the expression of proinflammatory and prothrombotic molecules [16-19]. Venous reflux was thought to be the cause of venous hypertension [20]. Its consequence was the reduction of shear stress, a key regulator of endothelial activation state [20,21], which promotes pathological change of venous wall and valve [7,22,23]. Shear stress reduces activation triggers of ECs and leukocytes, enhance expression of adhesion molecules and inflammatory cells infiltration into venous wall and leaflets. This established an environment promoted local inflammation [7,23]. Changing in normal signaling of ECs through different pathways induced production of proinflammatory mediators including IL-6, IL1, TNF-α, IFN, IL-8, MCP-1. On the other hand, endothelial cells promoted releasing of agents that stimulate thrombosis such as Von willebrand factor (Vwf) [24], plasminogen activator inhibitor-1 [25], and factor VIII [26], as well as inflammatory cytokine such as C Reactive Protein (CRP) [24] and Interleukin-6 (IL-6) [24]. These agents are biomarkers of endothelial dysfunction, and their levels correlated with a higher cardiovascular risk [27]. It was described that normal venous endothelium was able to become dysfunctional, and release prothrombotic and proinflammatory factors when exposed to increased endoluminal pressure [28]. Since CVI is a condition characterized by a sustained increasing in venous pressure, stasis or reversal of blood flow affected vessels; this may promote a prothrombotic state of endothelium. Recent study showed that releasing of prothrombotic agents from activated endothelium may explain correlation established between varicose veins and DVT [29].
This study focused on 2 inflammatory mediators, IL-6 and CRP. IL-6 was produced by monocyte, macrophages, helper T-cells, ECs, VSMCs, fibroblasts and stromal cells that effect to other cells in various functions. It effected to activate B-cell in transformation to plasma cell, and activated plasma cell in antibody secretion. It was a key role for acute phase of inflammatory response, and also effected to VSMC and endothelial cell in proliferation and proatherogenic effect [30].
The relationship of IL-6 and varicose vein had been studied by Christopher R. Lattimer [30] et al., compared serum inflammatory biomarker levels from varicose veins and antecubital vein of varicose veins patient and from leg vein and antecubital vein of healthy controls. They found that the most relevant inflammatory biomarkers in CVI were IL-6, IL-8, and MCP-1. IL-6 concentration was significantly greater in varicose vein compare to antecubital vein and healthy controls. This suggested that IL-6 released from the leg and diluted once it went to arm. Finding that IL-6 was elevated dominantly in the legs of varicose veins patients may explained that IL-6 was not directly related to systemic response CVI and may represent normal physiological response to higher venous pressures, typically in the gaiter region. This may reflected the higher sensitivity of IL-6 to local increased venous pressure contrast to IL-8 and MCP-1.
However, IL-8 and MCP-1 may be more specific for systemic inflammation in CVI. Age and disease severity were shown to correlate significantly with increases in IL-6 concentration [31].
CRP is one of the acute-phase proteins. CRP is predominantly secreted by the liver at 4- 6 hours after stimulation. It duplicates every 8 hours, and peaks within 36 to 50 hours. CRP has a plasma half-life of 19 hours, and even after a single stimulus, as in a trauma or surgery, it may take several days to return to the baselines [32]. There were 3 types of CRP assays consists of Conventional CRP, High sensitivity CRP (hsCRP) and Cardiac C-Reactive Protein (cCRP).
There were diversity of clinical use example hsCRP was detected lower level of CRP that bring to use for healthy individuals, cCRP was used for identify cardiovascular risk and conventional CRP assays were indicated for use for evaluation of infection, tissue injury, and inflammatory disorders. CRP assays provide information for the diagnosis, therapy, and monitoring of inflammatory diseases [33]. CRP is a more sensitive and more reliable indicator of acute inflammatory processes than the Erythrocyte Sedimentation Rate (ESR) and leukocyte count. Blood CRP levels rise more rapidly than ESR, and after the disease has subsided CRP values rapidly fall and reach the reference interval often days before ESR has returned to normal. Thus, CRP assays was applied to this study [34,35].
There is a correlation between increasing levels of IL-6 during inflammation and increasing levels of CRP [36], with IL-6 inducing the CRP gene [37]. CRP gene was protein from pentraxin family that encode to serum level of CRP , There were many studies that focus association of CRP gene variant to CRP level example that the minor alleles of rs1205 and rs1800947 are associated with lower CRP levels and that the minor allele of rs1130864 is associated with higher CRP levels, There were many studies that give priority association of variant type of CRP gene and many disease especially cardiovascular disease, stroke, hypertension and metabolic disease [38-40]. On the other hand, When CRP levels become elevated in atheroma, this leads to the induction of IL-6 by macrophages indicating that CRP may have a direct effect on IL-6 release [41].
CRP has half life of 19 hours and gets peak level at 36-50 hours [42], whereas IL-6 has half life of 2-3 hours and gets peak level at 12-18 hours [43]. Because half life of CRP is longer than IL-6, 24-hr postoperative level of CRP increased less than IL-6. However both CRP and IL- 6 level significantly increased 24 hour after surgery.
The inflammatory response (postoperative serum level - preoperative serum level of IL- 6 and CRP) between EV-RFA group and HL/VS group represented both of inflammatory cytokine could be predicted inflammatory response of both operation but significantly increasing in serum level of IL-6 due to shorter half life of this cytokine. In addition the little increasing of both inflammatory cytokine levels in EV-RFA group that implied minimal inflammatory response in this operation and hardly interfere inflammatory process in human.
Selection bias is main limitation of this study. There was no randomization of patients and no well control of postoperative program. Further well designed RCT need to confirm advantage of EV-RFA compare to other modalities in aspect of inflammatory response. However cost-effectiveness of each procedure is interesting issue to be concerned.
CONCLUSION
REFERENCES
- Shabani Varaki E, Gargiulo GD, Penkala S, Breen PP (2018) Peripheral vascular disease assessment in the lower limb: A review of current and emerging non-invasive diagnostic methods. Biomed Eng Online 17: 61.
- Rabe E, Pannier F (2012) Clinical, aetiological, anatomical and pathological classification (CEAP): Gold standard and limits. Phlebology 1: 114-118.
- Raffetto JD, Mannello F (2014) Pathophysiology of chronic venous disease. Int Angiol 33: 212-221.
- Atta HM (2012) Varicose veins: Role of mechanotransduction of venous hypertension. Int J Vasc Med 2012: 13.
- Mannello F, Ligi D, Canale M, Raffetto JD (2014) Omics profiles in chronic venous ulcer wound fluid: Innovative applications for translational medicine. Expert Rev Mol Diagn 14: 737-762.
- Satokowa H, Hoshino S, Igari T, Iwaya F, Midorikawa H (2002) The appearance of cytokines and adhesion molecules in saphenous vein valves in chronic venous insufficiency. Phlebology 16: 106-110.
- Takase S, Bergan JJ, Schmid-Schonbein G (2000) Expression of adhesion molecules and cytokines on saphenous veins in chronic venous insufficiency. Ann Vasc Surg 14: 427-435.
- Sidawy AP, Perler BA (2018) Rutherford's Vascular Surgery and Endovascular Therapy (9th Edn), Elsevier 2: 2832.
- Rasmussen L, Lawaetz M, Serup J, Bjoern L, Vennits B, et al. (2013) Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy, and surgical stripping for great saphenous varicose veins with 3-year follow-up. J Vasc Surg Venous Lymphat Disord 1: 349-356.
- Blake GJ, Ridker PM (2001) Novel clinical Markers of vascular wall inflammation. Circ Res 89: 763-771.
- Hosoi Y, Zukowski A, Kakkos SK, Nicolaides AN (2002) Ambulatory venous pressure measurements: new parameters derived from a mathematic hemodynamic model. J Vasc Surg 36: 137-142.
- Bonetti PO, Lerman LO, Lerman A (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168-175.
- Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, et al. (2003) New markers of inflammation and endothelial cell activation: Part I. Circulation 108: 1917-1923.
- Carrasco OF1, Ranero A, Hong E, Vidrio H (2009) Endothelial function impairment in chronic venous insufficiency: effect of some cardiovascular protectant agents. Angiology 60: 763-771.
- Constans J, Conri C (2006) Circulating markers of endothelial function in cardiovascular disease. Clin Chim Acta 368: 33-47.
- Nicolaides AN (2000) Investigation of chronic venous insufficiency: a consensus statement (France, March 5-9, 1997). Circulation 102: 126-163.
- White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJ, et al. (2011) Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol 226: 2841-2848.
- Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH (2012) The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res 49: 463-478.
- Takase S, Schmid-Schonbein G, Bergan JJ (1999) Leukocyte activation in patients with venous insufficiency. J Vasc Surg 30: 148-156.
- Poredos P, Spirkoska A, Rucigaj T, Fareed J, Jezovnik MK (2015) Do blood constituents in varicose veins differ from the systemic blood constituents? Eur J Vasc Endovasc Surg 50: 250-256.
- Blomgren L, Johansson G, Siegbahn A, Bergqvist D (2001) Coagulation and fibrinolysis in chronic venous insufficiency. Vasa 30: 184-187.
- Criado PR, Alavi A, Kirsner RS (2014) Elevated Levels of Coagulation Factor VIII in Patients with Venous Leg Ulcers. Int J Low Extrem Wounds 13: 130-134.
- Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, et al. (2009) Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol 54: 601-608.
- Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, et al. (2014) Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J 35: 448-454.
- Müller-Bühl U, Leutgeb R, Engeser P, Achankeng EN, Szecsenyi J, et al. (2012) Varicose veins are a risk factor for deep venous thrombosis in general practice patients. Vasa 41: 360-365.
- Sprague AH, Khalil RA (2009) Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539-552.
- Lattimer CR, Kalodiki E, Geroulakos G, Hoppensteadt D, Fareed J (2016) Are Inflammatory Biomarkers Increased in Varicose Vein Blood? Clin Appl Thromb Hemost 22: 656-664.
- Mitaka C (2005) Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta 351: 17-29.
- Kind CRH, Pepys MB (1984) The role of C-reactive Protein (CRP) Measurement in Clinical Practice. Int Med 5: 112-151.
- Dixon JS, Bird HA, Sitton NG, Pickup ME, Wright V (1984) C-reactive protein in the serial assessment of disease activity in rheumatoid arthritis. Scand J Rheumatol 13: 39-44.
- Gambino R (1989) C-Reactive Protein: An Underutilized Test. Lab Report for Physicians 11:41.
- Szalai AJ, van Ginkel FW, Dalrymple SA, Murray R, McGhee JR, et al. (1998) Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol 160: 5294-5299.
- Weinhold B, Bader A, Poli V, Rüther U (1997) Interleukin-6 is necessary, but not sufficient, for induction of the humanC-reactive protein gene in vivo. Biochem J 325: 617-621.
- Miller DT, Zee RY, Suk Danik J, Kozlowski P, Chasman DI, et al. (2005) Association of common CRP gene variants with CRP levels and cardiovascular events. Ann Hum Genet 69: 623-638.
- Suk Danik J, Chasman DI, Cannon CP, Miller DT, Zee RY, et al. (2006) Influence of genetic variation in the C-reactive protein gene on the inflammatory response during and after acute coronary ischemia. Ann Hum Genet 70: 705-716.
- Lee CC, You NC, Song Y, Hsu YH, Manson J, et al. (2009) Relation of genetic variation in the gene coding for C-reactive protein with its plasma protein concentrations: findings from the Women's Health Initiative Observational Cohort. Clin Chem 55: 351-360.
- Han KH, Hong KH, Park JH, Ko J, Kang DH, et al. (2004) C-reactive protein promotes monocyte chemoattractant protein-1--mediated chemotaxis through upregulating CC chemokine receptor 2 expression in human monocytes. Circulation 109: 2566-2571.
- Gerhartz C, Dittrich E, Stoyan T, Rose-John S, Yasukawa K, et al. (1994) Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur J Biochem. 223: 265-274.
- Kuribayashi T (2018) Elimination half-lives of interleukin-6 and cytokine-induced neutrophil chemoattractant-1 synthesized in response to inflammatory stimulation in rats. Lab Anim Res 34: 80-83.
- Kheirelseid EAH, Crowe G, Sehgal R, Liakopoulos D, Bela H, et al. (2018) Systematic review and meta-analysis of randomized controlled trials evaluating long-term outcomes of endovenous management of lower extremity varicose veins. J Vasc Surg Venous Lymphat Disord 6: 256-270.
- Proebstle TM, Alm J, Göckeritz O, Wenzel C, Noppeney T, et al. (2011) Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg 54: 146-152.
- Healy DA, Kimura S, Power D, Elhaj A, Abdeldaim Y, et al. (2018) A Systematic Review and Meta-analysis of Thrombotic Events Following Endovenous Thermal Ablation of the Great Saphenous Vein. Eur J Vasc Endovasc Surg 56: 410-424.
- Sermsathanasawadi N, Pitaksantayothin W, Puangpunngam N, Chinsakchai K, Wongwanit C, et al. (2018) Incidence, Risk Factors, Progression, and Treatment of Endovenous Heat-Induced Thrombosis Class 2 or Greater After Endovenous Radiofrequency Ablation. Dermatol Surg 1-8.
Citation: Taengsakul N, Saikaew T, Chaiaroon N, Urasuk T, Panoi A, et al. (2019) The Matter of Inflammatory Responses in Endovenous Radiofrequency Ablation and Conventional Venous Stripping. J Angiol Vasc Surg 4: 021.
Copyright: © 2019 Nawaphan Taengsakul, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

