
Stepwise Approach for Effectiveness and Outcome Impact of Repaired Moderate Rheumatic Mitral Regurgitation during Aortic Valve Replacement for Severe Aortic Stenosis
*Corresponding Author(s):
Ahmed SaberDepartment Of Cardiothoracic Surgery, Faculty Of Medicine, Cairo University, Cairo, Egypt
Email:ahmedsaber78@yahoo.com
Abstract
Objectives
The optimal management of rheumatic moderate mitral regurgitation in cases with severe aortic stenosis remains not well defined and it is frequently not corrected as it is claimed to improve after AVR and prompt myocardial remodeling. We evaluated the effectiveness and outcome impact of repaired mitral valve on clinical and echocardiographic parameters of the patient over follow-up of six-month duration.
Methods
This prospective comparable study was conducted between January 2016 and June 2018 in Egypt (Department of Cardiothoracic Surgery, Cairo University, and other open heart surgery centers). One hundred and thirty patients diagnosed with severe aortic stenosis and moderate rheumatic mitral regurgitation was involved in the study. Half of them (Group A) were offered aortic valve replacement and mitral valve repair with a remodeling ring annuloplasy, and the other half were offered aortic valve replacement only. Preoperative, intraoperative, postoperative, and at six-month post-surgery follow-up echocardiography was done as well as clinical correlation assessment. We excluded patients with echocardiographic evidence of mitral valve apparatus pathology (rheumatic or non-rheumatic) necessitating its replacement. Also patients with associated moderate-to-severe tricuspid valve regurgitation requiring concomitant repair or replacement were excluded. We also did not involve patients with concomitant coronary artery disease as well as those having aortic aneurysms or dissections necessitating intervention.
Results
Patients of both groups had properly-matching preoperative demographic data. Mean age was 40.34 ± 6.81 years in group A and 43.60 ± 6.53 in group B. Male patients represented 61.53% in group A and 53.84% for group B. Group A involved 48 (73.84%) patients in NYHA class III classification; versus 51 (78.46%) patients in group B. Mean preoperative LVEF% was 60.2 ± 5.1% for group A patients; versus 61.6 ± 7.5% for group B patients. Echocardiographic data obtained in the early postoperative and at 6-months follow-up were compared with the preoperative profile. We had the sum of 5 patients (3.84%) mortality in both groups. All patients expressed improvement of clinical symptoms of mitral regurgitation at time of hospital discharge. And at 6-months evaluation, clear statistical significance emerged where patients of group A had significant improvement of mitral regurgitation degree (improved in 75.38% compared to 50.77% of group B patients), significant improvement of MR jet area (3.91 ± 0.8 cm2compared to 4.78 ±0.9 cm2 in group B patients), and significant improvement of NYHA class (21.53% of group A in NYHA class I compared to none in group B, 78.46 % of group A compared to 46.15 % of group B in NYHA class II and 53.84% of group B remained in NYHA class III compared to none in group A).
Conclusion
The presence of moderate rheumatic mitral regurgitation in patients who undergo aortic valve replacement for severe aortic stenosis does not affect the immediate postoperative or early (6-months) follow-up outcome. However, statistically significant differences were found between both groups concerning the regression of the Moderate Rheumatic MR and improvement of the MR jet area; which reflects the effect of concomitant mitral valve repair on improving both the outcome of Moderate Rheumatic MR and the clinical status of the patients. However, operative parameters were statistically significant i.e. total operative time, total bypass time and total cross clamp time between both groups. Thus, patients with LV dysfunction should be considered carefully for this option selection. We recommend considering a procedure addressing the rheumatic mitral valve with moderate regurgitation in the setting of AVR for severe AS with great concern to those with a worse preoperative left ventricular profile.
Keywords
Aortic stenosis; Aortic valve replacement; Ring annuloplasty; Rheumatic mitral regurgitation
INTRODUCTION
The efficacy of Aortic Valve Replacement (AVR) for the treatment of patients with severe Aortic Stenosis (AS) has improved to the point where surgery has become the standard of care [1]. However, the appropriate management of concomitant rheumatic moderate Mitral Regurgitation (MR) in the setting of severe AS among patients submitted for AVR remains undefined. It is frequently not corrected because it is claimed that it may improve after AVR and subsequent myocardial remodeling; however, data supporting this assumption are sparse. Nevertheless, MR of varying degrees has been reported in up to 75% of patients undergoing AVR [2].
MR per se can be caused by a variety of pathological aetiologies. The post-acute rheumatic fever in eastern communities is as high as 100/100.000 compared to 2/100.000 (USA), is the most common type followed by the ischemic and the functional types [3].
Secondary (Functional) moderate MR (FMR) may improve after AVR and coexistent mitral replacement or repair may thus become not indicated [4]. However, if the mitral valve has signs of organic disease (rheumatic affection), coexistent mitral surgery (repair or replacement), at this moment, will be necessary. However, patients with severe AS and moderate MR who have a normal size left ventricle (< 45 mm in end-systolic diameter) before AVR are more likely to experience both persistent MR and the composite heart failure outcome. This suggests that Left Ventricular (LV) size remodeling is an important mechanism by which MR can improve in these patients postoperatively [5].
Relatively few clinical studies have examined the clinical impact and fate of Moderate Rheumatic MR in patients undergoing AVR for severe AS [6]. The decision to carry out combined surgical correction is still posing a surgical challenge, as the ability to predict the progress of preoperative MR following AVR still needs more proofs especially in the post-rheumatic aetiology. We are focusing on the beneficial value of concomitant mitral valve ring annuloplasty and its impact on the clinical and echocardiographic outcome.
PATIENTS AND METHODS
Study design
Inclusion and exclusion criteria
Management protocol
Intraoperatively: Operative data was analyzed for date of the operation, general anaesthetic technique, total bypass time, cross clamp time, total operative time, demand of inotropic support, haemodynamics, use of intraoperative Transoesophageal Echocardiography (TEE). All the patients were routinely scrubbed and draped exposing the chest. After standard median sternotomy, pericardiotomy and suspension of the pericardial edges, the ascending aorta was cannulated followed by bicaval cannulation in group A patients and common right atrial cannulation (by a two-stage common atrial cannula inserted through the right atrial appendage) in group B patients, for venous drainage. A two way cannula was inserted in the aortic root for venting and initial cardioplegia administration followed by selective coronary ostia perfusion immediately after aortotomy. Prior to cannulation, Heparine 350 U/Kg was administered. After institusion of cardiopulmonary bypass, cooling started to achieve systemic temperature of 28-30 centigrade. The aorta is then clamped and myocardial protection was achieved by selective intermittent antegrade infusion of cold crystalloid cardioplegia every 30-40 minutes. All patients were submitted for AVR but those of group A were submitted for combined mitral valve repair by ring annuloplasty sized 28-32 mm through left atriotomy incision. After completion of the procedure, the aorta was declamped and the patient was rewarmed to 37 centigrade and all electrolytes and acid-base imbalances corrected. The patient was then weaned off bypass. Protamine was then administered, followed by decannulation, haemostasis, placement of epicardial pacemaker wires and closure over retrosternal and may be pleural drains (if either pleura was opened). All monitoring lines and haemodynamic support were maintained during transfer of the patient to the Intensive Care Unit (ICU). All the patients were transferred mechanically ventilated.
Postoperatively: All patients were followed-up during ICU stay for haemodynamic status, duration of mechanical ventilation, postoperative ECG, the need and duration of inotropic support and total ICU stay. All patients were followed-up during postoperative hospital stay for perioperative mortality and morbidity, New York Heart Association (NYHA) functional class, total duration of hospital stay, medical treatment(s) needed and given, and routine early postoperative TTE (for comparable standard & specific measurements). At 6-months follow-up, data were obtained for delayed mortality and morbidity, NYHA functional class, medical treatment(s) needed and given, and TTE (for comparable standard & specific measurements).
Statistical analysis: All patients’ data were tabulated and processed using SPSS V10.0 (SPSS Inc., Chicago, IL) for Windows 98. Quantitative variables were expressed using mean and standard deviation, and were compared using t-student test. Qualitative variables were compared using Chi-square test or Fischer’s exact test when appropriate. Correlation between parameters was performed using Spearman’s rank correlation coefficient. In all tests, P value was considered significant when P < 0.05, highly significant when P < 0.01 and extremely significant when P < 0.001.
RESULTS
Preoperative data
|
Group A (n= 65) |
Group B (n= 65) |
P value |
Significance |
|
|
Age (years) |
40.34 ± 6.81 |
43.6 ± 6.53 |
> 0.05 |
NS |
|
Male/Female |
40/25 (61.53/38.46%) |
35/30 (53.84/46.15%) |
> 0.05 |
NS |
|
Positive family history |
11 (16.92%) |
13 (20.0%) |
> 0.05 |
NS |
|
Smoking |
30 (46.15%) |
27 (41.53%) |
> 0.05 |
NS |
|
Syncope |
10 (15.38%) |
14 (21.53%) |
> 0.05 |
NS |
|
Diabetes mellitus (DM) |
25 (38.46%) |
29 (44.61%) |
> 0.05 |
NS |
|
COPD |
0 (0.0%) |
2 (3.07%) |
> 0.05 |
NS |
|
NYHA III |
48 (73.84%) |
51 (78.46%) |
> 0.05 |
NS |
|
NYHA II |
17 (26.15%) |
14 (21.53%) |
> 0.05 |
NS |
|
MR murmur |
51 (78.46%) |
50 (76.92%) |
> 0.05 |
NS |
|
CHF(basal crepitations) |
3 (4.61%) |
2 (3.07%) |
> 0.05 |
NS |
|
AF |
15 (23.07%) |
13 (20.0%) |
> 0.05 |
NS |
|
Mean C/T ratio |
0.75 ± 0.03 |
0.66 ± 0.02 |
> 0.05 |
NS |
|
MR jet area (cm2) |
5.35 ± 0.3 |
5.27 ± 0.7 |
> 0.05 |
NS |
|
LVEDD (cm) |
5.83 ± 0.8 |
5.77 ± 0.7 |
> 0.05 |
NS |
|
LVESD (cm) |
4.45 ± 0.7 |
4.51 ± 0.7 |
> 0.05 |
NS |
|
LA (cm) |
4.4 ± 0.9 |
4.37 ± 0.8 |
> 0.05 |
NS |
|
EF (%) |
60.2 ± 5.1 |
61.6 ± 7.5 |
> 0.05 |
NS |
|
Max aortic gradient (mmHg) |
72 ± 3.2 |
71.6 ± 3.5 |
> 0.05 |
NS |
|
Mean aortic gradient (mmHg) |
54 ± 4.2 |
51.6 ± 2.2 |
> 0.05 |
NS |
|
Aortic valve area (cm2) |
0.57 ± 0.16 |
0.63 ± 0.23 |
> 0.05 |
NS |
Table 1: Preoperative Patients Characteristics.
Note: (Data were expressed as mean ± Standard Deviation (SD) or number (%), NS = Not Significant, t = Test of Significance).
Operative data
|
Group A |
Group B |
p value |
Significance |
|
|
Total operation time (hrs) |
5.2 ± 0.5 |
4.1 ± 0.5 |
< 0.01 |
Highly Significant |
|
Total bypass time (hrs) |
3.7 ± 0.5 |
2.3 ± 0.7 |
< 0.01 |
Highly Significant |
|
Total cross clamp time (hrs) |
1.6 ± 0.3 |
1.1 ± 0.4 |
< 0.01 |
Highly Significant |
|
Smooth weaning off CPB |
48 (73.84%) |
50 (76.92%) |
> 0.05 |
NS |
Table 2: Intraoperative Data.
Note: (Data were expressed as mean ± Standard Deviation (SD) or number (%), NS = Not Significant.
Postoperative data
|
Group A |
Group B |
p value |
Significance |
|
|
Mean period ICU stay(days) |
2.52 ± 0.4 |
2.17 ± 0.3 |
> 0.05 |
NS |
|
Duration of mechanical ventilation (hrs) |
6.1 ± 1.4 |
5.4 ± 2.5 |
> 0.05 |
NS |
|
Inotropic support (hrs) |
12.9 ± 4.6 |
12.2 ± 4.8 |
> 0.05 |
NS |
|
Total blood loss (ml) |
547.5 ± 411.1 |
431.17 ± 355.2 |
> 0.05 |
NS |
|
Reoperation for bleeding |
2 (3.07%) |
1 (1.53%) |
> 0.05 |
NS |
|
Wound infection |
3 (4.61%) |
2 (3.07%) |
> 0.05 |
NS |
|
Pneumonia |
1 (1.53%) |
0 (0.0%) |
> 0.05 |
NS |
|
Postoperative Mortality |
3 (4.61%) |
2(3.07%) |
> 0.05 |
NS |
|
Total hospital stay(days) |
8.47 |
8.24 |
> 0.05 |
NS |
|
LVEDD (cm) |
0.5 ± 5.64 |
0.3 ± 5.72 |
> 0.05 |
NS |
|
LVESD (cm) |
4.43 ± 0.7 |
4.48 ± 0.8 |
> 0.05 |
NS |
|
LA (cm) |
4.35 ± 0.8 |
4.30 ± 0.8 |
> 0.05 |
NS |
|
EF (%) |
5.8 ± 51.27 |
5.6 ± 53.47 |
> 0.05 |
NS |
|
MR jet area (cm2) |
4.83 ± 0.6 |
0.7 ± 5.07 |
< 0.01 |
Highly Significant |
|
MR grade 1+ (mild MR) |
16 (24.61%) |
0 (0%) |
< 0.01 |
Highly Significant |
|
MR grade 1+ to 2+(mild-moderate MR) |
33 (50.77%) |
17 (26.15%) |
< 0.01 |
Highly Significant |
|
MR grade 2+ (moderate MR) |
16 (24.61%) |
48 (73.84%) |
< 0.01 |
Highly Significant |
Table 3: Postoperative Data. Note: (Data were expressed as mean ± Standard Deviation (SD) or number (%), NS = Not Significant.
Follow-up data
All the patients had a TTE done in the follow-up period. Table 4 outlines the results of the 6-months follow-up evaluation TTE. As can be seen from the table, there was statistically significant difference regarding MR jet area between the two groups. Furthermore, there were statistically significant differences in the 6-months postoperative MR jet area, LVEDD, LVESD and EF as compared to the preoperative TTE in both groups (p < 0.01).
|
Group A |
Group B |
p value |
Significance |
|
|
MR jet area (cm2) |
3.91 ± 0.8 |
4.78 ± 0.9 |
< 0.01 |
Highly Significant |
|
LVEDD (cm) |
5.46 ± 0.7 |
5.57 ± 0.7 |
> 0.05 |
NS |
|
LVESD (cm) |
4.25 ± 0.7 |
4.33 ± 0.7 |
> 0.05 |
NS |
|
LA (cm) |
4.25 ± 0.9 |
4.30 ± 0.6 |
> 0.05 |
NS |
|
EF (%) |
66.27 ± 5.8 |
64.87 ± 6.5 |
> 0.05 |
NS |
|
MR grade 1+ (mild MR) |
25 (38.46%) |
0 (0%) |
< 0.01 |
Highly Significant |
|
MR grade 1+ to 2+(mild-moderate MR) |
24 (36.92%) |
(%50.77) 33 |
> 0.05 |
NS |
|
MR grade 2+ (moderate MR) |
16 (24.61%) |
32 (49.23%) |
< 0.01 |
Highly Significant |
Table 4: 6-months follow-up evaluation TTE.
Note: (Data were expressed as mean ± Standard Deviation (SD) or number (%), NS = Not Significant.
DISCUSSION
Despite the frequency with which MR is seen in cases with severe AS, there remains controversy regarding its management. There is general agreement (and necessity) that patient with severe (3+ or 4+) MR should undergo mitral valve surgery (repair or replacement) at the setting of AVR for severe AS. However, the importance of moderate (2+) MR in such patients is controversial and different clinical studies conflict regarding the correction of moderate MR at the time of AVR for severe AS based on the studies reporting that it may improve after AVR [8]. Some reports have shown improvement in MR in up to half of patients undergoing AVR only but the majority has involved relatively small sample sizes and is confounded by the inclusion of patients with FMR or ischemic heart disease [9]. Furthermore, relatively few studies have examined the clinical impact of MR in patients undergoing AVR. Most of these series were small, and none has focused on Heart Failure (CHF) outcomes [6]. For the previous rationale, and rarity of researches focusing selectively on rheumatic moderate MR in association with severe AS cases, we sought to track the course, magnitude of change and fate of moderate rheumatic MR in patients who require surgical treatment (AVR) for severe AS. We are focusing on the beneficial value of concomitant mitral valve ring annuloplasty and its impact on the clinical and echocardiographic outcome and our goal was to involve assessment of the impact of AVR on Moderate Rheumatic MR in the immediate and early postoperative (6-months follow-up) periods regarding patients survival and morbidity events. This effort is in a trial to reach any valuable recommendations (which are sparse in the literature) that facilitates the identification and selection of those patients with this particular rheumatic pathology most likely to benefit from concomitant mitral valve repair.
This prospective comparable study comprised 130 patients with severe AS and concomitant moderate rheumatic MR. 65 patients were submitted for combined AVR and mitral valve repair via remodeling ring annuloplasty (Group A). The other 65 patients were submitted for isolated AVR (Group B). Most prior studies were retrospective like those reported by Ruel, et al., [9], Wan, et al., [10], Koji, et al., [11]. We comparatively reviewed our results focusing on immediate & early postoperative (6-months follow-up) outcome of moderate rheumatic MR concomitant with severe AS in patients undergoing either combined AVR with mitral valve repair versus isolated AVR.
The preoperative baseline patient’s characteristics of both patients groups were similar with no statistically significant differences. It was more or less similar to patients investigated in other studies [9-11]. The majority of the patients in our study were in NYHA class III (73.84% of group A and 78.46% of group B) while the rest were in NYHA class II. This resembles the percentage of patients in NYHA class III stated by other authers like Wan, et al., [10] who had 78% of their patients in NYHA class III or IV. However, Ruel, et al., [9] had 18.7% of their patients in NYHA class IV and 81.3% in a less NYHA class. Moreover, in the study conducted by Wan, et al., congested patients constituted 72% of patients compared to 4.61% in our group A patients and 3.07% of group B patients. In the study by Wan, et al., 92% of the patients in their study had MR 2+ and 8% had MR 3+ as opposed to 100% of our patients with rheumatic MR 2+. 20% of their patients were in NYHA class IV as compared to 0% in our series. 28% of their patients had preoperative Atrial Fibrillation (AF) as opposed to 23.07% of our group A patients and 20.00% of group B. Finally, their patients had a mild LV dysfunction with a mean Left Ventricular Ejection Fraction (LVEF%) of 43 ± 16% as compared to 60.2 ± 5.1 % for group A patients and 61.6 ± 7.5% for group B patients of our series (preserved LVEF%). The series by Ruel and colleagues was conducted on 88.8% of the patients with MR 2+ (and the rest 11.2 % with MR 3+) and LVEF% of 49.5% (mildly impaired overall systolic function). However, they did not report on either using or the dosage of any preoperative anti-failure medications which could have favorably affected the symptomatology of such patients. Thus, our study population showed better preoperative profile as regards LVEF and CHF. Furthermore, 100% of our patients had only rheumatic mitral valve pathology. Thus, our study group population showed a more homogenous sample.
Our study group population- with median age 42.5 years- were younger than other series. The mean age of the study group by Wan, et al., was 74 ± 11 years and 45% were males and that by Ruel, et al., was 69 ± 11.6 years with 63.5 % males. Our study showed that the mean age for group A patients was 40.34 ± 6.81 years with 61.53 % males and for group B patients 43.6 ± 6.53 years with 53.84% males. This comparable male younger age study population might explain the lower incidence of perioperative co-morbidities.
Our preoperative TTE parameters showed no statistically significant differences among the two study groups with comparable data with other series. In the study by Wan and colleagues, they had LVEDD of 6.6 ± 1.0 cm and LVESD of 5.3 ± 1.3 cm. Koji, et al., demonstrated smaller Left Ventricular Dimensions with LVEDD of 5.5 ± 1.1 cm and LVESD of 3.8 ± 1.1 cm [11]. However, in our study, LVEDD was 5.83 ± 0.8 cm for group A and 5.77 ± 0.7 cm for group B. LVESD was 4.45 ± 0.7 cm for group A and 4.51 ± 0.7 cm for group B. Our patients had a preserved LVEF% (60.2 ± 5.1% for group A patients and 61.6 ± 7.5% for group B patients) as opposed to mild LV dysfunction faced in other reports (43 ± 16% in the study by Wan, et al., 49.5% in the series by Ruel and colleagues and 57 ± 12% in the study done by Koji and co-workers). MR jet area in our series was 5.35 ± 0.3 cm2 for group A patients and 5.27 ± 0.7 cm2 for group B with no statistically significant differences. Data and comment on this item was not given in prior reports. Moreover, in our study, no correlation was found between the preoperative left atrial dimension and the presence of moderate MR which is not an expected finding. Our logic explanation was in the fact that our patients had a preserved LV function, a point that was rarely discussed in previous studies.
Statistically significant differences have been concluded in our study operative results that merit mentioning. Comparing the lengths of total operative time, the total cardiopulmonary bypass time and the total cross clamp (ischemic) time between both groups showed high statistical significance (p < 0.01). This reflected a “longer time” needed for performing mitral valve ring annuloplasty repair concomitant with AVR as opposed to single aortic valve prosthesis. However, no statistically significant differences were found intraoperatively as regards weaning off CPB, the use and dosage of inotropic support, other adjunctive models to achieve weaning off bypass (eg: electrical cardioversion) and haemodynamic parameters (including heart rate, blood pressure control, urine output & CVP). These results proved that presence of moderate rheumatic MR did not additionally burden patients undergoing AVR in the operative setting as LVEF% in these patients is expected to suffer in accordance to the “longer time” needed for the composite surgery of AVR & mitral valve repair. Strangely enough, this observation has not been thoroughly studied in the literature. A possible reason is that most other studies were performed retrospectively. A point of concern that has to be discussed in our operative results is the downgrading of MR by the intraoperative TEE. It downgraded the degree of MR to mild-moderate degree in 33 patients (50.76%) of group A while it remained as it is (moderate MR) in group B population (p < 0.01) showing highly significant statistical difference. Data of intraoperative TEE and its importance were not addressed in prior reports.
Most authors addressed mid-term and long-term survival and the late improvement in functional class to illustrate the impact of MR and suffice with stating the in-hospital mortality to represent the immediate postoperative results [9-12]. Our post-operative in-hospital mortality rate was 4.61% in group A and 3.07% in group B comparable to 2.6% in the study by Koji, et al., and 5% in the study by Wan, et al., figures that might be attributed to long retrospective studies data they used. There were no statistically significant differences as regards period of mechanical ventilation, dosage or length of time patients were on inotropic support, total blood loss, perioperative morbidity or mortality and total period of ICU or hospital stay. Again, these results can conclude that the presence of moderate MR did not add an additional burden to patients undergoing AVR in the immediate postoperative period and total duration of hospital stay (Figures 1-3).
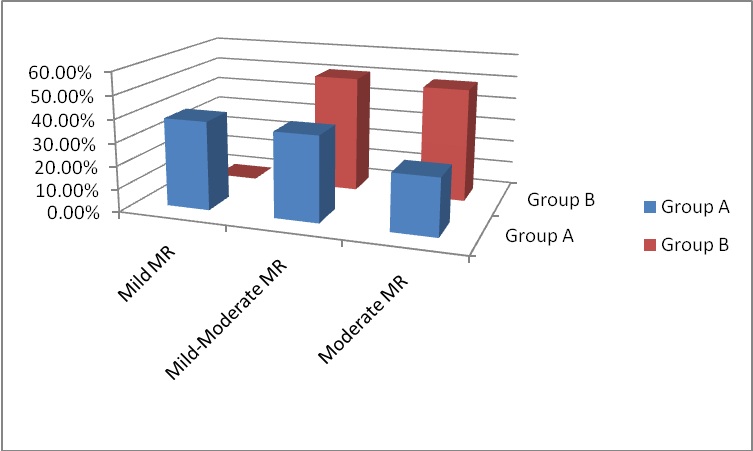 Figure 1: MR grading at 6-months follow-up.
Figure 1: MR grading at 6-months follow-up.
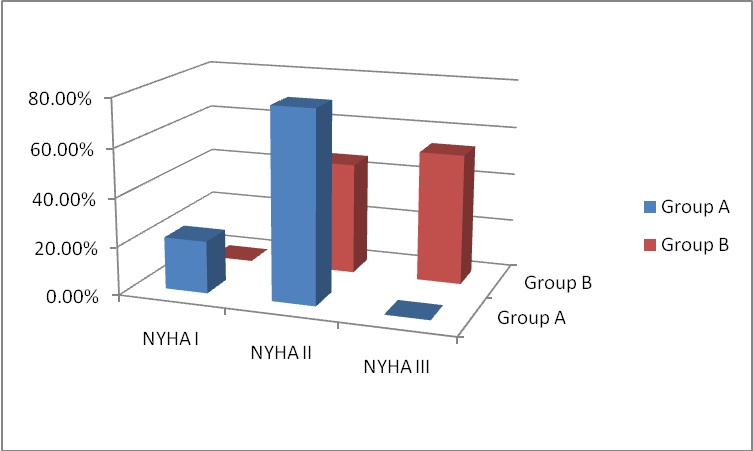 Figure 2: NYHA Class at 6-months follow-up.
Figure 2: NYHA Class at 6-months follow-up.
 Figure 3
Figure 3
The LA dimension, LVEDD and LVESD showed minute improvement in either group of patients without significant statistical differences. However, The MR jet area improved significantly in group A compared to the preoperative measurement with high statistical significance compared to group B in the early postoperative TTE results. In our opinion, this was reflected on the result of the magnitude of the moderate MR in the early postoperative period. Group A patients showed improvement of the moderate MR to mild grade (1+) in 24.61% of patients and to mild-moderate grade (1+ to 2+) 50.7 %. Group B patients showed improvement to mild-moderate grade (1+ to 2+) in 26.15 % and the remaining 73.84 % sustained moderate MR without significant effect on their survival. Although there is discrepancy in the literature as to agreement with these results, in their study, Wan and colleagues agree with our results. In their patients, 78% improved to mild grade MR, 17% unchanged moderate MR and 4% worsened to moderate-severe MR. Koji, et al., also agree with our results. They had 69.6% improvement to mild MR, 30.4% sustained moderate MR and 0% worsening of the moderate MR.
At 6-months follow-up, NYHA class improved in both study groups. This was statistically significant between the two groups and when compared to the preoperative data, where 78.46% and 21.53% of group A were in NYHA class II and I respectively; and 46.15% and 53.84% of group B in NYHA class II and III respectively (p < 0.01). Preoperatively, 73.84% of group A patients and 78.46% of group B patients were in NYHA class III and the rest were in NYHA class II. In the study by Wan, et al., 89% of their patients were in NYHA class I or II symptom. These results can conclude that repaired MR had an early good impact on the clinical status and symptomatology of the patients.
The 6-months follow-up TTE revealed significant improvement in the LVEF%, LA, LVEDD and LVESD of both patient groups compared to the preoperative baseline TTE with no statistically significant differences between the two groups. The MR jet area improved significantly between the two groups and as compared to the preoperative measurement showing statistically significant differences between both groups. Consequently, MR grade improved and the higher preoperative NYHA class showed significant regression with better survival. In group A patients, MR showed regression in 75.38% of patients (38.46% had MR 1+ and 36.92% had MR 1+ to 2+). Group B patients had 50.77% regression of MR 1+ to 2+). The rest 24.61% of group A patients and 49.23% of group B patients had remained moderate MR but without significant effect on survival. Thus, there were statistically significant differences among both groups again addressing the good impact of repaired MR. Wan and colleagues had follow-up echocardiographic data in 57% of their patients at mid-term and showed comparable results to ours. 67% of their patients showed improvement of the MR and 33% had sustained moderate MR. Koji, et al., demonstrated 73.3% improvement of MR, 52.4% had unchanged moderate MR and 6.7% had progression of it. Our patients with additional mitral valve repair showed higher incidence of freedom of the moderate MR and prevention of further progression at the 6-months postoperative evaluation.
Few studies have simultaneously examined the survival, functional outcome, and postoperative severity of MR. Barreiro, et al., examined quality of life and long-term survival in a mixed cohort that included structural as well as functional MR patients [6]. Repaired rheumatic moderate MR decreased in the majority of our patients postoperatively. This finding was most prevalent in the literature in patients with mild to moderate MR preoperatively, but the functional and echocardiographic impact of associated conditions was not examined. One study, which used routine preoperative and postoperative echocardiography, found preoperative left atrial diameter and pulmonary artery pressure to be related to postoperative MR severity. However, this study did not link echocardiographic data to functional outcomes and included patients with functional and structural MR [3]. The results of our study demonstrated that Moderate Rheumatic MR improves significantly in patients undergoing AVR for severe AS when it was repaired. Furthermore, progression of this moderate MR in this population seems to be uncommon, as is reoperation for MR. Consecuently, the NYHA functional status improved in the majority of these patients.
In conclusion, it was apparent from the above elucidated study results that the presence of Moderate Rheumatic MR in patients who undergo AVR for severe AS does not affect the immediate postoperative or early (6-months) follow-up outcome. However, statistically significant differences were found between both groups concerning the regression of the Moderate Rheumatic MR and improvement of the MR jet area and grading; which reflects the effect of concomitant mitral valve repair on improving both the outcome of Moderate Rheumatic MR and the clinical status of the patients. However, operative parameters were statistically significant i.e. total operative time, total bypass time and total cross clamp time between both groups. Thus, patients with LV dysfunction should be considered carefully for this option selection. We recommend considering a procedure addressing the rheumatic mitral valve with moderate MR in the setting of AVR for severe AS with great concern to those with a worse preoperative left ventricular profile.
STUDY LIMITATIONS
This study, however, is not free from limitations. The most important one is the short period of postoperative follow-up. Longer periods of time are needed to assess the impact of persisting postoperative moderate rheumatic MR on the functional status and survival of patients after AVR and to further assess repaired mitral valve. This may present a clue for addressing more patients to get benefit from concomitant mitral valve repair. Our study groups of population comprised younger age groups than other series with still preserved LV function. Thus, these patients may not have represented extremes of patient’s characteristics, so, the results of our study may not necessarily be generalizable to all patients with concomitant moderate MR at the time of AVR for severe AS. Yet, our findings represent considerable recommendations for a great section of those patients with rheumatic mitral valve affection.
REFERENCES
- McCarthy PM (2002) Aortic valve surgery in patients with left ventricular dysfunction. Semin Thorac Cardiovasc Surg 14: 137-143.
- Jeong DS, Park PW, Sung K, Kim WS, Yang JH, et al. (2011) Long-term clinical impact of functional mitral regurgitation after aortic valve replacement. Ann Thorac Surg 92: 1339- 1345.
- Moazami N, Diodato MD, Moon MR, Lawton JS, Pasque MK, et al. (2004) Does functional mitral regurgitation improve with isolated aortic valve replacement? J Card Surg 19: 444-448.
- Caus T, Rouviere P, Collart F, Mouly-Bandini A, Monties JR, et al. (2001) Late results of double-valve replacement with biologic or mechanical prostheses. Ann Thorac Surg 71: 261- 264.
- Ruel M, Rubens FD, Masters RG, Pipe AL, Bedard P, et al. (2004) Late incidence and predictors of persistent or recurrent heart failure in patients with aortic prosthetic valves. J Thorac Cardiovasc Surg 127: 149-159.
- Barreiro CJ, Patel ND, Fitton TP, Williams JA, Bonde PN, et al. (2005) Aortic valve replacement and concomitant mitral valve regurgitation in the elderly: Impact on survival and functional outcome. Circulation 112: 443-447.
- De Simone R, Glombitza G, Vahl CF, Meinzer HP, Hagl S (2000) Three-dimensional color Doppler flow reconstruction and its clinical applications. Echocardiography 17: 765-771.
- Lange A, Palka P, Donnelly E, Burstow DJ (2002) Quantification of mitral regurgitation orifice area by 3-dimensional echocardiography: Comparison with effective regurgitant orifice area by PISA method and proximal regurgitant jet diameter. Int J Cardiol 86: 87-98.
- Ruel M, Kapila V, Price J, Kulik A, Burwash IG, et al. (2006) Natural history and predictors of outcome in patients with concomitant functional mitral regurgitation at the time of aortic valve replacement. Circulation 114: 541-546.
- Wan CK, Suri RM, Li Z, Orszulak TA, Daly RC, et al. (2009) Management of moderate functional mitral regurgitation at the time of aortic valve replacement: is concomitant mitral valve repair necessary? J Thorac Cardiovasc Surg 137: 635-640.
- Koji T, Goro M, Taichi S, Shigeru M, Takashi Y, et al. (2010) Impact of untreated mild-to-moderate mitral regurgitation at the time of isolated aortic valve replacement on late adverse outcomes. Eur J Cardio-thorac Surg 37: 1033-1038.
- Bishay ES, McCarthy PM, Cosgrove DM, Hoercher KJ, Smedira NG, et al. (2000) Mitral valve surgery in patients with severe left ventricular dysfunction. Eur J Cardiothorac Surg 17: 213-221.
Citation: Saber A, Alkady H (2019) Stepwise Approach for Effectiveness and Outcome Impact of Repaired Moderate Rheumatic Mitral Regurgitation during Aortic Valve Replacement for Severe Aortic Stenosis. J Cardiol Stud Res 5: 014.
Copyright: © 2019 Ahmed Saber, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

