
Chronic Hyperplastic Candidiasis of the Oral Mucosa: Case Report
*Corresponding Author(s):
María Teresa Pérez-GraciaDepartment Of Pharmacy, Biomedical Sciences Institute, Universidad CEU Cardenal Herrera, Valencia, Spain
Tel:+34 00961395272,
Fax:+34 00961369000
Email:teresa@uch.ceu.es
Abstract
Oral candidiasis, mainly caused by Candida albicans, is of great importance in stomatology due to its frequency and clinical variety. This infection is frequently observed in people with different types of predisposing factors. The clinical forms of oral candidiasis are variable and different classifications have been used. The following case is that of a male patient with well demarcated whitish plaques that do not detach upon rasping, located on the cheek mucosa bilaterally, on both lip retro-commissures. Samples taken from the lesions were cultured and presence of C. albicans was identified, which confirmed the clinical diagnosis of chronic hyperplastic candidiasis. Antifungal therapy was applied (Miconazole). A control performed two months later revealed an improvement in the lesions and total remission was observed after six months. The importance of this case lies in the fact that this type of candidiasis has a very low prevalence when compared to other clinical types such as pseudo membranous or erythematous candidiasis. Moreover, its importance also lies in the fact that an exhaustive clinical monitoring must be conducted seeing as it has a risk of malignancy.
Keywords
Candida albicans; Hyperplastic Candidiasis; Oral Candidiasis
INTRODUCTION
Oral candidiasis is caused by Candida, which is present in the oral cavities of approximately 50% of healthy individuals as a commensal organism [1,2]. Transformation from commensal organism to pathogen depends on the intervention of different predisposing factors that modify the microenvironment of the oral cavity and favor the appearance of opportunistic infection [3,4]. Among these factors are chronic local irritants, inadequate care of appliances, corticosteroids, xerostomia, dietary factors, immunological and endocrine disorders, malignant and chronic diseases, severe blood dyscrasias, radiation to the head and neck, abnormal nutrition, age, hospitalization, oral epithelial dysplasia and heavy smoking [5].
Candida albicans is the most virulent and prevalent species, followed by C. tropicalis, C. glabrata, C. parapsilosis, C. guillermondii, C. krusei, C. kyfer and more recently C. dubliniensis [6-9].
C. albicans colonizes the oral surface and can cause damage through the expression of its virulence factors, including adherence to host cells, morphological transition, hydrophobicity and secretion of hydrolytic enzymes [10-12]. A major virulence factor of C. albicans is its ability to adapt to a variety of different habitats and the consequent formation of surface-attached microbial communities known as biofilms [13].
Candida infections of the oral mucosa can produce different clinical and histopathological manifestations. Currently, the most commonly used classification is the one developed by Holmtup and Axel [14], which contemplates the following presentations: pseudo membranous candidiasis (acute-chronic), erythematous candidiasis (acute-chronic), hyperplastic candidiasis, and associated lesions (prosthetic stomatitis, angle cheilitis, rhomboid glossitis) [15-19].
Erythematous candidiasis is characterized by localized erythema of the oral mucosa, with or without associated symptoms, that commonly occurs on the tongue and the palate and is associated with broad-spectrum antibiotics, corticosteroids, and HIV infection. In the tongue dorsum, erythematous candidiasis presents depapillated areas caused by the loss of filiform papillae. Histologically, this lesion is similar to pseudo membranous candidiasis. It is the most commonly recognized type of candidiasis, representing 60% of the cases [20].
Pseudo membranous candidiasis or thrush, which is characterized by white patches on the surface of the buccal mucosa, tongue, and the soft palate, occurs in patients using corticosteroids topically or by aerosol, in HIV-positive patients, and in other types of immune compromised patients. It represents about 35% of the cases [20].
Hyperplastic candidiasis is the least common of the triad of major clinical variants, with 5% of the cases. CHC can manifest in nodular form or as whitish plaques that cannot be attributed to any other disorder, do not detach upon rasping, and are typically located on the cheek mucosa and tongue, and especially bilaterally at both lip retro-commissures [21,22]. In this form of the infection the Candida hyphae are not only found at epithelial surface level but also invade deeper levels where epithelial dysplasia can be observed, with the associated risk of malignancy [20].
The diagnosis is often made based on clinical examination and thorough history. Additional adjunctive diagnostic methods such as biopsy and microbiological culture (Sabouraud dextrose agar and chromogenic media) are valuable in confirming the diagnosis [3].
Management of oral candidiasis should be directed towards identifying the underlying factors that could cause the disease, through clinical examination and history taking. If alteration or correction of the underlying predisposing factor is not possible or required, drug therapy is initiated [5]. The treatment is based in the use of topical polyene (nystatin or amphotericin) or azole antifungal agents (clotrimazole, miconazole, ketoconazole, fluconazole or itraconazole) [23,24]. The drug chosen depends on the clinical history of the patient, the oral symptoms and compliance.
CASE REPORT
A 50 year-old man made his first visit to the CEU-Cardenal Herrera Dentistry Clinic. The reason of the visit was to carry out a checkup and mouth cleaning seeing as it had been 3 years since his last visit to the dentist. The patient was smoker of 10 cigarettes per day, without other systemic diseases. We observed a painless, well demarcated whitish plaque that did not detach upon rasping, located on the cheek mucosa, and on both lip retro-commissures (Figure 1) the patient did not know how long he had had those lesions. The clinical features of the white lesions could be diagnosed as Chronic Hyperplastic Candidiasis (CHC). Therefore, differential diagnosis of all the white lesions that could not be removed should be made as leukoplakia. We counseled the patient about quitting smoking. To establish a definitive diagnosis we made a biopsy with local anesthesia (Figure 2) and a complementary microbiological culture.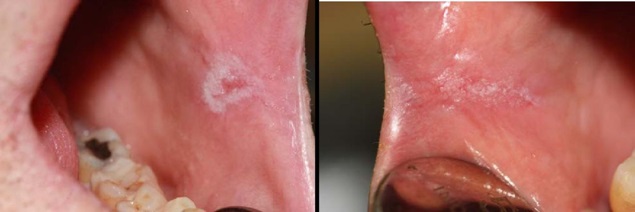
Figure 1: The intraoral image showing demarcated whitish plaques that did not detach upon rasping, located on the cheek mucosa, bilaterally at both lip retro-commissures.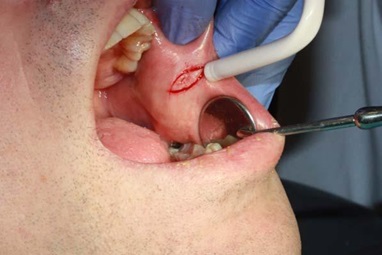
Figure 2: Image showing the moment of the biopsy.
The biopsy of the lesions is usually obtained and was stained with hematoxylin-eosin, the results showed pseudoepitheliomatous oral mucosa with very marked hyperkeratosis without dysplasia and superficial candidiasis associated with stromal inflammation psedoliquenoide pattern.
The result of the microbiological culture was a growth of Candida albicans colonies (Figure 3) we treated the lesions with miconazole gel 3 times a day for 2 months. The control made after two months revealed an improvement in the lesions (Figure 4) and total remission was observed after 6 months.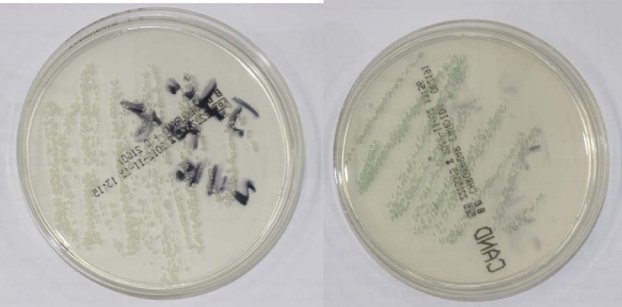
Figure 3: Candida albicans colonies in the Sabouraud dextrose agar (left) and CHROMagar Candida® (right) mediums.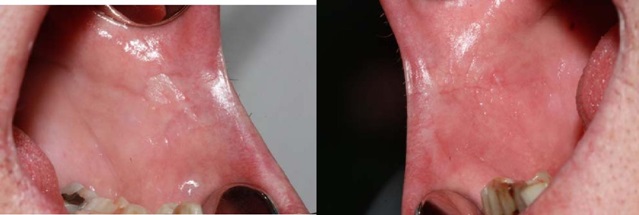
Figure 4: The intraoral image two months after antifungal treatment. No lesions are observed bilaterally at both lip retro-commissures.
MATERIAL AND METHODS
Swab samples were taken from the oral mucosa. The samples were spread in the Sabouraud dextrose agar medium and the CHROMagar Candida® (Becton Dickinson, Germany) medium and were incubated at 37°C during 48h. The results were observed after 24 and 48 hours.
Sabouraud dextrose agar (Becton Dickinson, Germany) is the medium most commonly used for the isolation of Candida spp. and other yeasts with clinical origin [24]. CHROMagar Candida® (Becton Dickinson, Germany) is a selective and differential culture medium that facilitates the isolation and presumptive identification of some clinically relevant species such as C. albicans, C. krusei, C. tropicalis and C. glabrata. This medium allows the specific identification of C. albicans colonies due to their green color; C. tropicalis colonies appear blue with a pink halo around them; C. krusei colonies are pink with a soft appearance and C. glabrata present a brown color [25,26].
Once the primary isolation of the yeast was made, fast methods such as the filamentation test in serum were applied to find C. albicans. Furthermore, nutrient assimilation methods were used to corroborate the identification (API 20 C AUX, bioMérieux, France).
RESULTS AND DISCUSSION
The clinical diagnosis was of hyperplastic candidiasis and was established using a biopsy from the lesion and the detection of Candida in the microbiological culture.
Chronic Hyperplastic Candidiasis (CHC) is a variant of oral candidiasis that typically appears as well-demarcated palpable, raised lesions that may vary from small translucent whitish areas to large opaque plaques that cannot be rubbed off. The most common site for these lesions is the buccal mucosa, especially the commissures areas [20]. The palate and tongue may also be involved, although less frequently. The major etiologic agent of the disease is the oral fungal pathogen Candida predominantly belonging to Candida albicans, although other systemic co-factors, such as vitamin deficiency and generalized immune suppression, may play a contributory role.
In this case, the patient had not taken antibiotics or steroids for an extended period of time. However, these are factors that are more commonly related to other types of oral candidiasis such as the acute erythematous candidiasis. In our case, the patient presented tobacco and deficient oral hygiene as risk factors. These risk factors are related with chronic hyperplastic candidiasis.
The smoking habit has a direct link to CHC, due to: induction of increased epithelial keratinization; reduction in salivary immunoglobulin A levels; and possible depression of polymorphonuclear leukocyte function [20].
Clinically, the lesions are symptomless and regress after appropriate antifungal therapy and correction of underlying nutritional or other deficiencies. The regression of a significant proportion of CHC lesions as a result of antifungal therapy is an indication that hyperplasia is a protective response of the host mucosa against a disseminated infection by Candida [20]. If the lesions are untreated, a minor proportion may demonstrate dysplasia and develop into carcinomas. In this case, the clinical improvement of the lesions was observed after two months of treatment with miconazole and total remission was observed after 6 months.
Histopathological examination of a suspected lesion is essential for the diagnosis of CHC [20]. Because this form may mimic other lesions, particularly squamous cell carcinoma, a biopsy is highly recommended. Histopathological examination will reveal epithelial parakeratosis with polymorphonuclear leukocytes in the superficial layers. In our case, the diagnosis was carried out with a biopsy and a microbiological culture.
This case was interesting for us because it is a type of candidiasis has a low prevalence if it is compared to other clinical types such as pseudo membranous or erythematous candidiasis. Moreover, its importance also lies in the fact that an exhaustive clinical monitoring must be conducted seeing as it has a risk of malignancy. Furthermore, this case is important because, in order to conduct the differential diagnosis of hyperplastic candidiasis, it is necessary to carry out a biopsy to discard other leukoplakia.
CONCLUSION
The diagnosis of CHC is complicated seeing as its characteristics are similar to those of a leukoplakia infected with Candida. For this reason, it is the key to carry out a differential diagnosis using a biopsy and a microbiological culture.
The total remission of the lesion after antifungal treatment confirms the diagnosis of CHC.
REFERENCES
- Barlow AJ, Chattaway FW (1969) Observations on the carriage of Candida albicans in man. Br J Dermatol 81:103-106.
- Samaranayake LP (1990) Oral candidosis: an old disease in new guises. Dent Update 17: 36-38.
- Coronado-Castellote L, Jimenez-Soriano Y (2013) Clinical and microbiological diagnosis of oral candidiasis. J Clin Exp Dent 5: e279-e286.
- Aguirre-Urízar JM (2002) Oral Candidiasis. Rev Iberoam Micol 19: 17-21.
- Scully C, el-Kabir M, Samaranayake LP (1994) Candida and oral candidosis: a review. Crit Rev Oral Biol Med 5: 125-157.
- Delgado ACD, de Jesus Pedro R, Aoki FH, Resende MR, Trabasso P, et al. (2009) Clinical and microbiological assessment of patients with a long-term diagnosis of human immunodeficiency virus infection and Candida oral colonization. Clin Microbiol Infect 15: 364-371.
- Luque AG, Biasoli MS, Tosello ME, Binolfi A, Lupo S, et al. (2009) Oral yeast carriage in HIV-infected and non-infected populations in Rosario, Argentina. Mycoses 52: 53-59.
- Ribeiro PM, Bacal F, Koga-Ito CY, Junqueira JC, Jorge AOC (2011) Presence of Candida spp. in the oral cavity of heart transplantation patients. J Appl Oral Sci 19: 6-10.
- Junqueira JC, Vilela SFG, Rossoni RD, Barbosa JO, Costa ACBP, et al. (2012) Oral colonization by yeasts in HIV-positive patients in Brazil. Rev Inst Med Trop Sao Paulo 54: 17-24.
- Schaller M, Borelli C, Korting HC, Hube B (2005) Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 48: 365-377.
- Mishra NN, Prasad T, Sharma N, Payasi A, Prasad R, et al. ( 2007) Pathogenicity and drug resistance in Candida albicans and other yeast species. A review. Acta Microbiol Immunol Hung 54: 201-235.
- Junqueira JC, Fuchs BB, Muhammed M, Coleman JJ, Suleiman JM, et al. (2011) Oral Candida albicans isolates from HIV-positive individuals have similar in vitro biofilm-forming ability and pathogenicity as invasive Candida isolates. BMC Microbiol 11: 247.
- Silva S, Henriques M, Oliveira R, Williams D, Azeredo J (2010) In vitro biofilm activity of non-Candida albicans Candida species. Curr Microbiol 61: 534-540.
- Holmstrup P, Axell T (1990) Classification and clinical manifestations of oral yeast infections. Acta Odontol Scand 48: 57-59.
- Samaranayake LP, Keung-Leung W, Jin L (2009). Oral mucosal fungal infections. Periodontol 2000 49: 39-59.
- Farah CS, Lynch N, McCullough MJ (2010) Oral fungal infections: an update for the general practitioner. Aust Dent J 55: 48-54.
- Williams DW, Kuriyama T, Silva S, Malic S, Lewis MA (2011) Candida biofilms and oral candidosis: treatment and prevention. Periodontol 2000 55: 250-265.
- Martin R, Wächtler B, Schaller M, Wilson D, Hube B (2011) Host-pathogen interactions and virulence-associated genes during Candida albicans oral infections. Int J Med Microbiol 301: 417-422.
- Gow NA, Hube B (2012) Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol 15: 406-412.
- Sitheeque MAM Samaranayake LP (2003) Chronic hyperplastic candidosis/candidiasis (Candidal Leukoplakia). Clin Rev Oral Biol Med 14: 253-267.
- González-García RJ, Sastre-Pérez MF, Muñoz-Guerra L, Naval-Gías FJ, Rodríguez-Campo C et al. (2006) Candidiasis hiperplásica crónica de la mucosa oral. Rev Esp Cir Oral y Maxilofac 28.
- Liu X, Hua H (2007) Oral manifestation of chronic mucocutaneous candidiasis: seven case reports. J Oral Pathol Med 36: 528-532.
- Rhodus NL (2012) Treatment of oral candidiasis. Northwest dentistry 91: 32-33.
- Odds FC (1991) Quantitative microculture system with standardized inocula for strain typing, susceptibility testing and other physiologic measurements with Candida albicans and other yeasts. J Clin Microbiol 29: 2735-2740.
- Baumgartner C, Freydiere AM, Gille Y (1996) Direct identification and recognition of yeast species from clinical material by using Albicans ID and CHROMagar Candida plates. J Clin Microbiol 34: 454-456.
- Pfaller MA, Houston A, Coffmann S (1996) Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J Clin Microbiol 34: 58-61.
Citation: Gracia MTP, Fernández CMH, Cebrian BM, García BS (2014) Chronic Hyperplastic Candidiasis of the Oral Mucosa: Case Report. J Clin Stud Med Case Rep, 1: 001.
Copyright: © 2014 María Teresa Pérez-Gracia, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

