
Hemorrhoidal Thrombosis: Conservative Treatment
*Corresponding Author(s):
Morelos Adolfo García SanchezSurgical Department, Colonic And Rectal Surgeon, General Hospital Specialized Medical Unit, National Autonomous University Of Mexico, México City, Mexico
Tel:+1 52 55245900,
Email:morelosadolfo@hotmail.com
Abstract
Hemorrhoidal thrombosis is a common complication of the hemorrhoidal disease, its symptoms are common and well known, still the diagnosis is usually straight forward. The objective is to present a case of a massive hemorrhoidal thrombosis (internal and external hemorrhoid involvement), with an infectious process, in which a medical conservative treatment was given with an auspicious response. Nevertheless, even nowadays with medical and technological advances available, the acute treatment of the aforementioned entity, in which any medical or surgical emergency is present, remains controversial, not so in the definite treatment which is surgical in all cases, on the contrary high recurrence will be the typical scenario.
Keywords
Abbreviations
Hemorrhoidal disease (HD)
Hemorrhoidal thrombosis (HT)
Introduction
Hemorrhoidal disease (HD) is one of the most common proctological pathologies, given that its incidence varies between 10 and 14.7% of the population [1,2]. Hemorrhoids are described as normal anatomical structures present since birth, formed by arteriovenous connections between anal conduct and mucosa with squamous epithelium fixed by connective tissue. There are accounts of the disease since 400 AD in which Hippocrates described the treatment with sizzling iron for the prolapsing ones in his treatise, something not very distant to what is performed in modern times [3].
Hemorrhoidal Thrombosis (HT) is a common complication of the HD, and is even what motivates the patient to seek medical attention, if its not the presenting symptom. The purpose of this paper is to present the experience on one case with massive hemorrhoidal thrombosis which involved internal and external hemorrhoids in addition of an infectious process, submitted to conservative medical treatment.
Case Report
The patient is a 34 year old male, with an unremarkable medical history, who started its actual disease 10 years ago, he came to the emergency department complaining of intense and incapacitating persistent pain in anal region and rectal bleeding with an evolution of 48 hours, unmodified by defecation, with tumour sensation in the same zone, fever, asthenia and adynamia. At physical exam with pallor, and prolonged capillary refill at 7 seconds. Proctologic inspection in Sims’ position revealed an evident massive mixed hemorrhoidal thrombosis with ischemia and necrosis with fibrin and mucous, with frigidity of tissue and ready bleeding, as well as intense pain. Instrumental exam and rectal examination is missed out.
Laboratories reported Hemoglobin of 7.1 mg/dl, Hematocrit 23, and WBC 21,000 (Figure 1).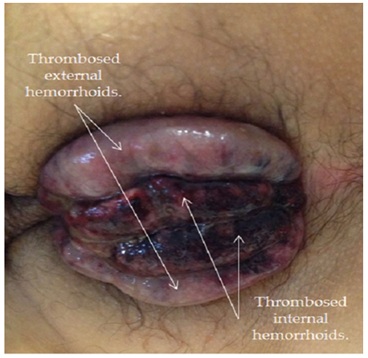 Figure 1: Massive hemorrhoidal thrombosis.
Figure 1: Massive hemorrhoidal thrombosis.
The patient is admitted and a conservative medical treatment consisting in NPO, sitz baths every 4 hours for 20 minutes with cold water and next with medical colostomy (Medical colostomy is defined as the lack of a physical colostomy on the patient, but obtaining the bennefits of a real colostomy; this benefits are: Bypassing the fecal matter to avoid infection, nourishing or feeding the patient even though its pathology, rest of the affected anatomic area, avoiding abrasion, increase in pressure and pain in the patient, accelerated recovery, psychologic benefit given the patient is using the enteral route, at least for liquids, ingestion of some sort of calories, avoids overload by intravenous solutions. Furthermore the medical colostomy allows to avoid morbility and provides early recovery; by avoiding both the first surgery for the colostomy and the second for the reconnection). And consists of: Liquid hypercaloric diet and Loperamide for 1 week, double scheme antibiotics was used (metronidazole and ciprofloxacine), as well as NSAIDS (diclofenac) and analgesics. Ointment of topical steroids like fluorcinolone for 10 days.
Finally the patient is discharged 10 days later and continuously seen at office with a satisfactory evolution (Figure 2).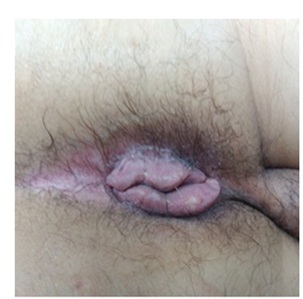
Discussion
The HT is a common complication of the HD, to make a proper diagnosis the patient must be explored, given that it is a clinical diagnosis [2]; the treatment is what remains controversial nowadays [4]. It is clear that the surgical complications increase considerably if, taking in account the intense pain as an emergency [5], the surgeon decide to operate in acute. Between the complications to consider we have in case of the acute ones: important transoperatory bleeding conducting to hypovolemic shock, accidental sphincter lesion and secondary infection even more in ischemic, inflamed and edematous tissues; and the chronic ones: anal stenosis, incontinence including its different grades and disease recurrence as a result of deformed anatomical structures [6]; all these are considered by the authors and is poorly described in the literature.
The HT has several management lines, and the tendency for conservative treatment is not improper [2,5,6,7]; however most of the authors prefer thrombectomy (elliptical excision) with local anesthetics [1-5,8]. Is important to mention the role of phlebotonics as diosmine, troxerutin and hesperidin with encouraging results but not definite yet [9]. The ligation guided by doppler has no applicability for HT, given that it is only indicated on minor grades haemorrhoids and even in that instance it fails having complications as pain, hemorrage and hemorrhoidal prolapse [10].
Other option is the so called “emborrhoid” technique which consists in embolisation of superior rectal hemorrhoidal arteries with clinical success of 72% but limited to only 14 patients and non specific for HT [11]. Its is also to mention the use of recombinant streptokinase suppositories for HT at a 100, 000 to 200,000 UI dose with sodium salicilate which offer a significative clinical improvement of symptoms in 5 days, although it is promising cost must be evaluated as well as mid and long term complications [12]. Lastly the use of the Merocel anal pack that its a polyvinyl alcohol sponge biocompatible and highly absorbent that is used for epistaxis treatment, accelerating the reduction on the internal hemorrhoids prolapse in acute phase but with partial results and still in research [13]. As noted, the management of HT is multiple and it will depend on each individual case, as well as the physician’s experience.
Conclusion
The treatment of the HT is wide and multiple; however we conclude that it must satisfy the basic ethical principles of healing without harming or putting at risk the patients heath unnecessarily. On the other hand its clear that each behaviour will depend on the experience and knowledge of the galenical work.
REFERENCES
- Mirhaidari SJ, Portes JA, Slezak FA (2016) Thrombosed external hemorrhoids in pregnacy: a retrospective review of outcomes. Int J Colorectal Dis 31: 1557-1559.
- Estalella L, López-Negre JL, Parés D (2013) Enfermedad hemorroidal. Med Clin 140: 38-41
- Abarca-Aguilar F, Alfonzo-Nuñez R, Anido-Escobar V, Aillaud II L, Fidel Llorente F, et al. (2010) Rev Mex Coloproctología 16: 4-14.
- Chan KKW, Arthur JDR (2013) External haemorrhoidal thrombosis: evidence for current management. Tech Coloproctol 17: 21-25.
- Rodríguez-Wong U (2009) Trombosis hemorroidaria: opciones de tratamiento. Rev Hosp Jua Mex 76: 81-83.
- Barrionuevo-López M, Perren A, García R, Cardozo D, Olivato C, et al. (2015) Terapéutica de la fluxión hemorroidal. Rev Arg Coloproct 26: 54-58.
- Lohsiriwat V (2012) Hemorrhoids: From Basic pathophysiology to clinical management. World J Gastroenterol 18: 2009-2017.
- Trompetto M, Clerico G, Cocorullo GF, Giordano P, Marino F, et al. (2015) Evaluation and management of hemorrhoids: Italian society of colorectal surgery (SICCR) consensus statement. Tech Coloproctol 19: 567-575.
- Giannini I, Amato A, Basso L, Tricomi N, Marranci M, et al. (2015) Flavonoids mixture (diosmin, troxerutin, hesperidin) in the treatment of acute hemorrhoidal disease: a prospective, randomized, triple-blind, controlled trial. Tech Coloproctol 19: 339-345.
- Scheyer M, Antonietti E, Rollinger G, Lancee S, Pokorny H (2015) Hemorrhoidal artery ligation (HAL) and rectoanal repair (RAR): retrospective analysis of 408 patients in a single center. Techniques in Coloproctology 19: 5-9.
- Vidal V, Sapoval M, Sielezneff Y, De Parades V, Tradi F, et al. (2015) Emborrhoid: a new concept for the treatment of hemorrhoids with arterial embolization: the first 14 cases. Cardiovasc Intervent Radiol 38: 72-78.
- Hernández-Bernal F, Valenzuela-Silva CM, Quintero-Tabío L, Castellanos-Sierra G, Monterrey-Cao D, et al. (2013) Recombinant streptokinase suppositories in the treatment of acute haemorrhoidal disease. Multicentre randomized double-blind placebo-controlled trial (THERESA-2). Colorectal Dis 15: 1423-1428.
- Tan WJ, Yusof S (2014) The Merocel anal pack: an innovative use for symptomatic hemorrhoids. Tech Coloproctol 18: 1127-1128.
Citation: García SMA, De La Fuente GM, Gómez PEM, Cano VG, Cruz MAD, et al. (2017) Hemorrhoidal Thrombosis: Conservative Treatment. J Clin Stud MedCase Rep 4: 043.
Copyright: © 2017 Morelos Adolfo García Sanchez, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

