
Anaemia in the Older Person
*Corresponding Author(s):
Chua ELondon North West University Healthcare, Northwick Park Hospital, England, United Kingdom
Tel:+44 02088692601,
Fax:+44 02088992241
Email:eddy.chua@nhs.net
Abstract
Anaemia is a common entity and a marker of morbidity and mortality in the older person (defined as 65 years and older). For the older person, anaemia of inflammation and chronic disease is by far more prevalent than iron deficiency. Differentiation between the two remains a challenge despite emergence of newer biochemical tests as the aetiology of anaemia is often multifactorial. Dose regimen for iron replacement therapy is changing to help promote absorption and reduction in side effects from oral iron. Intravenous iron is utilised for non-oral responders, in mixed anaemia such as inflammatory bowel disease, heart failure patients and in chronic renal disease. Finally, despite extensive tests, the aetiology can remain unknown, the unexplained anaemia of the elderly.
ABBREVIATIONS
ACD: Anaemia of Chronic Disease
AICD: Anaemia of Inflammation and Chronic Disease
AID: Absolute Iron Deficiency
CBC: Complete Blood Count
CHr: Haemoglobin in reticulocytes
CKD: Chronic Renal Disease
DNA: Deoxy-Ribonucleuic Acid
DMT1: Divalent Metal Transporter 1
ELISA: Enzyme Link Radioimmunoassay
EPO: Erythropoietin
ESA: Erythropoietin Stimulating Agents
FCM: Food Cobalamin Malabsorption
FCM: Ferric Carboxymaltose
FID: Functional Iron Deficiency
FN: Ferritin
Fr: Ferroportin
GI: Gastro-Intestinal Tract
HA: Haemolytic Anaemia
Hb: Haemoglobin
IBD: Inflammatory Bowel Disease
IDA: Iron Deficiency Anaemia
ID: Iron Deficiency
IV: Intravenous
IM: Intramuscular
LDH: Lactate Dehydrogenase
MCV: Mean Corpuscular Volume
MDS: Myelodysplastic Syndrome
MMA: Methyl Malonic Acid
NHANES III: The third National Health and Nutrition Examination Survey
PA: Pernicious Anaemia
RA: Rheumatoid Arthritis
RBC: Red Blood Cell
RC: Red Cell
RDW: Red cell Diameter Width Distribution
RE: Reticuloendothelial System
RNA: Ribo- Nucleic Acid
SI: Serum Iron
TIBC: Total Iron Binding Capacity
TNF: Tissue Necrosis Factor
TSAT: Transferrin Saturation
TfN: Transferrin
sTfR: Serum Transferrin Receptor
WHO: World Health Organization
UAE: Unexplained Anaemia in old people
ZPP: Zinc Protoporhyrin
INTRODUCTION
Anaemia in the older person (defined as >65 years old) is common and important as it is associated with greater morbidity from falls, cognitive decline, greater likelihood of hospitalizations with longer length of stay and mortality. However, its evaluation in the older person remains challenging as in many cases multiple conditions may be contributing factors. Indeed, in some instances, no cause may be found, despite continued investigation. Several criteria have been created in an attempt to define anaemia. The most widely known is the World Health Organization (WHO) criteria [1]. It uses the standard of a Hemoglobin level (Hb) of <130 g/L for men and <120 g/dl for women. Alternatively, data from community dwellers in United States, the third National Health and Nutrition Examination Survey (NHANES III), utilises an Hb level at 132 g/L and 122 g/L for older white men and women and 127 g/L and 115 g/L for older black men and women respectively [2]. These are arbitrary sub-divisions to separate subjects into anaemic and non-anaemic subsets. The lower Hb threshold may not necessarily influence morbidity or mortality although, in one study on anaemia in hospitalised subjects, a value less than 140 g/L for men and 130 g/L for women was associated for all causes of mortality [3]. With no uniformly agreed definition of anaemia, there is wide variability in reported prevalence of the condition. What is known, however, is that anaemia becomes consistently more prevalent in the older non-institutionalised adult and this is most seen in the oldest bracket of this subset (>80 years). Nursing home residents are also at risk of anaemia with the highest risk noted in hospitalized older adults.
As a strategy for investigation, the above criteria are impractical for use in clinical practice. In our recent audit of 309 consecutive older patients (169 females, mean age 84.7 years and 140 males, mean age 82.6 years) admitted to the Geriatric ward, utilisation of the WHO criteria, suggested that 55% of females and nearly 70% of male patients were anaemic, the Joosten criteria (Hb<115 g/l for both sexes) is a viable alternative at 48% and 41%, but a more pragmatic approach might be the “rule of 10” (Hb around 100 g/L or less) at 33% and 35% respectively [4]. For the purpose of this review, we will focus on an isolated anaemia, the type that is most frequently encountered in daily clinical practice. The “autoimmune crytopenias” (anaemia with low neutrophil and platelet count) and anaemia due to haematogical malignancies will not be discussed as it warrants specialised management.
IRON DEFICIENCY ANAEMIA
Worldwide, Iron Deficiency Anaemia (IDA) is the most common cause of anaemia at around 12%, although its prevalence can vary between population groups. In the older person, Anaemia of Inflammation and Chronic Disease (AICD) is more common, with up to 80% of this population being affected [5]. The later figure is very likely to be an over estimate as it would have included the Unexplained Anaemia in the older person (UAE). Table 1 summarizes the types of anaemia in the older person. An overview of iron metabolism will aid understanding on the current tools and their limitations when evaluating anaemia.
|
Aetiology of Anaemia |
Prevalence |
|
Anaemia of chronic disease/inflammation |
40-45% |
|
Chronic renal disease |
5-10% |
|
Haematological |
Up to 5% |
|
Endocrine |
Up to 5% |
|
Iron deficiency |
20% |
|
Vitamin B12 or folate deficiency |
5-10% |
|
Unexplained anaemia in elderly |
15% |
Table 1: Approximate prevalence on types of anaemia in the older person.
IRON METABOLISM
Non haem iron absorption
Haem iron absorption
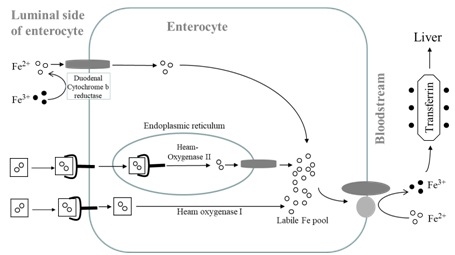 Figure 1: Absorption of haem and non haem iron. Postulated haem receptors, one in which the receptor is internalised via endocytic route, iron is released from haem by haem oxygenase II and transported to the labile iron pool by DMT I. Alternatively haem receptor takes haem iron and iron is released from haem by haem oxygenase I and enters the same labile iron pool [9]. Iron can be mobilised by ferroportin when required.
Figure 1: Absorption of haem and non haem iron. Postulated haem receptors, one in which the receptor is internalised via endocytic route, iron is released from haem by haem oxygenase II and transported to the labile iron pool by DMT I. Alternatively haem receptor takes haem iron and iron is released from haem by haem oxygenase I and enters the same labile iron pool [9]. Iron can be mobilised by ferroportin when required.Within the portal circulation, iron is transported and bound to the TfN. Iron enters the hepatocytes via Transferrin Receptors (TfR), a transmembrane protein located on the surface of liver cells. In the liver, iron is stored as FN or mobilised to the systemic circulation via the iron exporter Fr bound to the iron transport protein TfN (caeruloplasmin assumes the role of hephaestin). Iron bound to TfN is eventually released and enters Recticulo-Endoethelial cells (RE) via TfR. In the bone marrow, iron enters via TfR in an endocytic process where both iron and TfR are internalised. Iron is released to be utilised for erythropoiesis. TfR is recycled to the surface which prevents its depletion [10]. In the spleen, as Red Blood Cells (RBC) come to the end of their life span, they are degraded by macrophages and this iron is reutilised. Here, the iron exporter protein Fr together with caeloplasmin modulates the mobilisation of iron via the hormone hepcidin (See below).
IRON HOMEOSTASIS
There is no dedicated mechanism for iron excretion. Therefore, iron regulation must occur at the site of absorption to prevent overload. However it may be noted that the body can passively lose a small amount of iron through shedding of skin cells and the cells of the Gastrointestinal (GI) tract when mucosal cells are shed into the intestinal lumen as well as during excretion of sweat and urine. In pre-menopausal women, there is additional iron loss caused by menstruation. The activity of the iron exporter protein, Fr, is regulated by the hormone hepcidin (also known as hepcidin antimicrobial peptide) produced in the liver. Binding of hepcidin to Fr induces its internalisation followed by Fr degradation, thereby decreasing the rate at which iron enters the circulation from the enterocyte and macrophages [11]. Thus control of hepcidin synthesis is of great importance in order to govern blood iron levels.
Several factors can modulate hepcidin expression, including inflammation (cytokines), hypoxia, need for erythropoiesis and body iron stores [12]. Whereas on the one hand, when body iron stores are replete (as occurs in iron overload), hepcidin production is enhanced. Its interaction with Fr will down regulate iron export from enterocyte and macrophages to the circulation. On the other hand, when iron stores become depleted (as is the case in ID), hepcidin production is reduced thus facilitating iron release from enterocytes and also releasing the “RE lock” for iron export to the systemic circulation. Simultaneously, there is an upregulation of TfR’s in marrow cells to internalise iron released from serum TfN for erythropoiesis. This is summarised in figure 2.
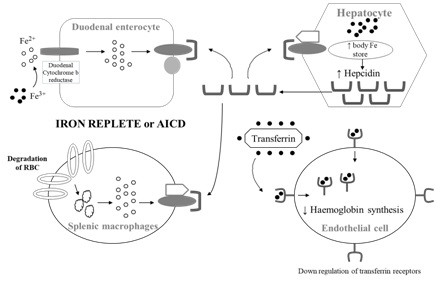 Figure 2: Top figure, in the iron replete state, anaemia of inflammation and chronic disease or in excess iron, hepcidin expression is enhanced which blocks ferroportin from exporting iron from enterocytes and RC cells. TfR are also down regulated to reduce uptake of iron for erythropoiesis. In the iron deficient state (bottom figure), hepcidin expression is reduced, ferroportin transports iron out from enterocyte and hepatocytes. There is upregulation of TfR in the endothelial cells to internalise iron for haemoglobin synthesis.
Figure 2: Top figure, in the iron replete state, anaemia of inflammation and chronic disease or in excess iron, hepcidin expression is enhanced which blocks ferroportin from exporting iron from enterocytes and RC cells. TfR are also down regulated to reduce uptake of iron for erythropoiesis. In the iron deficient state (bottom figure), hepcidin expression is reduced, ferroportin transports iron out from enterocyte and hepatocytes. There is upregulation of TfR in the endothelial cells to internalise iron for haemoglobin synthesis.
IRON DEFICIENCY (ID)
AID can result from three main mechanisms
• Reduced absorption in the small intestine due to malabsorption, e.g., coeliac disease
• Increased blood loss (chronic) from the intestine
Iron bioavailability (defined as the percentage of ingested iron that is available for absorption and for body function) is also important. Foods can be categorized into low, medium and high bioavailability with mean absorption at 5%, 10% and 15% respectively [13]. Low bioavailability foods are predominately found in cereals and roots vegetables consumed predominately in developing countries. Intermediate bioavailability foods are similar to low, but with some protein (meat) and foods with some ascorbic acid. High bioavailability foods are those found in western diet with meat, poultry, fish and foods with high levels of ascorbic acid.
With migration, ethnic minorities living in western countries are at risk of ID where they continue to consume diets with low iron bioavailability. Cereals and roots vegetables contain natural inhibitors of iron absorption by forming insoluble complexes with iron. Examples include phytate (found in whole grains, legumes and nuts), oxalic acid (in spinach and rhubarb), polyphenols, phosphates and tannins (in coffee and plant tissues) [14,15]. Therefore, haem iron, as a source of dietary iron should not underestimated due to its higher iron bioavailability [16]. In western countries, haem iron accounts for only 10-15% of ingested iron, but contributes to one third of the absorbed iron due its higher bioavailability [17,18] with 20-30% of haem iron being absorbed compared to only 10% for non-haem iron [19]. A persistent low dietary iron intake results in depletion of FN stores as iron is utilised for erythropoiesis. These stores initially compensate but as they become depleted with insufficient iron for Hb synthesis, anaemia occurs. As such, anaemia is a relatively late feature in ID. The depletion of iron stores and FN is the earliest feature which is often followed by changes in red cell morphology; this is frequently seen before signs of the anaemia itself.
ANAEMIA OF INFLAMMATION AND CHRONIC DISEASE (AICD)
Aetiology
There are several pathophysiologic features of AICD. These include (i) a reduced life span of the red blood cell RBC (ii) impaired iron metabolism (iii) impaired Erythropoietin (EPO) production and (iv) amuted response of the bone marrow to EPO.
The most prevalent theory on AICD suggests an interference with iron homeostasis due to the heightened presence of inflammatory cytokines specifically Interleukin 6 (IL-6), Tissue Necrosis Factor (TNF) and Interleukin 1 Beta (IL-1β) [21]. IL-6 has been shown to impair erythroid development, although the exact mechanism is unclear. It also increases hepatic production of hepcidin which switches off iron export from the duodenal enterocyte and the recticulo-endothelial cells through inhibition of Fr. This is sometimes referred to as the “RE lock” [22]. Although these cells have ample iron, blood circulation is depleted of iron, causing FID.
IL-6 has also been shown to upregulate DMT1 expression resulting in increased uptake of ferrous iron from macrophages and enterocytes [23]. However, most of this iron will be retained in these cells and stored as FN. With the degradation of hepatic and splenic tissue during inflammation, FN is released into the circulation; this explains its elevated levels as observed in AICD. FN in the enterocyte will be lost when mucosal cells are shed into the intestinal lumen. IL-1β and TNF-α can also depress EPO production and the efficacy of erythropoiesis as well as increase breakdown of erythrocytes (causing their reduced life span) resulting in an anaemia [24]. This has been summarised in figure 3. Prior to discussion of the diagnosis of AICD, it will be helpful to review the current tools to evaluate anaemia.
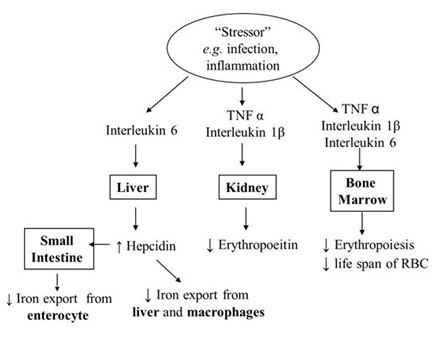 Figure 3: Pathophysiology anaemia of inflammation and chronic disease.
Figure 3: Pathophysiology anaemia of inflammation and chronic disease.TOOLS TO EVALUATE ANAEMIA
|
Indices |
IDA |
AICD |
MA |
Ageing |
|
Haemoglobin |
↓ |
↓ |
↓ |
↓ |
|
Serum Iron |
↓ |
↓ |
↓ |
↓ |
|
Transferrin Saturation |
↓ |
Normal or ↓ |
Normal or ↓ |
? Normal |
|
Serum Transferrin |
↑ |
Normal or ↓ or ↑ |
Normal or ↓ |
? Normal |
|
Serum Ferritin |
↓ |
↑ |
↑ |
↑ |
|
Serum transferrin receptors |
↓ |
Normal |
Normal or ↑ |
? Normal |
|
Red cell zinc Protoporphyrin |
↑ |
↑ |
↑ |
↑ |
Table 2: Biochemical indices of anaemia in IDA, AICD, MA and Ageing.
IDA: Iron Deficiency Anaemia; AICD: Anaemia of Inflammation and Chronic Disease; MA: Mixed Anaemia
Bone marrow
Peripheral blood film and red cell characteristics
RDW, though not a specific indicator for any individual anaemia, can be useful clinically. An elevated RDW should prompt the clinician to check the haematinics (ferritin, B12 and folate) as it is superior to MCV when used as a diagnostic criterion for iron, vitamin B12 and folate deficiency even in the absence of anaemia [28]. The anaemic older person with an elevated RDW appears to have an association with greater mortality [29]. Blood film evaluation although useful, is usually not required in a case of straight forward haematinic deficiency. It is labour intensive and requires a fresh sample. It can be a follow on investigation when evaluating an unexplained anaemia e.g., differentiating mirocytosis of ID from other haemoglobinopathies [30].
Serum Ferritin (FN)
Iron studies
TSAT is the ratio of SI and TIBC and multiplying this by 100. As it is a ratio, it suffers from the same handicaps observed for SI. Nevertheless, typically in absolute IDA, SI is low, TIBC is high and TSAT is low. In AICD, iron studies also pose diagnostic limitations as SI level is dependent on recycling of iron in the RE system which is often reduced during inflammation due to RE block and serum TfN expression can be unchanged, reduced (as is the case in malnutrition and chronic disease) or even elevated (Table 2).
Zinc Protoporhyrin (ZPP)
Serum Transferrin Receptor (sTfR)
Serum Erythropoietin (EPO)
DIAGNOSIS OF AICD AND MIXED ANAEMIAS
AICD is most often normochromic, normocytic to microcytic associated with a mild anaemia and SI and TSAT are usually low [5]. TfN can remain low, unchanged or even elevated. Serum ferritin is often raised as CRP. Bone marrow examination will reveal reduced sideroblasts with raised intracellular iron. Reticulocyte count may be reduced for the degree of anaemia and serum hepcidin may be raised. AICD and IDA are common bedfellows, as is the case in a Mixed Anaemia (MA), making a distinction of the former from the latter difficult. The sTfR-FN index is superior to sTfR alone and may help in the diagnosis of the mixed anaemia (IDA and AICD) [40]. The rationale being that in IDA, sTfR is increased and the log FN is reduced. Therefore an index ratio of >2 denotes IDA. In AICD, sTfR is unchanged but FN is increased and an index ratio of <1 denotes AICD. For those with a MA, the index ratio will also be >2. Traditionally the two groups of patients cited to have mixed anaemias were patients with Rheumatoid Arthritis (RA) and Inflammatory Bowel Disease (IBD).
POTENTIAL FUTURE TOOLS FOR DIAGNOSIS OF ANAEMIA
Reticulocyte indices
Serum hepcidin
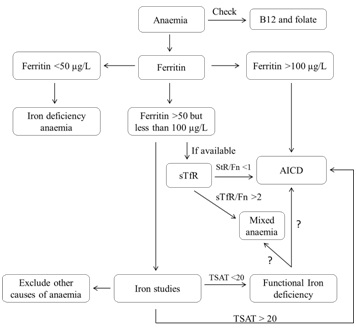 Figure 4: Algorithm for investigating anaemia in the older person.
Figure 4: Algorithm for investigating anaemia in the older person.MANAGEMENT OF ID WITH ORAL IRON SUPPLEMENTATION
The recommended dose to treat IDA is approximately 200 mg/day of elemental iron; as such 200 mg of ferrous sulphate (65 mg elemental iron) taken three times a day meets the requirement. This aims for an optimum rise in haemoglobin concentration of about 2 g/dl over 2-4 weeks and equates to an additional daily requirement of 20 mg of elemental iron. However, the absorptive apparatus in the intestinal epithelium is easily saturated, absorbing only 10-15% of ingested iron per day (20-30 mg of elemental iron). Thus remaining 180 mg of unabsorbed elemental iron often leads to GI upset (e.g., abdominal pain, nausea, constipation and diarrhoea) especially in the older person, occurring in as many as 70% of cases, reducing drug compliance [50]. This suggests that one should rethink the thrice daily regimen as being optimal in the treatment ID.
From a historical perspective, about 75 years ago, Hahn observed that absorption of radioactive iron was greatly increased in iron deficient dogs when compared to dogs that were iron-replete [51]. Granick later proposed the existence of a hypothetical block within the enterocyte that prevented the absorption of unwanted iron and which disappeared in ID [52]. Forty years on, Fairweather-Tait confirmed the existence of this ‘block’, presenting rodent intestinal mucosa with a high iron content meal lead to a reduction in iron absorption from a test meal given subsequently [53,54].
In the early part of the 21st century, Rimon et al., gave different iron dosing regimens to ID octogenarians three times a day. Liquid iron preparation was used to titrate to a lower dose of daily elemental iron. At 3 months, similar efficacy was observed in normalizing haemoglobin irrespective of the dose of iron but the fewest side effects were observed in the group on the lowest elemental iron preparation [55]. More recently, in young female iron deficient subjects, using an iron traceable isotope to monitor absorption and measurement of serum hepcidin, optimum iron absorption was observed with lower doses of iron not exceeding 80 mg of elemental iron per day. Higher iron dose or doses more than once a day, amplified circulating hepcidin which impeded iron absorption the following day. This hepcidin effect lasted for 48 hours before iron absorption returned to its initial baseline [56].
The same group followed up the study to examine iron absorption and hepcidin measurements in ID female subjects using a daily iron and alternate day iron regimen. Iron absorption was greater in the alternate day regimen which was coupled with lower serum hepcidin and with fewer GI side-effects. This suggests that alternate day, low dose iron is likely to be superior [57]. However, it is unclear if this alternate day regimen is applicable to subjects with more severe anaemia. Alternate day, low dose iron may also reduce drug adherence. For optimum efficacy, iron supplementation should be taken with half a glass of orange juice or 250 mg ascorbic acid. For uncomplicated IDA, a response in haemoglobin is usually seen within 7 to 10 days although it may take up to 12 weeks or longer to fully replenish depleted stores. For those who are still intolerant to oral iron, Intravenous Iron (IV) is an alternative. Several preparations of IV iron are available in the United Kingdom: Low Molecular Weight Iron Dextran (LMWID), Ferric Carboxymaltose (FCM) and Ferric Gluconate all have similar efficacy and adverse events but with different costs and frequency of administration.
Despite being safer (anaphylaxis rate at 0.02%) than the traditional high molecular weight iron dextran, IV iron needs to be administered in a facility where resuscitation facilities are available. IV iron is effective in ID subjects (subjects often have higher hepcidin levels) who have failed to respond to oral iron and superior than oral iron in the management of IDA in IBD patients [47]. Oral iron may worsen bowel activity in IBD. Indeed the European consensus on the management of IBD stipulates that IV iron should be the first lime in the management of IBD subjects who are anaemic (haemoglobin <10 g/dl) with active IBD or previous intolerance to oral iron (ii) there is often an inflammatory component such that oral absorption will be impaired and (iii) IV iron can partially overcome the iron restricted erythropoiesis associated with the inflammation caused by IBD [58].
IV iron is also useful in the management of ID in heart failure, anaemia due to CKD on erythropoietin stimulating agents ESA and may reduce post-operative transfusion requirements in certain abdominal and orthopedic surgeries [59,60]. IV iron can lead to misinterpretation of body iron status. A blood check on iron status should not be under taken until at least 2 weeks following initiation of IV iron, as interpretation of iron status would be inaccurate.
MANAGEMENT OF ACID
• The initial management of AICD if appropriate is to treat the underlying chronic condition e.g., the use of corticosteroids and immunosuppressant therapy for anaemia in IBD or the use of ESA for CKD
• Blood transfusion: This will elevate Hb level but will not address the underlying condition. It can be utilised to avert crisis, but is not a suitable long solution [61]
• Using multiple tests in parallel to help define iron status and trial of iron: Two models have been proposed (a) MCV model which utilises MCV, TSAT and ZPP (b) FN model, which utilizes FN, ZPP and TSAT [62]. If any two are abnormal, then the subject has impaired iron status and a therapeutic trial of iron could be undertaken. TSAT levels are often difficult to interpret in inflammatory states but a TSAT of <20 in the context of a raised CRP may indicate ID and a therapeutic trial of iron may be considered (c) As inflammation often leads to a raised FN, a useful rule is to divide the patients serum FN by three [63]. If FN is less than 50 mg/L a decision may be taken to initiate iron supplementation (d) Serum TfR /ferritin index: The utility of this index has been discussed and several commercial ELISAs are available. This is, however, not routinely utilised for large volume analysis due to lack of a common reference material for standardization.
Therapeutic trial of iron
Anaemia due to folate and vitamin B12 deficiency
Vitamin B12 is mainly stored in the liver with stores ranging from 2 to 5mgs body such that one can remain replete for 2 to 5 years. Its principle role is in the synthesis of Deoxyribose Nucleic Acid (DNA) and Ribo- Nucleic Acid (RNA). Bone marrow cells are the most active in cell division and will be most sensitive to deficiency resulting in morphological changes on the RBC’s. These include cell distortion, typically hypersegmentation of neutrophils, macrocytosis and eventually anaemia. Vitamin B12 deficiency also disrupts erythropoiesis causing pre-mature cell death (apoptosis) via haemolysis as observed in prolonged Pernicious Anaemia (PA). Serum bilirubin and Lactate Dehydrogenase (LDH) are often elevated, whereas serum haptoglobulin and reticulocyte count are reduced.
The most common cause of B12 deficiency in the older person is not PA but Food Cobalamin Malabsorption (FCM) at over 60% [66]. Unlike PA, FCM subjects can absorb crystalline vitamin B12 but lack the ability to liberate dietary vitamin B12 bound to protein. The older person is susceptible to B12 deficiency for several reasons (a) chronic gastric acid suppression therapy, e.g. proton pump inhibitors usage (b) atrophic gastritis with age and (c) chronic H-pylori infection all reduce gastric juice and pepsin secretion. The reduction in the former encourages colonic bacterial overgrowth into the terminal ileum disrupting intrinsic factor -B12 complex affecting B12 absorption.
Many individuals with FCM are not anaemic and diagnosed using routine geriatric blood tests. This has been referred to as subclinical B12 deficiency without the megaloblastic features on the blood film. This may explain that macrocytosis is a poor reflector of vitamin B12 status [28,67]. Methyl Malonic Acid (MMA) assay may be superior serum B12 but assay is not available in most clinical centres in the United Kingdom. The gold standing to confirm PA is the Schillings test but it is rarely performed in the older person. Antibodies to IF are present in up 70 percent of individuals with PA [68]. The management of FCM is oral replacement or Intramuscular (IM). Improvements in anaemia are similar in both routes. IM is the favoured route if there is an issue with compliance. If oral replacement is undertaken, it is prudent to repeat the vitamin level several weeks into the treatment as a subset of B12 deficiency may have PA. There is also increased risk of gastric carcinoma in PA so evaluation of symptoms pre and post treatment is required.
Vitamin B12 supplementation is safe from toxicity since it is water soluble. On the provision that no other causes for the anaemia are involved, the following response should occur following supplementation (a) fall in markers of haemolysis (LDH and bilirubin) within 2-3 days (b) increase marrow activity (reticulocytes) within 3 to 4 days (c) resolution of morphological changes in blood within 1-2 weeks and (d) normalization of anaemia within 4-8 weeks. Resolution of neuropsychiatric symptoms (very rarely seen in the 21st century) may take longer and may be incomplete.
Total body folate stores vary from 0.5 to 20 mg, but unlike vitamin B12, folate stores are more easily depleted lasting from only 2 to 6 months. In clinical practice, both vitamin assay should be conducted to avoid replacement of folate in undiagnosed PA which can potentially lead to irreversible nerve damage. Diagnosis of folate deficiency is via a blood test. There is debate on the superior test, Red Cell (RC) folate or serum folate [69]. There are several analytical issues with RC folate e.g., (a) reduced oxygen saturation and haematocrit may falsely elevate RC folate and (b) analysis may incur higher costs as specimens require pre-treatment.
In B12 deficiency, serum folate may be a superior index than RCF. Vitamin B12 is needed for uptake of 5 Methyl- Tetrahydrofolate (5-MTF) for developing RBC’s [70]. Vitamin B12 deficiency may falsely reduce RC folate in the absence of folate deficiency since with vitamin B12 supplementation, RC folate was increased but not serum folate [71-74]. During dialysis, if blood sampling was taken after dialysis, serum folate may be lowered but not RC folate [75]. This can be overcome by taking the blood sample pre-dialysis. Serum folate appears to be the better test in terms of cost and provision of clinical information. The management of deficiency is oral replacement but for the older person, there may be no improvement in anaemia as aetiology of anaemia are can be multifactorial.
OTHER TYPES OF ANAEMIA
Multiple myeloma
Myelodysplastic Syndrome (MDS) and Haemolytic Anaemia (HA)
HAEMOLYTIC ANAEMIA (HA)
Haemolysis should be suspected in the context of a macrocytic anaemia and in conjunction with a raise serum bilirubin, LDH and reticulocyte count and a low haptoglobin. An increased LDH and reduced haptoglobulin has a 90 percent certainty to indicate haemolysis [76]. Likewise, a normal LDH and haptoglobulin will exclude haemolysis [77]. A positive direct antiglobulin test indicates immune haemolysis. Blood film may reveal RBC fragments (schistocytes), spherical RBC (spherocytes) and chip RBC (bite cells).
IRON DEFICIENCY (ID) AND ANAEMIA IN HEART FAILURE
Iron deficiency” without anaemia
Cardio-renal anaemia syndrome
ANAEMIA IN CHRONIC KIDNEY DISEASE (CKD)
Anaemia in renal impairment is a common entity encountered in daily geriatric practice, although the initiation of ESA and its management is specialised. Anaemia is a risk factor for the development of Left Ventricular Hypertrophy (LVH) in CKD patients. LVH in CKD has a major impact on cardiovascular morbidity and mortality [83,84].
The Kidney Disease Outcome Quality Initiative Anaemia Working Group recommend that serum FN and the TSAT should be the main biochemical tools for the management of anaemia in CKD and in End Stage Renal Replacement Therapy. ID correlates reasonably well with at TSAT<20%, serum ferritin<100 µg/L and iron overload, TSAT>50% and serum FN of 800 µg/L. FID is defined as a response to IV iron as reflected by an increase in Hb or a reduced requirement for ESA [85,86]. ID is the most common cause of poor response to ESA in patients with end stage renal failure. Animal studies reveal that oral iron therapy may potentially have adverse side effects, including inflammation and further kidney damage. This may be mitigated through adequate dosing of IV iron. In the presence of ESA, IV is superior to oral iron as oral therapy may be insufficient to keep pace with the increased iron requirement from the hyper-stimulated bone marrow due to elevated erythropoiesis [87]. Although the management of anaemia is individualized, for most CKD patients, ESA therapy can usually be initiated when the TSAT>25% and serum FN>200 µg/L.
As stated previously, FN is an acute phase reactant. TSAT has some acute-phase activity as it is a ratio dependant on SI and TIBC. During inflammation, serum TFN may be elevated. On the one hand with a constant circulating iron level, TSAT will be lowered. On the other hand, chronic disease can suppress TfN synthesis and with a constant circulation of iron, TSAT will be raised. This is a frequent encountered entity in patients with advanced CKD. The availability of an alternative test to help define body iron status before initiation of ESA may aid management. The sTfR/Ferritin index can be a useful adjunct if available locally. This index gives good correlation in the estimation of body iron status [88]. However, it is still limited as serum FN expression is influenced by inflammation although sTfR can be used to detect concurrent iron deficiency with inflammation [39,40].
ANAEMIA IN THE OLDER PERSON
Anaemia in the older person can have several aetiologies. In a prospective study on 191 old patients (>70 years old) in a geriatrics department, 56 patients fulfill the criteria for IDA (serum FN<50 µg/L, TSAT<20%) but only 23 eventually filled the criteria of pure IDA with a CRP<5 mg/L, normal renal function EGFR>30, normal thyroid, folate and B12 [89]. For ACD, 135 subjects initially fulfilled the criteria (serum FN>50 mcg/L, TSAT<20 percent, CRP>5). These conditions consisted of autoimmune conditions, malignancy, acute and chronic infections. However, 21 from this cohort had renal impartment, 8 were hypothyroid, and 1 had both with 105 patients categorized as ACD. Ultimately, 23 (12%) had pure IDA, 105 (55%) as having ACD and 167 (87 %) as AICD.
UNEXPLAINED ANAEMIA IN THE ELDERLY (UAE) OR IDIOPATHIC ANAEMIA OF AGEING
Unexplained Anaemia in the Elderly (UAE) can occur between 20 to 30% in the old community dwellers. In the NHANES III cohort, in anaemic old subjects (WHO criteria for anaemia) in subjects >65 years, approximately one third was due to a nutrient deficiency (of which, 50% was to ID), a third due to chronic diseases and the final third was idiopathic [90]. A Belgium study on anaemic subjects (Joosten criteria for anaemia) in hospitalized older subjects reveal up to 17% had an unexplained anaemia [91].
SUMMARY
Biochemical tools to help establish the cause of anaemia should be undertaken in conjunction with clinical history and examination. For the older person, oral iron supplementation in IDA should utilize a low dose alternating day iron dosing to help reduce GI side effects but with no compromise in efficacy compared to the traditional 3 times daily dosing. IV iron should be utilized for those intolerant to oral preparation. IV iron should be the first line management in certain medical conditions such as ID in IBD. A significant proportion of the anaemia in the older person is AICD. Currently, there is no test that can reliably diagnose AICD. Using multiple tests in parallel may help differentiate the type of anaemia, in particular, the mixed anaemias. For anaemic subjects with functional iron deficiency, a trial of oral or IV iron could be undertaken.
Anaemia in the older person is common and often due to several aetiologies. Addressing a specific nutrient deficiency e.g., in anaemia with CKD, may not necessarily improve the haemoglobin level. The search for the cause of the anaemia in the frail older person should involve of a clinician who has a clear clinical overview or when it is not possible due to cognitive impairment discussing with a relative or spouse. As is the case in good medical practice, a balance needs to be addressed between trying to establish a definitive diagnosis by subjecting a frail person to invasive investigations without necessarily improving the clinical outcome against no investigations and then accepting and living with the uncertainty on the cause of the anaemia. For some, with the progression of time, the aetiology of the anaemia may become apparent e.g., in myelodysplasia. However, there remains a proportion in the old person, the UAE, where despite extensive investigations, aetiology of anaemia remains elusive.
REFERENCES
- [No authors listed] (1968) Nutritional Anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 405: 5-37.
- Beutler E, Waalen J (2006) The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 107: 1747-1750.
- Culleton BF, Manne BJ, Zhang J, Tonnelli M, Klarenbach S, et al. (2006) Impact of anaemia on hospitalisation and mortality in older adults. Blood 107: 3841-3846.
- Joosten E, Pelemans W, Hiele M, Noyen J, Verhaeghe R, et al. (1992) Prevalence and Causes of Anaemia in a Geriatric Hospitalized Population. Gerontology 38: 111-117.
- Konijn AM (1994) Iron metabolism in inflammation. Baillieres Clin Haematol 7: 829-849.
- MacKenzie EL, Iwasaki K, Tsuji Y (2008) Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 997-1030.
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, et al. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482-488.
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, et al. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090-2093.
- West AR, Oates PS (2008) Mechanisms of heme iron absorption: Current questions and controversies. World J Gastroenterol 14: 4101-4110.
- Lim BC, Morgan EH (1985) Transferrin endocytosis and iron uptake by developing erythroid cells in the chicken (Gallus domesticus). Journal of Comparative Physiology B 155: 201-210.
- Ganz T (2006) Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program 29-35.
- Zhao N, Zhang AS, Enns CA (2013) Iron regulation by hepcidin. J Clin Invest 123: 2337-2343.
- Fairweather-Tait SJ (1992) Bioavailability of trace elements. Food chem 43: 213-217.
- Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, et al. (1983) The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr 49: 331-342.
- Morris ER, Ellis R (1982) Phytate, Wheat Bran, and Bioavailability of Dietary Iron. In: Keis C (ed.). ACS symposium series. American Chemical Society, USA. 203: 121-141.
- Martinez-Torres C, Romano E, Layrisse M (1981) Effect of cysteine on iron absorption in man. Am J Clin Nutr 34: 322-327.
- Cook JD, Morck TA, Skikne BS, Lynch SR (1982) The importance of animal products in human iron nutrition. In: Beitz DC (ed.). Animal Products in Human Nutrition, Elsevier, New York, USA. Pg no: 322-336.
- Bezwoda WR, Bothwell TH, Charleton RW, Torrence JD, Macphail AP, et al. (1983) The relative dietary importance of haem and non-haem iron. S Afr Med J 64: 552-556.
- Gassmann B (1988) Requirements of vitamin A, iron, folate, and vitamin B12: report of a joint FAO/WHO expert consultation. Food and Agriculture Organization of the United Nations, Rome, Italy. Pg No: 128.
- Cartwright GE (1966) The anemia of chronic disorders. Semin Hematol 3: 351-375.
- Raj DS (2009) Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum 38: 382-388.
- Galushko EA (2014) [The clinical significance of hepcidin detection in the patients with anemia and rheumatoid arthritis]. Klin Med (Mosk) 92: 21-27.
- Zhou S, Du X, Xie J, Wang J (2017 Interleukin-6 regulates iron-related proteins through c-Jun N-terminal kinase activation in BV2 microglial cell lines. PLoS One 12: 0180464.
- Jenkins K (2012) Measuring quality of life in anaemia management. Journal of Renal Nursing 4: 26-27.
- Cash JM, Sears DA (1989) The anaemia of chronic disease: spectrum of associated diseases in a series of unselected hospitalized patients. Am J Med 87: 638-644.
- Lipschitz DA (1990) The anaemia of chronic disease. Am Geriatr Soc 38: 1258-1264.
- Thompson WG, Meola T, Lipkin M Jr, Freedman ML (1988) Red cell distribution width, mean corpuscular volume, and transferrin saturation in the diagnosis of iron deficiency. Arch Intern Med 148: 2128-2130.
- Bathol S, Wang Q, Qureshi S, Chua E (2013) The red cell diameter width distribution, the forgotten haematological parameter for anaemia in the older person. European Geriatric Medicine 4: 1-4.
- Lam AP, Gundabolu K, Sridharan A, Jain R, Msaouel P, et al. (2013) Multiplicative interaction between mean corpuscular volume and red cell distribution width in predicting mortality of elderly patients with and without anaemia. Am J Hematol 88: 245-249.
- Thompson W, Cassino C, Babitz L, Meola T, Berman R, et al. (1989) Hypersegmented neutrophils and vitamin B12 deficiency. Hypersegmentation in B12 deficiency. Acta Haematol 8: 186-1891.
- Guyatt GH, Patterson C, Ali M, Singer J, Levine M, et al. (1990) Diagnosis of iron-deficiency anaemia in the elderly. Am J Med 88: 205-209.
- Casale G, Bonora C, Migliavacca A, Zurita IE, de Nicola P (1981) Serum Ferritin and Ageing. Age Ageing 10: 119-122.
- Worwood M (1997) The laboratory assessment of iron status--an update. Clin Chim Acta 259: 3-23.
- Elwood PC, Shinton NK, Wilson CI, Sweetown P, Frazel AC (1971) Haemoglobin Vitamin B12 and folate levels in the elderly. BR Journal Haematol 21: 557-563.
- Rodger RS, Fletcher K, Fail BJ, Rahman H, Sviland L, et al. (1987) Factors influencing haematological measurements in healthy adults. J Chronic Dis 40: 943-947.
- Chua E, Clague JE, Sharma AK, Horan MA, Lombard M (1999) Serum transferrin receptor assay in iron deficiency anaemia and anaemia of chronic disease in the elderly. QJM 92: 587-594.
- Hastka J, Lasserree JJ, Schwartzbeck A, Strach M, Hehmann R. (1993) Zinc protoporphyrin in anaemia of chronic disorders. Blood 81: 1200-1204.
- Joosten E, Van Loon R, Billen J, Blanckaert N, Fabri R, et al. (2002) Serum transferrin receptor in the evaluation of the iron status in elderly hospitalised patients with anaemia. Am J Hematol 69: 1-6.
- Ferguson BJ, Skinkne BS, Simpson KM, Baynes RD, Cook JD (1992) Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med 119: 385-390.
- Punnoen K, Irjala K, Rajamäki A (1997) Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 89: 1052-1057.
- Genc S, Erten N, Karan, MA, Besisik, SK, Saka B, et al. (2004) Soluble Transferrin Receptor and Soluble Transferrin Receptor-Ferritin Index for Evaluation of the Iron Status in Elderly Patients. Tohoku J Exp Med 202: 135-142.
- Thomas C, Thomas L (2002) Biochemical markers and hematological indices in the diagnosis of functional iron deficiency. Clin Chem 48: 1066-1076.
- Brugnara C (2003) Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem 49: 1573-1578.
- Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, et al. (2013) Guidelines for the laboratory diagnosis of functional iron deficiency. Br J Haematol 161: 639-648.
- Michels KR, Zhang Z, Bettina AM, Cagnina RE, Stefanova D, et al. (2017) Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2: 92002.
- Stefanova D, Raychev A, Arezes J, Ruchala P, Gabayan V, et al. (2017) Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non–transferrin-bound iron. Blood 130: 245-257.
- Bregman DB, Morris D, Koch TA, He A, Goodnough L (2013) Hepcidin levels predict nonresponsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol 88: 97-101.
- Thomas C, Kobold U, Thomas L (2011) Serum hepcidin-25 in comparison to biochemical markers and haematological indices for the differentiation of iron restricted erythropoiesis. Clin Chem Lab Med 49: 207-213.
- Joosten E, Meeuwissen J, Vandewinckele H, Hiele M (2008) Iron status and colorectal cancer in symptomatic elderly patients. Am J Med 121: 1072-1077.
- Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ (2015) Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS One 10: 0117383.
- Hahn PF, Bale WF, Ross JF, Balfour WM, Whipple GH (1943) Radioactive iron absorption by gastro-intestinal tract: influence of anemia, anoxia, and antecedent feeding distribution in growing dogs. J Exp Med 78: 169-188.
- Granick S (1946) Protein Apoferritin and Ferritin in Iron Feeding and Absorption. Science 103: 107.
- Fairweather-Tait SJ, Wright AJ (1984) The influence of previous iron intake on the estimation of bioavailability of Fe from a test meal given to rats. Br J Nutr 51: 185-191.
- Fairweather-Tait SJ, Swindell TE, Wright AJA (1985) Further studies in rats on the influence of previous iron intake on the estimation of bioavailability of Fe. Br J Nutr 54: 79-86.
- Rimon E, Kagansky N, Kagansky M, Mechnick L, Mashiah T, et al. (2005) Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med 118: 1142-1147.
- Moretti D, Goede JS, Zeder C, Jiskra M, Chatzinakou V, et al. (2015) Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 126: 1981-1989.
- Stoffel NU, Cercamondi CI, Brittenham G, Zeder C, Geurts-Moespot AJ, et al. (2017) Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol 4: 524-533.
- Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, et al. (2015) European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 9: 211-222.
- Froessler B, Palm P, Weber I, Hodyl NA, Singh R, et al. (2016) The Important Role for Intravenous Iron in Perioperative Patient Blood Management in Major Abdominal Surgery: A Randomized Controlled Trial. Ann Surg 264: 41-46.
- Muñoz M, Gómez-Ramírez S, Cuenca J, García-Erce JA, Iglesias-Aparicio D, et al. (2013) Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion 54: 289-299.
- Cavill I, Auerbach M, Bailie GR, Barrett-Lee P, Beguin Y, et al. (2006) Iron and the anaemia of chronic disease: a review and strategic recommendations. Curr Med Res Opin 22: 731-737.
- [No authors listed] (1985) Summary of a report on assessment of the iron nutritional status of the United States population. Am J Cin Nutr 42: 1318-1330.
- Cook JD (1982) Clinical evaluation of iron deficiency. Semin Hematol 19: 6-18.
- Auerbach M, Witt D, Toler W, Fierstein M, Lerner RG, et al. (1988) Clinical use of the total dose intravenous infusion of iron dextran. J Lab Clin Med 111: 566-570.
- Grasso P (1973) Sarcoma after intramuscular iron injection. Br Med J 2: 667.
- Andrès E, Affenberger S, Vinzio S, Kurtz JE, Noel E, et al. (2005) Food-cobalamin malabsorption in elderly patients: Clinical manifestations and treatment. Am J Med 118: 1154-1159.
- Hin H, Clarke R, Sherliker P, Atoyebi W, Emmens K, et al. (2006) Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing 35: 416-422.
- Carmel R (2008) Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patient age and race. Clin Exp Immunol 89: 74-77.
- Farrell CJ, Kirsch SH, Herrmann M (2013) Red cell or serum folate: what to do in clinical practice? Clin Chem Lab Med 51: 555-569.
- Tisman G, Herbert V (1973) B(12) dependence of cell uptake of serum folate: an explanation for high serum folate and cell folate depletion in B 12 deficiency. Blood 41: 465-469.
- de la Iglesia S, Martin P, Garcia G, Lopez J, Luzardo H, et al. (2007) Comparison between serum and erythrocyte folate in patients with anemia and/or macrocytosis. Haematologica 92: 428.
- Klee GG (2000) Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin Chem 46: 1277-1283.
- Snow CF (1999) Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med 159: 1289-1298.
- Flynn MA, Singh A, Slaughter J, King P, Krause G, et al. (2003) Interrelationship of homocysteine-cobalamin-folate indices in human subjects of various ages: can hyper-homocyteinemia be relieved with B-12 supplementation? Mo Med 100: 155-158.
- Heinz J, Domrose U, Westphal S, Luley C, Neumann KH, et al. (2008) Washout of water-soluble vitamins and of homocysteine during haemodialysis: effect of high-flux and low-flux dialyser membranes. Nephrology (Carlton) 13: 384-389.
- Marchand A, Galen RS, Van Lente F (1980) The Predictive Value of Serum Haptoglobin in Hemolytic Disease. JAMA 243: 1909-1911.
- Galen RS (1982) Application of the predictive value model in the analysis of test effectiveness. Clin Lab Med 2: 685-699.
- Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, et al. (2013) Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 165: 575-582.
- Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, et al. (2014) Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 35: 2468-2476.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, et al. (2015) Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J 36: 657-668.
- Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, et al. (2004) Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 292: 1555-1562.
- Litton E, Xiao J, Ho KM (2013) Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ 347: 4822.
- Stewart GA, Gansevoort RT, Mark PB, Rooney E, McDonagh TA, et al. (2005) Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney int 67: 217-226.
- Middleton RJ, Parfrey PS, Foley RN (2001) Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 12: 1079-1084.
- Am J Kidney Dis (2001) IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2000. Am J Kidney Dis 37: 182-238.
- Fishbane S, Maesaka JK (1997) Iron management in end-stage renal disease. Am J Kidney Dis 29: 319-333.
- Macdougall IC (2017) Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin Kidney J 10: 16-24.
- Cook JD, Flowers CH, Skikne BS (2003) The quantitative assessment of body iron. Blood 101: 3359-3364.
- Joosten E, Lioen P (2015) Iron deficiency anemia and anemia of chronic disease in geriatric hospitalized patients: How frequent are comorbidities as an additional explanation for the anemia? Geriatr Gertontol Int 15: 931-935.
- Guralnik JM, Eisenstaedt RS, Ferruci L, Klein HG, Woodman RC (2004) Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 104: 2263-2268.
- Joosten E, Pelemans W, Hiele M, Noyen J, Verhaeghe R, et al. (1992) Prevalence and causes of anaemia in a geriatric hospitalized population. Gerontology 38: 111-117.
Citation: Chua P, Wang Q, Chua E (2018) Anaemia in the Older Person. J Gerontol Geriatr Med 4: 020.
Copyright: © 2018 Chua P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

