
Patient-Reports of Fatigue and Cognitive Changes in Cancer Survivors
*Corresponding Author(s):
Christine Hill-KayserDepartment Of Radiation Oncology, University Of Pennsylvania, Philadelphia, Pennsylvania, Australia
Tel:+1 2156627771, +1 2158507903
Fax:2153495445
Email:hill@uphs.upenn.edu
Abstract
INTRODUCTION
METHODS
Older cancer survivors utilized an Internet-based resource for creation of survivorship care plans by their own volition, creating a convenience sample frame. The care plan program allows patients to answer a series of questions in order to generate a detailed survivorship care plan, the details of which have been described previously [5]. Created in 2007 by a team of oncology nurses and physicians, the tool has undergone several revisions and modifications to include questions regarding patient-related outcomes and late sequela in order to provide more focused information for the user. Survivors are queried regarding their experience with perceived cognitive changes via the question “are you concerned about cognitive changes, such as memory loss, difficulty with short-term memory, concentration, or learning new skills?” and about pervasive fatigue via the question “Are you experiencing fatigue (overwhelming physical, mental or emotional exhaustion)?” Responses to these questions included “yes,” “no,” and “don't know” and were collapsed into two categories (“yes” versus “no” and “don't know”) for analysis. Survivors also provide information on demographics (age, race, gender), cancer diagnosis and cancer treatments received. We used Fisher's exact tests to assess the statistical significance of comparisons; statistical significance was defined as p<0.05. Institutional Review Board approval was obtained prior to any study proceedings. For this study, data were obtained from the survivor user database for all survivors with a diagnosis age of 75 years or older.
RESULTS
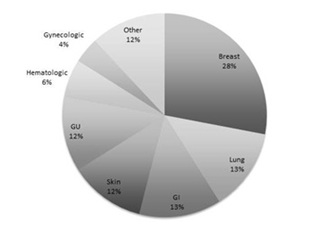 Figure 1: Distribution of cancer diagnosis among 174 older cancer survivors utilizing an Internet-based tool for creation of survivorship care plans.
Figure 1: Distribution of cancer diagnosis among 174 older cancer survivors utilizing an Internet-based tool for creation of survivorship care plans.
| Survivor Characteristics | N (%) |
| Gender | |
| Male | 56 (32%) |
| Female | 118 (68%) |
| Race | |
| Caucasian | 144 (83%) |
| African-American | 13 (2%) |
| Other | 7 (4%) |
| Age | |
| Median | 79 years |
| Range | 75->100 years |
| Current | |
| Median | 80 years |
| Range | 76->100 years |
| Time Since Diagnosis | |
| Median | 1 year |
| Range | <1–16 years |
| Treatments Received | |
| Radiation | 91 (52%) |
| Breast/chest wall | 30 (32%) |
| Thorax | 11 (12%) |
| Pelvis | 10 (11%) |
| Head and neck | 6 (3%) |
| Skin | 6 (3%) |
| Brain | 1 (1%) |
| Other | 27 (30%) |
| Chemotherapy | 85 (49%) |
| Surgery | 118 (68%) |
Table 1: Characteristics of “old-old” cancer survivors utilizing an Internet-based tool for creation of survivorship care plans and providing patient-reported information on late effects.
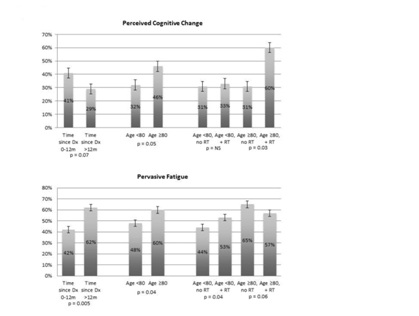 Figure 2: Patient reports of perceived cognitive change (upper panel) and pervasive fatigue (lower panel) in older cancer survivors utilizing an Internet-based tool for creation of survivorship care plans based on time since diagnosis, age and receipt of radiotherapy.
Figure 2: Patient reports of perceived cognitive change (upper panel) and pervasive fatigue (lower panel) in older cancer survivors utilizing an Internet-based tool for creation of survivorship care plans based on time since diagnosis, age and receipt of radiotherapy. Dx: Diagnosis; RT: Radiotherapy
DISCUSSION
CONFLICTS OF INTEREST
There are no conflicts of interest to disclose on the part of any author.
ACKNOWLEDGMENT
This work was funded in part by a grant from the LIVESTRONG Foundation.
REFERENCES
- National Institute on Aging (2001) Exploring the Role of Cancer Centers for Integrating Aging and Cancer Research. National Institute on Aging, Baltimore, Maryland, USA.
- Rose JH, Bowman KF, Deimling GT, Stoller EP (2005) Health Maintenance Activities and Lay Decision-Making Support: A Comparison of Young-Old and Old-Old Long-Term Cancer Survivors. J Psycho Oncol 22: 21-44.
- Laugsand EA, Sprangers MA, Bjordal K, Skorpen F, Kaasa S, et al. (2010) Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes 8: 104.
- Nekolaichuk CL, Bruera E, Spachynski K, MacEachern T, Hanson J, et al. (1999) A comparison of patient and proxy symptom assessments in advanced cancer patients. Palliat Med 13: 311-323.
- Hill-Kayser CE, Vachani C, Hampshire MK, Jacobs LA, Metz JM (2009) An internet tool for creation of cancer survivorship care plans for survivors and health care providers: design, implementation, use and user satisfaction. J Med Internet Res 11: 39.
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, et al. (2000) SEER Cancer Statistics Review, 1975-2000. National Cancer Institute, Maryland, USA.
- Uyar D, Frasure HE, Markman M, von Gruenigen VE (2005) Treatment patterns by decade of life in elderly women (> or =70 years of age) with ovarian cancer. Gynecol Oncol 98: 403-408.
- Taphoorn MJ, Stupp R, Coens C, Osoba D, Kortmann R, et al. (2005) Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol 6: 937-944.
- Ahles TA, Root JC, Ryan EL (2012) Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 30: 3675-3686.
- Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, et al. (2006) Cognitive Function of Older Patients Receiving Adjuvant Chemotherapy for Breast Cancer: A Pilot Prospective Longitudinal Study. J Am Geriatr Soc 54: 925-931.
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, et al. (2010) Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 28: 4434-4440.
- Koch L, Bertram H, Eberle A, Holleczek B, Schmid-Höpfner S, et al. (2014) Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship--a multi-regional population-based study. Psychooncology 23: 547-554.
- Debess J, Riis JØ, Engebjerg MC, Ewertz M 2010) Cognitive function after adjuvant treatment for early breast cancer: A population-based longitudinal study. Breast Cancer Res Treat 121: 91-100.
- Quesnel C, Savard J, Ivers H (2009) Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat 116: 113-123.
- Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D (2005) The effects of adjuvant chemotherapy on cognition in women with breast cancer--preliminary results of an observational longitudinal study. Breast 14: 142-150.
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, et al. (2006) A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94: 828-834.
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, et al. (2007) Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer 109: 1905-1913.
- 18. Von Ah D, Storey S, Crouch A, Johns SA, Dodson J, et al. (2017) Relationship of Self-reported Attentional Fatigue to Perceived Work Ability in Breast Cancer Survivors. Cancer Nurs 40: 464-470.
- Cheng KK, Lee DT (2011) Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol 78: 127-137.
- Prue G, Rankin J, Allen J, Gracey J, Cramp F (2006) Cancer-related fatigue: a critical appraisal. Eur J Cancer 42: 846-863.
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, et al. (2006) Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 106: 751-758.
- Thong MS, Mols F, Wang XS, Lemmens VE, Smilde TJ, et al. (2013) Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Euro J Cancer 49: 1957-1966.
- van Geest Q, Westerik B, van der Werf YD, Geurts JJ, Hulst HE (2017) The role of sleep on cognition and functional connectivity in patients with multiple sclerosis. J Neurol 264: 72-80.
- Wiseman SJ, Bastin ME, Hamilton IF, Hunt D, Ritchie SJ, et al. (2017) Fatigue and cognitive function in systemic lupus erythematosus: associations with white matter microstructural damage. A diffusion tensor MRI study and meta-analysis. Lupus 26: 588-597.
- Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, et al. (2008) Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer 16: 399-405.
- Zhang HS, Li Y, Mo HY, Qiu DX, Zhao J, et al. (2017) A community-based cross-sectional study of sleep quality in middle-aged and older adults. Qual Life Res 26: 923-933.
- Chiu HY, Lai FC, Chen PY, Tsai PS (2016) Differences Between Men and Women Aged 65 and Older in the Relationship Between Self-Reported Sleep and Cognitive Impairment: A Nationwide Survey in Taiwan. J Am Geriatr Soc 64: 2051-2058.
Citation: Frick M, Vachani C, Bach C, Arnold-Korzienowski K, Metz J, et al. (2018) Patient-Reports of Fatigue and Cognitive Changes in Cancer Survivors. J Gerontol Geriatr Med 4: 018.
Copyright: © 2018 Melissa Frick, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

