
Effect of Sodium Tripolyphosphate on Polished and Roughened Bovine Enamel
*Corresponding Author(s):
Changxiang WangSchool Of Dentistry, University Of Birmingham, 5 Mill Pool Way, Edgbaston, Birmingham B5 7EG, United Kingdom
Tel:+44 1214665528,
Fax:+44 1214665491
Email:c.wang@bham.ac.uk
Abstract
Objectives: Sodium Tripolyphosphate (STP) is commonly incorporated into toothpastes for stain removal where its mild chelating properties interfere with stained pellicle integrity. However, these chelating properties may negatively impact on enamel surface finish. This study investigated the effects of STP treatment on the surface finish of polished and roughened enamel with and without tooth-brushing.
Methods: Bovine enamel specimens (n=8/group) were prepared to either 1200-grit SiC and 3µm diamond finish (Polished group), P800-ground finish, or P320-ground finish and soaked or brushed (Oral B P35), using an in vitro tooth-brushing simulator (5-60m brushing), in STP solutions of concentrations (w/w) 2.5%, 5.0% or 10.0% STP. Gloss changes were measured with a Novo-Curve glossmeter and surface roughness and wear depth determined by profilometry.
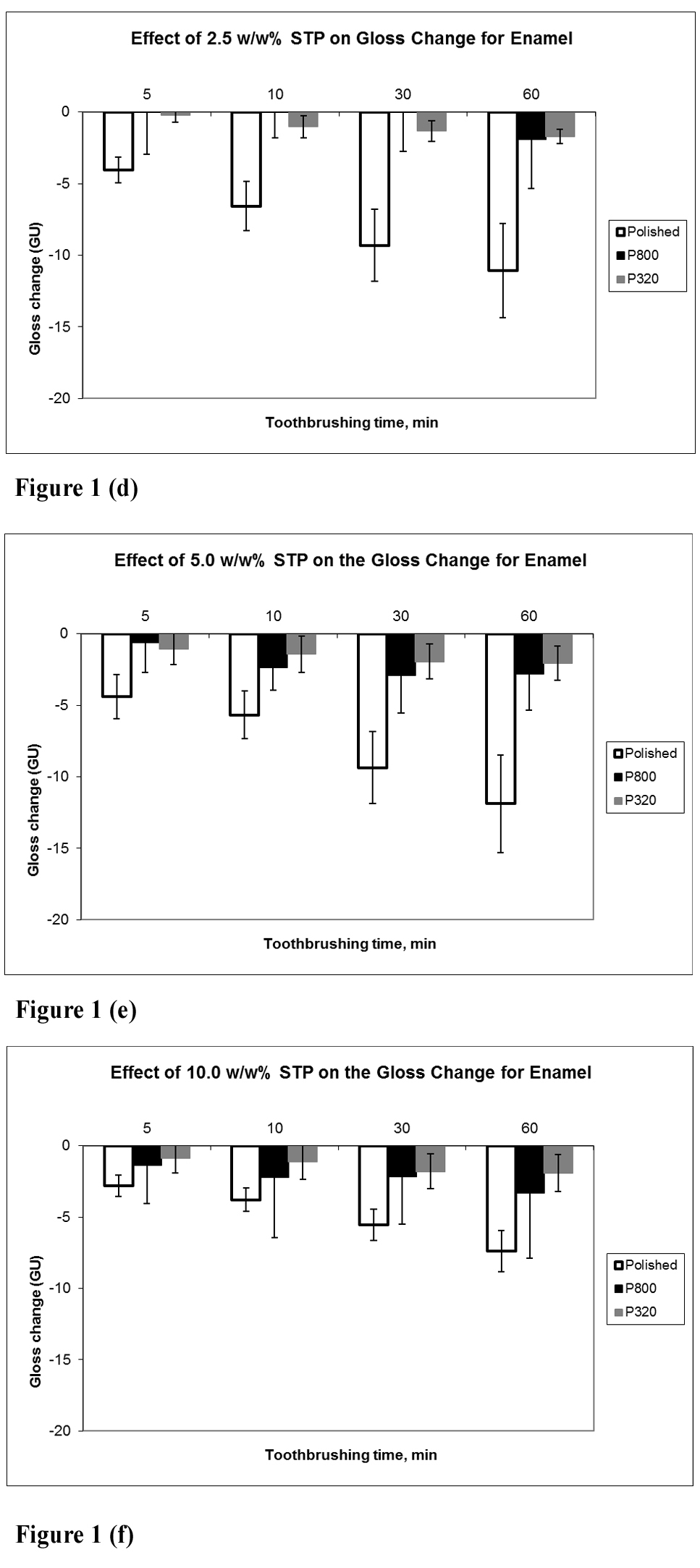
Results: There were no significant changes in surface roughness and wear depth after brushing in STP for 5-60m. Small gloss decreases occurred for all polished and roughened specimens, which were of greater magnitude with prolonged soaking or brushing. Brushing in STP did not exacerbate the gloss loss relative to soaking for equivalent times. There was no clear linear relationship between STP concentration and gloss change after soaking or brushing reflecting the small gloss changes taking place.
Conclusion: Minor decreases in enamel surface gloss following extensive soaking in STP did not cause greater susceptibility to wear during tooth-brushing. Only minimal changes in enamel surface finish occurred after exposure to STP, even with highly polished surfaces, and its stain removal properties potentially provide positive benefits for oral hygiene.
Keywords
INTRODUCTION
Effective cleaning of the tooth surface through good oral hygiene is central to both the prevention of oral and dental disease and the appearance of the dentition. Clear causal links are well established between the presence of dental plaque, caries and periodontal disease. The appearance of the teeth, particularly whiteness, is of great importance to patients and tooth discolouration is a common dental complaint. It has been reported, depending on the population examined, that personal dissatisfaction with the appearance of the dentition ranges from 17.9 to 52.6% [1-5]. Extrinsic staining of the tooth can arise from a variety of sources including smoking, red wine consumption, cationic compounds such as chlorhexidine or stannous salts [6-9]. Stain removal can be challenging, thus, oral hygiene strategies need to address both the removal of dental plaque and extrinsic stain.
While a variety of tooth whitening procedures are used professionally [10-13], they are costly and labour intensive [14], and some of these procedures do not contribute to oral hygiene. There is considerable demand for ‘Over-The-Counter’ (OTC) tooth-whitening products that can be integrated into a normal oral hygiene regime, and whitening toothpastes are the dominant delivery format [15]. In general, whitening toothpastes provide tooth whitening by abrasive removal of extrinsic stain and dental plaque and the chemical action of constituents such as Sodium Tripolyphosphate (STP), Sodium pyrophosphate, sodium hexametaphosphate, hydrogen peroxide, as well as the enzymes papain and bromelain [3].
Sodium Tripolyphospahte (STP) is a linear condensed phosphate that is commonly incorporated in whitening toothpastes for effective stain removal [16], where its mild chelating properties interfere with stained pellicle integrity [17-19]. However, increasing interest in enamel surface finish and polishing raises questions as to whether these chelating properties may negatively impact enamel surface finish. There are not many studies in the literature regarding the effect of STP on the surface roughness and gloss at varying concentrations. The present in vitro study investigates the effects of STP treatment on the surface finish of polished and roughened bovine enamel, with and without tooth brushing.
MATERIALS AND METHODS
Effects of STP on enamel without tooth brushing
Effect of STP on enamel with tooth brushing
Linear profiles (2D) were taken of the brushed specimen surfaces using a Talysurf Series 2 inductive gauge profilometer (Taylor-Hobson, UK), and wear depth and roughness values were calculated using Talysurf software and 3D surface analysis of selecting specimens was conducted. Gloss measurements were taken before and after brushing with a Novo-Curve small area glossmeter, at intervals of 90 degrees rotation about the centre point of each specimen.
Statistical analysis of the data
RESULTS
Effect of STP on enamel without tooth-brushing

Effect of STP on enamel with tooth-brushing
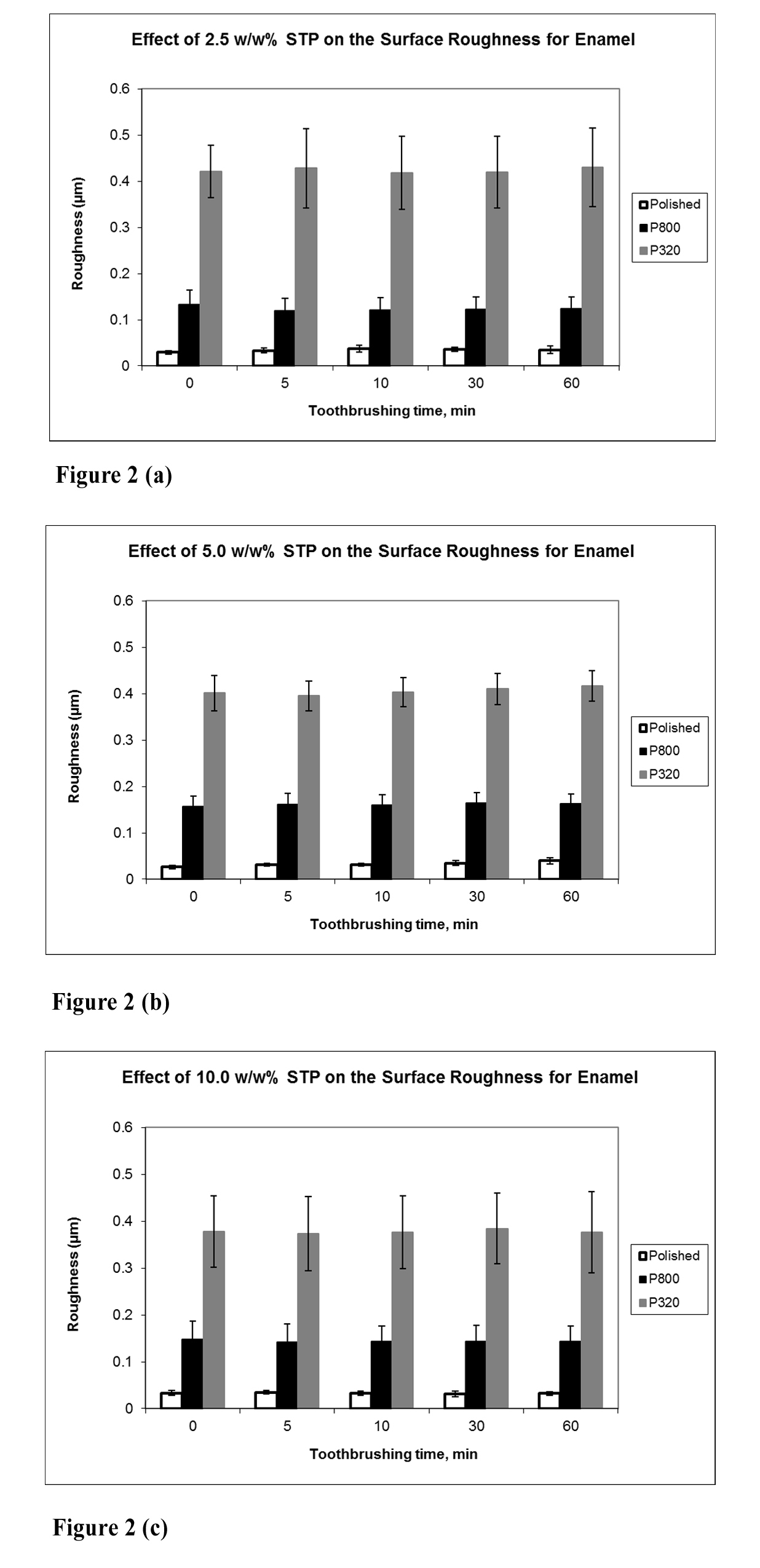
The mean surface roughness for the polished and roughened bovine enamel surfaces did not change following brushing for up to 60 mins (7200 strokes) and STP concentration had no influence on this parameter (Figure 2: a-c).
DISCUSSION
Bovine enamel has been used in this pilot study due to the reported and extensive similarities between bovine and human teeth. Indeed a recent study, which compared teeth from several species, indicated that on the basis of their chemical and morphological composition, bovine teeth should be the first choice as substitutes for human teeth in research [21].
Sodium tripolyphosphate, a sodium salt of triphosphoric acid, has been widely used for water treatment, detergency and in the food industry [22,23]. Its surfactant and chelating properties have led to its use in whitening toothpastes for stain removal. In vitro studies with crystalline Hydroxyapatite (HA) powder showed that STP was effective in removing existing stain and inhibiting stain formation through inhibition of the adsorption of salivary protein or tea stain and the desorption of existing protein and stain from HA surfaces [15,24]. In vivo trials also reported significant extrinsic stain removal efficacy for a dentifrice containing STP versus the baseline [18] and a reduction in dental stain by a chewing gum containing STP [25]. These beneficial effects for stain removal and inhibition depend in part on the chelating properties of STP, the latter of which may, however, negatively impact on enamel surface finish.
In the present study, the effects of STP on several parameters of enamel surface finish have been examined to determine whether its chelating properties have any adverse effects. Furthermore, exposure of enamel to STP has been investigated both by simple soaking of specimens in STP solutions and also, following tooth-brushing with STP solutions under clinically relevant exposure time conditions. Any significant chelating action of STP may be expected to show exaggerated enamel surface loss when exposure occurs under brushing conditions due to the physical abrasion from brushing on a softened surface. Surface gloss of enamel represents a very sensitive parameter of change to the surface finish of this tissue. Soaking or brushing of enamel specimens in STP for periods of 5 – 60 mins resulted in only small decreases in surface gloss, with little difference between the two treatments, suggesting that any chelating action of STP caused minimal tissue loss from the enamel surface. Both surface roughness and wear depth values did not show any change after exposure of specimens to STP for up to 60 mins. The starting finish of an enamel surface (polished vs ground) influences its available surface area, but the lack of influence of this on gloss, surface roughness or wear depth emphasizes the minimal effects of STP on enamel surface finish, as does the lack of influence of STP concentration. Interestingly, a lack of influence of STP concentration on stain desorption from hydroxyapatite has also been reported [24].
Mechanistically, it has been suggested that the main action of STP in both the inhibition of salivary protein adsorption to hydroxyapatite and desorption of bound salivary proteins is through competitive binding to the crystal surface, although the chelating action of STP has been proposed as a possible additional factor [24]. Data from the present study indicate that chelating effects of STP on intact enamel are minimal under clinically relevant exposure times, implying that chelation may be of minor importance when considering adsorption/desorption of salivary proteins and stains to enamel.
In summary, the present study has demonstrated that exposure of enamel to STP, either by soaking or with brushing, results in only minimal changes to surface gloss and has no statistically significant effect on surface roughness or wear depth. Thus, several parameters of enamel surface finish indicate that any chelating action of STP on enamel is minimal under clinically relevant conditions underpinning the positive benefits from its use for stain removal from teeth.
ACKNOWLEDGEMENT
The authors gratefully acknowledge financial support from GSK Consumer Healthcare for this research.
COMPETING INTEREST
The authors are not aware of any competing interests for this study.
REFERENCES
- Joiner A (2010) Whitening toothpastes: A review of the literature. J Dent 38: 17-24
- Alkhatib MN, Holt R, Bedi R (2004) Prevalence of self-assessed tooth discolouration ion the United Kingdom. J Dent 32: 561-566
- Alkhatib MN, Holt R, Bedi R (2005) Age and perception of dental appearance and tooth colour. Gerodontology 22: 32-36
- Xiao J, Zhou XD, Zhu WC, Zhang B, Li JY, et.al. (2007) The prevalence of tooth discolouration and the self-satisfaction with tooth colour in a Chinese urban population. J Oral Rehabil 34: 351-360
- Odioso LL, Gibb RD, Gerlach RW (2000) Impact of demographic, behavioural, and dental care utilization parameters on tooth colour and personal satisfaction. Compend Contin Educ Dent Suppl 21: 35-41
- Joiner A (2006) Review of the extrinsic stain removal and enamel/dentine abrasion by a calcium carbonate and perlite containing whitening toothpaste. Int Dent J 56: 175-180
- Watts A, Addy M (2001) Tooth discolouration and staining: a review of the literature. Br Dent J 190: 309-316.
- Ten Bosch JJ, Coops JC (1995) Tooth colour and reflectance as related to light scattering and enamel hardness. J Dent Res 74: 374-380
- Nathoo SA (1997) The chemistry and mechanisms of extrinsic and intrinsic discolouration. J Am Dent Assoc 128: 6-10
- Joiner A (2006) The bleaching of teeth: A review of the literature. J Dent 34: 412-419
- Griffiths CE, Bailey JR, Jarad FD, Youngson CC (2008) An investigation into most effective method of treating stained teeth: An in vitro study. J Dent 36: 54-62
- Sarrett DC (2002 ) Tooth whitening today. J Am Dent Assoc 133: 1535-1538
- Lynch CD, McConnell RJ (2003) The use of microabrasion to remove discoloured enamel: A clinical report. J Prosthet Dent 90: 417-419
- Walsh TF, Rawlinson A, Wildgoose D , Marlow I, Haywood J, et.al. (2005) Clinical evaluation of the stain removal ability of a whitening dentifrice and stain controlling system. J Dent 33: 413-418
- Langford J, Pavey KD, Olliff CJ, Cragg PJ, Hanlon GW, et.al. (2002) Real-time monitoring of stain formation and removal on calcium hydroxyapatite surfaces using quartz crystal sensor technology. Analyst 127: 360-367
- American Dental Association (2001) Whitening toothpastes. Journal of the American Dental Association 132: 1146-1147
- Sharif N, MacDonald E, Hughes J, Newcombe RG, Addy M (2000) The chemical stain removal properties of whitening toothpaste products: studies in vitro. Br Dent J 188: 620-624
- Hughes N, Maggio B, Sufi F, Mason S, Kleber CJ (2009) A comparative clinical study evaluating stain removal efficacy of a new sensitivity whitening dentifrice compared to commercially available whitening dentifrice. J Clin Dent 20: 218-22
- Soparkar P, Rustogi K, Zhang YP, Petrone ME, DeVizio W, et.al. (2004) Comparative tooth whitening and extrinsic tooth stain removal efficacy of two tooth whitening dentifrice: six-week clinical trial. J Clin Dent 15: 46-51
- Parry J, Harrington E, Rees GD, McNab R, Smith AJ (2008) Control of brushing variables for the in vitro assessment of toothpaste abrasivity using s novel laboratory model. J Dent 36: 117-124
- Teruel JD, Alcolea A, Hernández A, Ruiz AJ (2015) Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Bio 60: 768-775
- Rashchi F, Finch JA (2000) Polyphosphates: A review. Their chemistry and application with particular reference to mineral processing. Minerals Engineering 13: 1019-1035
- Hourant P (2004) General properties of the alkaline phosphates: major food and technical applications. Phosphorus Research Bulletin 15: 85-94
- Shellis RP, Addy M, Rees GD (2005) In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. J Dent 33: 313-324
- Porciani PF, Perra C, Grandini S (2010) Effect on dental stain occurrence by chewing gum containing sodium tripolyphosphate—a double-blind six-week trial. J Clin Dent 21: 4-7
Citation: Wang C, Lucas R, Cooper PR, Smith AJ (2017) Effect of Sodium Tripolyphosphate on Polished and Roughened Bovine Enamel. J intern Med Prim Healthcare 2: 004
Copyright: © 2017 Changxiang Wang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

