Water Treatment through Mwcnt Nanomembranes
*Corresponding Author(s):
Jorge I CifuentesSchool Of Mechanical Engineering And Engineering Research Center, University Of San Carlos Of Guatemala, Guatemala
Tel:+502 2418 9133,
Email:jicifuentes@ing.usac.edu.gt
Abstract
Keywords
INTRODUCTION
CARBON NANOTUBES (CNTS)
ELECTRODEPOSITION
This research was performed under the norm “COGUANOR NGO 29 001” which contains markers and parameters to have optimal potable water [9].
MWCNT MEMBRANES AND GENERAL ASSEMBLY
Figure 1 shows the placement of all the components in a diagram. The cotton pads were moistened with rubbing alcohol and filled with Mwcnt to create membranes the Mwcnt gives the membrane the capacity to conduct electricity.
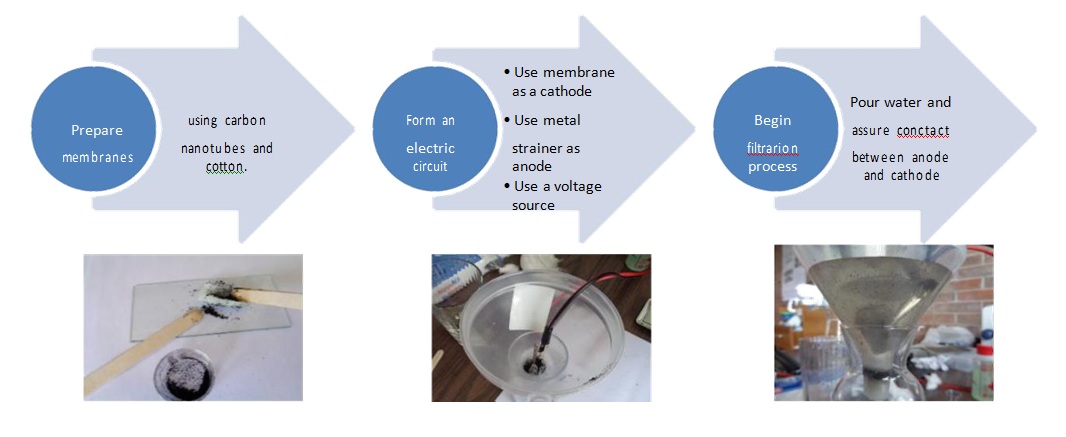
Figure 1: Fabrication process diagram.
The bottom of the funnel was filled with three layers before place the cotton membrane, in the following way:
From bottom to top a cotton pad a thick layer of Mwcnt acting as nano filter and membrane and another cotton pad. The plastic funnel is placed into the glass container to continue with the process.
With the membranes ready a copper wire was connected to it using an alligator clip and then placed it above the three-layer membrane detailed before, as shown in figure 2. This step is very important because the cotton and Mwcnt act as a membrane and they should restrict the passage of water to act as a filter.

Later the aluminum strainer is attached with an alligator clip to a copper wire, as shown in figure 3 and then attached to the top of the funnel taking care to leave a space between the strainer and the membrane.
The two wires were connected to a pair of 9v batteries which at that time had 9.48v in total. The tap water was poured into the funnel. The water acted as a conductor between the conducting membrane containing Mwcnt and the aluminum strainer both connected individually to the batteries.
In this process while the water was energized the Mwcnt acted as the cathode and the strainer as the anode. Most of the contaminants that were present in the water got attached to the nanotubes by the same principle of electrodeposition.
RESULTS AND DISCUSSION
|
|
Pathogenic Escherichia coli/ total/100 ml |
|
Before nano filtration |
6.8 |
|
After Mwcnt nano membranes filtration |
2.0 |
|
Normal test |
Presumptive test |
Confirmative test |
|
|
Gas Formation |
|||
|
Sown quantity |
Gas formation - 35 Celsius degrees |
total |
Fecal - 44.5 Celsius degrees |
|
10,00 cm3 |
+ + + + + |
+ + - - - |
- - - - - |
|
01,00 cm3 |
+ + - - - |
+ - |
- - |
|
00,10 cm3 |
- - - - - |
unnecessary |
unnecessary |
|
Normal test |
Presumptive test |
Confirmative test |
|
|
Gas Formation |
|||
|
Sown quantity |
Gas formation - 35 Celsius degrees |
total |
Fecal - 44.5 Celsius degrees |
|
10,00 cm3 |
+ + - - - |
+ - |
- - |
|
01,00 cm3 |
- - - - - |
unnecessary |
unnecessary |
|
00,10 cm3 |
- - - - - |
unnecessary |
unnecessary |
Table 3: Bacteriological analysis results after filtration.
In table 4, all the physicochemical parameters that were improved are shown.
|
Water pollutants |
Before filtration |
After filtration |
|
Total coliforms |
6.8 per 100 ml |
2.0 per 100 ml |
|
pH |
6.87 mg/l |
6.44 mg/l |
|
Turbidity |
4.04mg/l |
0.23mg/l |
|
Total hardness |
450 mg/l |
132 mg/l |
|
Color |
58units |
1.0unit |
|
Magnesium |
93.14 mg/l |
13.62 mg/l |
|
chlorides |
18.5mg/l |
17.50mg/l |
|
Calcium |
30.46 mg/l |
27.25 mg/l |
|
Sulfates |
7.0mg/l |
6.0mg/l |
|
Manganese |
0.132 mg/l |
0.08 mg/l |
|
Alkalinity and bicarbonates |
126 mg/l |
120 mg/l |
Table 4: Physiochemical analysis.
The number of coliform germs could be better but different factors affected the experiment during the process. An example of these factors was that the membrane was not secured properly and the water passed too quickly through it avoiding the treatment.
The heat and oxidation of the nanotubes created a bluish color in the water which must be taken into consideration in future experiments.
ACKNOWLEDGEMENT
REFERENCES
- Cifuentes J (2015) Improved health by nanotechnology for water treatment, qualitative research. PhD Climate Change and Sustainability. ResearchGate 2-9.
- Rusnano (2008) Nanomembranes technology for water filtration. Rusnano, Russia.
- NanoCeram (2014) Nanoceramic and nanofiltration water technologies, USA.
- Rafiee J, Mi X, Gullapalli H, Thomas A, Yavari F, et al. (2012) Wetting transparency of graphene. Nat Mater 11: 217-222.
- Mahdy A, Elkhatib E, LIN Z (2013) Effects of drinking water treatment residuals on soil solution composition and phosphorus speciation in biosolid-amended soils of Kafr El-Dawar, Egypt, and Troy, USA. Agrochimica 57: 315-336.
- Alex PC (2010) Carbon nanotubes. Journal of Information, Technology and Society 10-14.
- Hilder M, Winther-Jensen B, Li D, Forsyth M, MacFarlane DR (2011) Direct electro-deposition of graphene from aqueous suspensions. Physical Chemistry Chemical Physics 13: 9187-9193.
- Osborne K (2017) Electroplating. Perry Metal Protection Ltd, Auckland, New Zealand.
- http://www.ada2.org/sala-prensa/publicaciones/doc_view/28-cogua¬nor-29001-99
Citation: Cifuentes JI, Argueta CM (2018) Water Treatment through Mwcnt Nanomembranes. J Nanotechnol Nanomed Nanobiotechnol 5: 021.
Copyright: © 2018 Jorge I Cifuentes, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.