
An Unusual Case of Acute Kidney Injury with Edematous Kidneys and Venous Micro Thrombi: A Case Report
*Corresponding Author(s):
Rakesh MadhyasthaDepartment Of Nephrology, Medical Subspecialties Institute, Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates
Tel:+971589914919,
Email:Madhyap@clevelandclinicabudhabi.ae
Abstract
This case report describes a young patient with Acute Kidney Injury (AKI) stage 3, low grade micro-hematuria (3-5 dysmorphic red blood cells in each high power field [RBC/HPF]), low grade proteinuria (Urine Protein To Creatinine (UPCR) of around 1 gm/gm), edematous kidneys on ultrasound, and unusual histologic finding on kidney biopsy. The main complaints on presentation were low back and flank pains for a few days. He took 10 tablets of acetaminophen and 2 tablets of ibuprofen over the last few days before the symptoms started. He did not have any alcohol or illicit drugs use. Investigations have not revealed a cause for this AKI including Doppler ultrasound of the renal arteries and veins, and serologic testing. Anti dsDNA was positive, although ANA and anti-histones were negative.
The kidney biopsy revealed mild irregular interstitial edema with limited inflammation. There was no significant interstitial fibrosis or tubular atrophy. There were microthrombi filling distended small veins throughout the renal cortex. Small arteries and arterioles are within normal limits. The patient recovered renal function within 3 weeks with supportive care. There was no need for dialysis.
Keywords
Acute kidney injury; Edematous kidneys; Microthrombi
BACKGROUND
CASE HISTORY
Vital signs were normal. Physical examination was positive for mild costovertebral angle tenderness on both sides. Otherwise, the rest of the physical exam was unremarkable. He had no skin rash.
Initial laboratory testing revealed a serum creatinine of 341 umol/L (3.8 mg/dl), and urinalysis showed proteinuria (2+), and 0-3 and 3-5 RBC/HPF. Urine protein to creatinine ratio was 94 mg/mmol. Urine microscopy revealed few white blood cells, few dysmorphic red blood cells, and granular casts. The patient received intravenous fluids. He then had a chest X-ray which showed lower lobes atelectasis, with small bilateral pleural effusions. Renal ultrasound showed mildly enlarged and edematous kidneys of 14-14.5 cm, cortical thickness of 2.3-2.4 cm, and mildly increased echogenicity. Doppler ultrasound of both renal arteries and veins was normal. Serological studies showed negative Anti-neutrophilic Cytoplasmic Antibody (ANCA), and Anti-glomerular Basement Antibody (anti-GBM). Anti-double stranded DNA antibody was low positive. The titer was 10 IU/ml (Ref range 0-9). C3 complement was within the normal range and anti-histone antibody were negative. No drug or alcohol testing was done. The patient was mildly anemic with lowest hemoglobin (Hb) of 113 g/L but Hb and Platelets were relatively stable throughout the course. He had a normal Hb electrophoresis. Peripheral smear was never done. Renal function continued to deteriorate despite supportive care, and a kidney biopsy was performed on day 3 of his admission.
Histopathologic finding Sections cut at multiple levels and stained with H&E (Figure 1A), PAS (Figure 1B), Jones and trichrome stains were examined. They showed renal cortex in which approximately 30 glomeruli were present. Medulla was not included. The glomeruli were mostly normocellular, largely bloodless with sparse leukocytes in occasional poorly expanded capillary lumens. The tubulointerstitial compartment showed mild irregular interstitial edema with patchy accentuation. The proximal tubules had subtle fraying of their luminal brush border best seen on PAS (Figure 1B). A small number of distal tubules profiles had a few damaged or apoptotic cells. Limited inflammation including a few lymphocytes, histiocytes and sparse neutrophils was present in patchy foci of mildly accentuated interstitial edema with reactive stromal cells often in the vicinity of distended veins. There was no significant interstitial fibrosis or tubular atrophy.
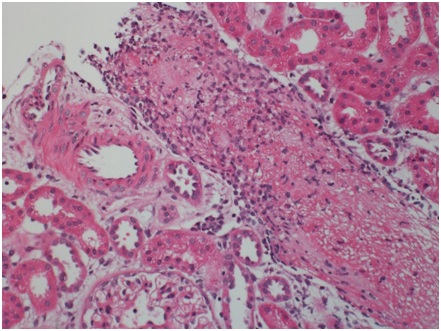
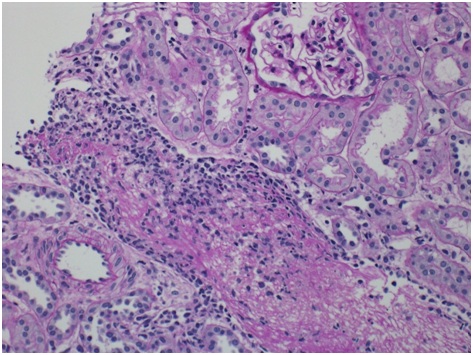
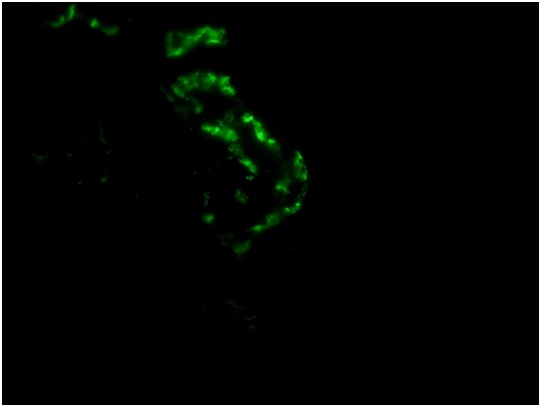
The most striking feature was the presence of a microthrombus filling a distended vein branch across the width of one core. The vein wall was difficult to identify but was highlighted by immunehistochemical stain for CD31. There were also mononuclear cells aggregated at the periphery of the microthrombus that were, at least focally, in continuity with flat endothelium. Other distended small veins were present throughout the renal cortex. There were 2 microscopic foci of leaked proteinaceous or extruded thrombus material with associated reactive macrophages and monocytes. Small arteries and arterioles were within normal limits. The scouting sections for direct immunofluorescence showed the presence of up to 5-6 glomeruli of which one had early tuft retraction and capsular fibrosis suggestive of some ischemic damage(Figure 1C).
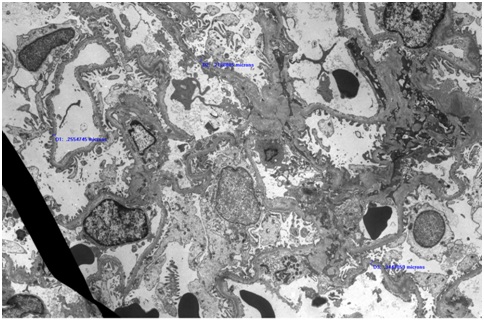
There was evidence of ATN in the distal tubules and the proximal tubules had subtle fraying of their luminal brush border. However there was no significant interstitial fibrosis or tubular atrophy. On electron microscopy (Figure 1D), section examined had 4 glomeruli. 2 glomeruli had poorly expanded capillary loops. At the ultrastructural level, the glomerular basement membrane was within the normal limit for thickness. Occasional loops had mild widening of the largely lucent subendothelial layer in which scant flocculent particles were noted. There was a patchy limited broadening of podocyte foot processes.
Subsequent progress: Serum creatinine continued to deteriorate and peaked at 635 umol/L. There was no indication to start dialysis. The patient was treated conservatively with intravenous hydration, pain medications and low salt/low potassium diet. Creatinine started improving slowly and was down to 88 umol/L (0.9 mg/dl) on day 21 after the presentation. Proteinuria has also resolved completely. He had no further back or flank pains.
DISCUSSION
The literature review has revealed limited information and reports that describe similar presentations and close histologic findings in the setting of illicit drugs use such as ecstasy or a large amount of alcohol consumption. Our patient denied both. We suspect an immunologically mediated process which caused endothelial injury. This injury may have been compounded by hypercoagulability, or abnormal blood flow possibly due to dehydration, could have potentially triggered those microthrombi. Additionally, NSAIDs may have also affected the intrarenal blood flow and contributed to this presentation. Redfern A et al., describes a case series on similar unexplained pathologic finding of renal arcuate venous microthrombi with associated localized inflammatory response. In case series of 6 patients, all of them reported to have consumed alcohol and notably 4 out of 6 patients were either using NSAID or Acetaminophen for pain. Hematuria and proteinuria along with negative immunology screen was reported [11]. The other striking features are the presence of flank pain as a presenting complaint and demographically young patients [11-13]. Most often patients have had successful renal recovery with conservative management however there have been reports where temporary dialysis has been necessary [11,14]. The possibility of post-infectious glomerulonephritis was entertained but he had normal C3 and negative cultures. Anti-streptolysin O titer was normal.
The above are speculations, and the exact cause of this AKI remains uncertain. The first mechanism of Acute Kidney Injury (AKI) from NSAIDs is due to reduced renal plasma flow caused by a decrease in prostaglandins, which regulate vasodilation at the glomerular level. NSAIDs disrupt the compensatory vasodilation response of renal prostaglandins to vasoconstrictor hormones released by the body [15]. Inhibition of renal prostaglandins results in acute deterioration of renal function after ingestion of NSAIDs. The second mechanism of AKI is acute interstitial nephritis (AIN), which is characterized by the presence of an inflammatory cell infiltrate in the interstitium of the kidney. AIN is caused by an immunological reaction after NSAID exposure of about a week [16]. AIN is now recognized as a major cause of drug induced AKI and accounts for about 15% of all patients with unexplained AKI [17]. As an entity, AIN due to NSAIDs is under recognized as well. In our patient the biopsy did not show evidence of AIN and there was absence of widespread Acute Tubular Necrosis (ATN) as well Increased awareness of such presentation and further identification of similar cases may help clarify this entity and explain its etiology and pathophysiology.
CONCLUSION
REFERENCES
- Lameire N, Van Biesen W, Vanholder R(2006) The changing epidemiology of acute renal failure. Nat ClinPractNephrol 2: 364-377.
- Lameire N, Van Biesen W, Vanholder R(2006) The rise of prevalence and the fall of mortality of patients with acute renal failure: What the analysis of two databases does and does not tell us. J Am SocNephrol 17: 923-925.
- Lameire N, Van Biesen W, Vanholder R (2005) Acute renal failure. Lancet 365: 417-430.
- Cerda´ J, Lameire N, Eggers P, Pannu N, Uchino S, et al. (2008) Epidemiology of Acute Kidney Injury. Clin J Am SocNephrol 3: 881-886.
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, et al. (2006) Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am SocNephrol17: 1135-1142.
- Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM (2006) Declining mortality in patients with acute renal failure, 1988 to 2002. J Am SocNephrol 17: 1143-1150.
- Liano F, Pascual J(1996) Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811-818.
- Arije A, Kadiri S, Akinkugbe OO (2000)The viability of hemodialysis as a treatment option for renal failure in a developing economy. Afr J Med MedSci; 29: 311-314.
- Vachvanichsanong P, Dissaneewate P, Lim A, McNeil E (2006) Childhood acute renal failure: 22-Year experience in a university hospital in southern Thailand. Pediatrics 118: 786-791.
- Khakurel S, Satyal PR, Agrawal RK, Chhetri PK, Hada R (2005) Acute renal failure in a tertiary care center in Nepal. JNMA J Nepal Med Assoc 44: 32-35.
- Redfern A, Mahmoud H, McCulloch T, Shardlow A, Hall M, et al. (2015) Renal Arcuate Vein Microthrombi-Associated AKI. Clin J Am SocNephrol 10: 180-186.
- Eldehni MT, Roberts SD, Naik R, Vaux E (2010) Case report of ecstasy-induced renal venous thrombosis. NDT Plus 3: 459-460.
- Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D (2013) AKI associated with synthetic cannabinoids: A case series. Clin J Am SocNephrol 8: 523-526.
- Rhidian R, Babu A (2013)Acute kidney injury requiring haemodialysis following ingestion of mephedrone. BMJ Case Rep.
- Whelton A (1999)Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am J Med106:13-24.
- Ulinski T, Guigonis V, Dunan O, Bensman A(2004) Acute renal failure after treatment with non-steroidal anti-inflammatory drugs. Eur J Pediatr163:148-150.
- Clarkson M, Giblin L, O’Connell F, Kelly P, Walshe J,et al. (2004) Acute interstitial nephritis: Clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 19:2778-2783.
Citation: Hijazi F, Attallah N, Madhyastha R (2019)An Unusual Case of Acute Kidney Injury with Edematous Kidneys and Venous Micro Thrombi: A Case Report. J Nephrol Renal Ther 4: 023.
Copyright: © 2019 Fadi Hijazi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

