
Journal of Nuclear Medicine Radiology & Radiation Therapy Category: Medical
Type: Case Report
Pearls and Pitfalls of 18F Sodium Fluoride PET-CT Bone Imaging for Evaluation of Back Pain and Spine Pathology
*Corresponding Author(s):
David PouldarAlbany Medical College, New York, United States
Tel:+310 483 3074,
Email:davidpouldar@gmail.com
Received Date: Aug 07, 2016
Accepted Date: Nov 17, 2016
Published Date: Nov 30, 2016
Abstract
Study design: The following paper is a review article with case presentations showing how to order, perform and interpret 18F sodium fluoride PET/CT of the spine. It discusses its advantages and limitations in evaluating the orthopedic spine patient.
Objective: The purpose of this article is to aid physicians and technologists in performing, analyzing, ordering, and preparing 18F sodium fluoride PET/CT in the spine surgery setting for patients suffering from back pain.
Summary of background data: The evaluation of patients who have already undergone spine surgery who present with persistent pain has always been a diagnostic challenge in the orthopedic setting. While CT and MRI are conventionally used to aid diagnosis, 18F sodium fluoride PET/CT has been an undervalued tool in the setting of the complex diagnosis of back pain especially following surgical fusion. The aim of this paper is to educate radiologists, orthopedic surgeons and clinicians in use of PET/CT bone scan in the orthopedic setting.
Methods: In our review article, we adopt the oncology protocol of 18F sodium fluoride PET/CT and modify it to fit the CT spine protocol. We determine which radiologic parameters are most effective in image acquisition and describe the steps involved in preparing the patient for imaging, imaging acquisition and interpretation. We illustrate this article with cases describing how 18F sodium fluoride PET/CT can alter diagnosis and help manage difficult cases.
Results: 18F sodium fluoride PET/CT is a valuable resource in the orthopedic surgeon’s acumen to identify pain generators of the spine. Additionally, this article further describes the use of PET/CT in the imaging of the spine by discussing PET/CT’s advantages and limitations compared to other imaging modalities, the clinical indications for when to use PET/CT, and the proper patient preparation, radiopharmaceutical preparation, imaging acquisition, data processing and physician analysis and reporting. In our experience PET/CT bone imaging is often superior to CT alone in demonstrating potential post-surgical complications including loosening and healing changes.
Conclusion: This article aids physicians and technologists in understanding what is involved in performing PET/CT studies of the spine. In several cases, we described PET/CT’s advantages in the orthopedic setting in identifying the pain generator of the spine. By using these guidelines, 18F sodium fluoride PET/CT and its potential benefits in the spine surgery setting can be further explored.
Objective: The purpose of this article is to aid physicians and technologists in performing, analyzing, ordering, and preparing 18F sodium fluoride PET/CT in the spine surgery setting for patients suffering from back pain.
Summary of background data: The evaluation of patients who have already undergone spine surgery who present with persistent pain has always been a diagnostic challenge in the orthopedic setting. While CT and MRI are conventionally used to aid diagnosis, 18F sodium fluoride PET/CT has been an undervalued tool in the setting of the complex diagnosis of back pain especially following surgical fusion. The aim of this paper is to educate radiologists, orthopedic surgeons and clinicians in use of PET/CT bone scan in the orthopedic setting.
Methods: In our review article, we adopt the oncology protocol of 18F sodium fluoride PET/CT and modify it to fit the CT spine protocol. We determine which radiologic parameters are most effective in image acquisition and describe the steps involved in preparing the patient for imaging, imaging acquisition and interpretation. We illustrate this article with cases describing how 18F sodium fluoride PET/CT can alter diagnosis and help manage difficult cases.
Results: 18F sodium fluoride PET/CT is a valuable resource in the orthopedic surgeon’s acumen to identify pain generators of the spine. Additionally, this article further describes the use of PET/CT in the imaging of the spine by discussing PET/CT’s advantages and limitations compared to other imaging modalities, the clinical indications for when to use PET/CT, and the proper patient preparation, radiopharmaceutical preparation, imaging acquisition, data processing and physician analysis and reporting. In our experience PET/CT bone imaging is often superior to CT alone in demonstrating potential post-surgical complications including loosening and healing changes.
Conclusion: This article aids physicians and technologists in understanding what is involved in performing PET/CT studies of the spine. In several cases, we described PET/CT’s advantages in the orthopedic setting in identifying the pain generator of the spine. By using these guidelines, 18F sodium fluoride PET/CT and its potential benefits in the spine surgery setting can be further explored.
Keywords
Spine Pathology
INTRODUCTION
Back pain, especially chronic low back pain, is a common symptom that affects the majority of the adult population at one time in their lives being a major cause of morbidity and disability in the United States healthcare system. The cause of back pain is often multifactorial and includes muscular problems, intervertebral disc degeneration, ligament and joint loosening, inflammation, nerve impingement, fracture, spondylolisthesis and other abnormalities. Other more serious causes include osteomyelitis, discitis, metastatic disease and cauda equina syndrome [1]. Most patients with back pain are initially evaluated by only history and physical with imaging reserved for more serious cases. The majority of patients are managed conservatively with physical therapy, medication and patient education. Other therapies include manipulation, traction, acupuncture, bracing and injections with variable results [2].
In the chronic back pain patient, if back pain symptoms persist or progress despite conservative treatment or there is a significant impact on the quality of life, then surgery of the spine becomes an option. The current invasive procedures include artificial disc replacement, decompression surgery, fracture repair, laminectomy, motion preservation surgeries and spinal fusion. Surgical fusion in particular may be performed for trauma, tumor, deformity, infection, recurrent disk herniation and stenosis with instability, spondylolisthesis and other conditions [3]. Complications following surgical development of pain include implant failure, infection, pseudarthrosis, degenerative changes, bone fracture and ligamentous instability, among others [4].
Plain radiographs of the spine are often the first imaging obtained to look for gross abnormalities that are generating the back pain such as vertebral compression fracture. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are recommended with those suspected of serious underlying condition such as infection, malignancy, radiculopathy or spinal stenosis. CT and MRI also provide structural information about the vertebrae, disc space and spinal canal and provide valuable information prior to surgery [3].
Additional imaging modalities include technetium based nuclear medicine bone imaging with planar and Single Photon Emission Tomography (SPECT). Both planar and SPECT bone imaging has been limited by low sensitivity, low spatial resolution and lack of specificity. With the advent of bone imaging with SPECT-CT, both sensitivity and specificity have increased allowing better anatomical correlation [5]. Although many centers have SPECT-CT scanners, they still have a lower resolution compared to PET-CT imaging. With the widespread use of PET-CT scanners for oncological imaging and the availability of 18F-labeled sodium fluoride, the utilization of PET-CT for evaluation of back pain and spinal pathology is more feasible. In our practice 18F-fluoride PET-CT bone imaging has become a useful adjunct in the evaluation of spine pathology especially when traditional imaging such as CT or MRI is inconclusive.
In the chronic back pain patient, if back pain symptoms persist or progress despite conservative treatment or there is a significant impact on the quality of life, then surgery of the spine becomes an option. The current invasive procedures include artificial disc replacement, decompression surgery, fracture repair, laminectomy, motion preservation surgeries and spinal fusion. Surgical fusion in particular may be performed for trauma, tumor, deformity, infection, recurrent disk herniation and stenosis with instability, spondylolisthesis and other conditions [3]. Complications following surgical development of pain include implant failure, infection, pseudarthrosis, degenerative changes, bone fracture and ligamentous instability, among others [4].
Plain radiographs of the spine are often the first imaging obtained to look for gross abnormalities that are generating the back pain such as vertebral compression fracture. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are recommended with those suspected of serious underlying condition such as infection, malignancy, radiculopathy or spinal stenosis. CT and MRI also provide structural information about the vertebrae, disc space and spinal canal and provide valuable information prior to surgery [3].
Additional imaging modalities include technetium based nuclear medicine bone imaging with planar and Single Photon Emission Tomography (SPECT). Both planar and SPECT bone imaging has been limited by low sensitivity, low spatial resolution and lack of specificity. With the advent of bone imaging with SPECT-CT, both sensitivity and specificity have increased allowing better anatomical correlation [5]. Although many centers have SPECT-CT scanners, they still have a lower resolution compared to PET-CT imaging. With the widespread use of PET-CT scanners for oncological imaging and the availability of 18F-labeled sodium fluoride, the utilization of PET-CT for evaluation of back pain and spinal pathology is more feasible. In our practice 18F-fluoride PET-CT bone imaging has become a useful adjunct in the evaluation of spine pathology especially when traditional imaging such as CT or MRI is inconclusive.
TECHNICAL INFORMATION
Positron Emission Tomography (PET) is a tomographic scintigraphic technique that computes the 3-dimensional distribution of radioactivity based on the annihilated 511-keV photons which are emitted by positron emitting labeled radiotracers [6,7]. In PET bone imaging, the radiotracer used is 18F-labeled sodium fluoride. The distribution of 18F follows that of regional blood flow, bone formation and bone metabolism. Retention of the radiotracer is a 2-step process. The 18F ion first covalently exchanges with OH- ion on the surface of hydroxyapatite matrix of bone, and then the 18F ion migrates to the crystalline matrix where it is retained until the bone is remodeled [6,8,9]. Uptake is higher in osteoid or new bone because of higher availability of these binding sites [6,9,10]. Because bone with inflammation, osteoblastic neoplasm, infection and trauma are undergoing a high rate of reformation, 18F accumulates in these areas making focal increased uptake of 18F-labeled NaF a useful marker for these pathological processes. Because imaging reflects bone formation, purely lytic lesion without osteoblastic activity would not be visualized by sodium fluoride [6,11].
In comparison to traditional nuclear bone imaging with technetium 99m Methylene Diphosphate (MDP) or technetium 99m Hydroxymethylene Diphosphonate (HDP), 18F sodium fluoride has better pharmacokinetic qualities including higher uptake in bone and blood clearance rate [7,9,10]. This lends to improved target-to-background ratio, better resolution, higher diagnostic accuracy, and quicker clearance from the circulation. Furthermore, in comparison to MDP which has significant protein binding, 18F minimally binds serum proteins, which leads to shorter clearance time of radiotracer bound proteins and shorter uptake times between radiotracer administration and imaging (3-4 hours for MDP, 2-3 hours for HDP and 1 hour in 18F) [6]. In PET imaging with 18F sodium fluoride, a positron particle is emitted forming a nuclear reaction with an opposite charged electron producing two high energy annihilation photons of 511 KeV, while Tc-99m bone imaging works by direct gamma radiation emission at a lower energy level of 140 KeV detected by a gamma or SPECT camera. The physical half life of 18F sodium fluoride is 110 min compared to technetium-99m of 6 hours, although both radiotracers have a lower biological half life due to early urinary excretion of the radiotracers.
A prescription requesting an 18F sodium fluoride PET-CT examination should include sufficient medical information to warrant medical necessity for the procedure. The request should include the diagnosis, patient history, and questions to be answered [10]. The technologist or physician involved in performing the exam should elicit any additional history from the patient in regards past surgeries and dates of surgeries, other invasive procedures, location and characteristics of pain, other studies performed, and any other relevant information.
Patients should be instructed to be well hydrated before their examination which promotes rapid excretion of the radiotracer to decrease radiation dose and improve image quality [7]. Patients should be informed that they do not need to fast and may take all their usual medications. Unless contraindicated, patients should drink 2 or more 8 oz. glasses of water within 1 hour of the examination and another 2 or more 8 oz. glasses of water after administration of the radiotracer this improves image quality and reduces radiation exposure to the bladder [10].
18F sodium fluoride radiotracer is administered intravenously. Although in the literature doses of 18F sodium fluoride may vary between 185-370 MBq (5-10 mCi), we routinely give 370 MBq of NaF which provides better imaging characteristics. An obese patient may require an even higher dose. Imaging protocols may vary from 30-120 minutes; we find that 1 hour is sufficient wait time before imaging to produce good quality images.
Arms along the torso may produce beam-hardening or scatter artifacts so arms should be raised and supported above their head [12,13]. We find that both lateral and Anterior-Posterior (AP) tomogram are the most effective. From the tomograms, the CT axial imaging is determined and the patient is immediately moved into the CT field for the transmission scan. The position of choice for imaging of the spine is for the patient to be in a supine position, which ensures patient comfort and reduces movement.
In contrast to other CT imaging studies, PET-CT of the spine does not require breath holding. CT imaging of the spine also does not require IV or oral contrasting agents. CT of the spine is should be acquired with higher radiation doses for better image resolution [7,14,15]. The effective radiation dose for 18F sodium fluoride radiation dose is 10 mSv (1 rem) which is approximately half the effective radiation dose for the CT portion of the study which may vary depending on the type of CT scanner and acquisition, body habitus of patient, age and sex, among other factors [10]. In imaging pediatric patients, radiation dose exposures need to be considered.
CT parameter settings determine image quality and radiation dose. Generally, high kV and mAs settings, thin collimation and low pitch result in the best image quality, but also lead to a relatively high radiation exposure to the patient and lengthened examination time. Collimation settings are ideally 1.5 mm or less and pitch has to be less than 2. Lower pitch settings usually mean better image quality, pending the patient stays still. Slimmer and younger patients could be imaged with a lower kV and mAs [16]. Higher CT energy also reduces metal artifact from spinal implants. The gantry angle is fixed on a PET-CT camera (Tables 1 and 2).
In comparison to traditional nuclear bone imaging with technetium 99m Methylene Diphosphate (MDP) or technetium 99m Hydroxymethylene Diphosphonate (HDP), 18F sodium fluoride has better pharmacokinetic qualities including higher uptake in bone and blood clearance rate [7,9,10]. This lends to improved target-to-background ratio, better resolution, higher diagnostic accuracy, and quicker clearance from the circulation. Furthermore, in comparison to MDP which has significant protein binding, 18F minimally binds serum proteins, which leads to shorter clearance time of radiotracer bound proteins and shorter uptake times between radiotracer administration and imaging (3-4 hours for MDP, 2-3 hours for HDP and 1 hour in 18F) [6]. In PET imaging with 18F sodium fluoride, a positron particle is emitted forming a nuclear reaction with an opposite charged electron producing two high energy annihilation photons of 511 KeV, while Tc-99m bone imaging works by direct gamma radiation emission at a lower energy level of 140 KeV detected by a gamma or SPECT camera. The physical half life of 18F sodium fluoride is 110 min compared to technetium-99m of 6 hours, although both radiotracers have a lower biological half life due to early urinary excretion of the radiotracers.
A prescription requesting an 18F sodium fluoride PET-CT examination should include sufficient medical information to warrant medical necessity for the procedure. The request should include the diagnosis, patient history, and questions to be answered [10]. The technologist or physician involved in performing the exam should elicit any additional history from the patient in regards past surgeries and dates of surgeries, other invasive procedures, location and characteristics of pain, other studies performed, and any other relevant information.
Patients should be instructed to be well hydrated before their examination which promotes rapid excretion of the radiotracer to decrease radiation dose and improve image quality [7]. Patients should be informed that they do not need to fast and may take all their usual medications. Unless contraindicated, patients should drink 2 or more 8 oz. glasses of water within 1 hour of the examination and another 2 or more 8 oz. glasses of water after administration of the radiotracer this improves image quality and reduces radiation exposure to the bladder [10].
18F sodium fluoride radiotracer is administered intravenously. Although in the literature doses of 18F sodium fluoride may vary between 185-370 MBq (5-10 mCi), we routinely give 370 MBq of NaF which provides better imaging characteristics. An obese patient may require an even higher dose. Imaging protocols may vary from 30-120 minutes; we find that 1 hour is sufficient wait time before imaging to produce good quality images.
Arms along the torso may produce beam-hardening or scatter artifacts so arms should be raised and supported above their head [12,13]. We find that both lateral and Anterior-Posterior (AP) tomogram are the most effective. From the tomograms, the CT axial imaging is determined and the patient is immediately moved into the CT field for the transmission scan. The position of choice for imaging of the spine is for the patient to be in a supine position, which ensures patient comfort and reduces movement.
In contrast to other CT imaging studies, PET-CT of the spine does not require breath holding. CT imaging of the spine also does not require IV or oral contrasting agents. CT of the spine is should be acquired with higher radiation doses for better image resolution [7,14,15]. The effective radiation dose for 18F sodium fluoride radiation dose is 10 mSv (1 rem) which is approximately half the effective radiation dose for the CT portion of the study which may vary depending on the type of CT scanner and acquisition, body habitus of patient, age and sex, among other factors [10]. In imaging pediatric patients, radiation dose exposures need to be considered.
CT parameter settings determine image quality and radiation dose. Generally, high kV and mAs settings, thin collimation and low pitch result in the best image quality, but also lead to a relatively high radiation exposure to the patient and lengthened examination time. Collimation settings are ideally 1.5 mm or less and pitch has to be less than 2. Lower pitch settings usually mean better image quality, pending the patient stays still. Slimmer and younger patients could be imaged with a lower kV and mAs [16]. Higher CT energy also reduces metal artifact from spinal implants. The gantry angle is fixed on a PET-CT camera (Tables 1 and 2).
| Technical Parameters for Spinal PET-CT 16-slice | |
| Position / Landmark | Supine position |
| Scouts | 2 Scouts / AP: 120kVp/10mALateral: 120kVp/40mA |
| Scan | Helical Full rotation 0.8 seconds |
| Pitch/Mode, Speed (mm) | 1.75:1, 17.50 mm |
| Detector Width x Rows = Beam Collimation | 0.625 x 16 detectors = 10 mm collimation |
| kV/mA/Rotation Time (sec) | 140 kV/ 360 mA / 0.8 sec (360mA for lumbar, 320mA for cervical, and 360mA for thoracic spine) |
| Slice Thickness | Recon : 2.5 mm |
| SFOV | Large |
| Scan Delay (sec) | 20-50 sec |
| Recon 1 Algorithm | Standard |
| Recon 2 Algorithm | DFOV 14 Detail |
| Technical Parameters for Spinal PET-CT 16-slice | |||
| PET Output: | Image | Corrections: Well Counter File: | Default |
| Recon Method: | OS-EM | Well Counter: | Sensitivity & Activity |
| Subsets: | 28 | Randoms: | Delayed Event Subtraction |
| Iterations: | 2 | Normalization: | Default |
| Z-axis Filter: | Standard | Decay: | Yes |
| Post filter: | 6.00 FWHM (mm) | Deadtime: | Yes |
| Diameter: | 70 | Scatter: | Yes |
| Attenuation Type: | Measured | 8-10 min. acquisition time depending on patient size | |
| Transmission Scan: | Most recent | ||
PET emission images should be obtained at 1 hour after radiopharmaceutical administration in patients with normal renal function [10,12]. If comparing two PET studies, one should keep 18F uptake times constant whenever possible [10,12].
After completion of the CT transmission scan, the patient bed is moved into the field of view of the PET scanner, at the rear of the gantry. Images may be acquired in 2D or 3D mode, although 3D is recommended. Scanning is performed in the caudocranial direction with image acquisition starting at the thighs to limit 18F uptake artifacts from excretion from the urinary system [7]. Combined scanning time for CT and PET scans are usually within 30 minutes. Acquisition times may vary due to injected radioactivity, PET sensitivity, and the patient’s BMI, however, typical acquisition times are 2-5 minutes per bed position [10,11,17].
PET images are typically acquired in a 128 x 128 matrix, while CT images are acquired in a 512 x 512 matrix, or 256 x 256 in older scanners [10]. Software packages for reconstruction are widely available. Optimal reconstruction parameters ultimately depend on patient factors and camera settings. Maximum-intensity images may be useful in facilitating the detection of lesions and increased uptake. Integrated PET/CT systems include software that provides aligned CT, 18F sodium fluoride PET and fusion images. Fused images are sent directly to the PACS where they can be displayed in sagittal, coronal and transaxial planes.
INDICATIONS AND INTERPRETATION OF PET-CT BONE IMAGING
PET-CT bone imaging has many advantages in evaluating patient having undergone spine surgery with recurrent back pain after previous laminectomies, fusions and disc replacements. Detection of new bone formation (i.e., trabecularization and cortical remodeling) is difficult to detect on other imaging modalities [11]. The combination of accurately aligned anatomical (CT) and metabolic (PET) information could have a significant advantage in determining the pain generator over conventional CT, MRI, bone scan, SPECT and pain management methods. PET-CT is a useful alternative in assessing regional bone blood flow, mineralization, loosening, nonunion and bone graft incorporation with high anatomical precision and accuracy [9,11]. In various instances, 18F sodium fluoride PET-CT has been proven a valuable modality by displaying information not available through other imaging studies and to be useful in altering the spine surgeons planned treatment course and decision making process. For example, PET-CT is useful in diagnosing nonunion in patients who have previously had surgery in a patient with a normal CT scan. Because of the added physiological information provided by PET, PET-CT is also useful in diagnosing inflammatory processes from hardware impingements which may appear normal on conventional MRI and CT scans [9,12,18].
Normal physiological uptake of 18F can be seen in every viable tissue. Increased uptake of 18F can be seen in benign and malignant tumors, infections, healing sites and other inflammatory processes. 18F radiotracer localization depends on regional blood flow and well as new bone formation [10,12]. The radiologist or clinician interpreting the 18F sodium fluoride bone should look for focal areas of increased uptake that is greater than the similar background activity. For example, uptake at the insertion site of a vertebral screw should be compared to its contralateral side, as well as, other insertion sites at different vertebral levels. 18F uptake and CT findings should also be correlated with timing and type of surgical history, physical examination, localization of pain, and other relevant clinical data. In addition, any other imaging modality should be directly visualized and not to be reliance on the official report. Recent surgeries can have intense uptake in the first few weeks in the postoperative period. Care must be taken in interpreting high osteoblastic activity from bone healing from high osteoblastic activity due to bone pathology. In some cases, a follow up PET-CT may be required if the interpretation is not clear. When viewing images, beware that at times tiny focal areas may be more prominent for no clear reason and should be treated as imaging noise rather than true pathologies especially when the site is distant from the region of true pathology. In addition, as patients adjust their posture and gait in relation to back pain, large areas of the spine may have generalized uptake due to muscle use inequality as well as inflammation. Because of the difficulty of reliable quantitative indices for 18F sodium, we do not rely on standardized uptake value or other method for quantification of bone uptake.
Younger patients that have not undergone surgery should have relative homogenous distribution of the radiotracer throughout the vertebral structures. To look for homogeneity within the osseous structures, the best images to view is the rotating maximum intensity projection. In the older patients, marked age related degenerative changes will develop with various amount of increased radiotracer activity that may not be clinically significant to the underlying problem. These areas of increased uptake can be confused with pathological areas of spinal distress and pain. All areas of the spine undergo degenerative changes. These areas of non-significant uptake include disc degenerative processes, facet joint arthritis and osteophytes formation. Other normal aging process may include mild degrees of disc herniation, degenerative spondylolisthesis, spondylolysis and stenosis [19,20]. Severe osteoporosis can cause quite heterogenous distribution of the radiotracer that can often be confusing with myeloinfiltrative processes as well as metastases. All benign pathologies found on a technetium labeled bone scan can also be encountered with PET bone scan [21-23].
Following spinal surgery, areas of implants and repair have increased radiotracer activity that can last up to a year and sometimes longer. Vertebral bodies that have undergone kyphoplasty have prominent radiotracer uptake lasting longer than a year [24]. Areas of screw insertion or other type of insertion into the bone start with intense uptake during the first few weeks and gradually change to a small mild area surrounding the screws or other metal [25-27].
When evaluating patients with prior surgical spinal fusion, the radiologist must confirm intact surgical fusion on the CT scan without abnormal focal FDG uptake in comparison to other similar sites [28]. All sites of the surgical implant must be analyzed with emphasis on the sites of recurring pain. The PET-CT scan must look for abnormal uptake that may be caused by hardware impingement or other complications of surgical fusion [22,29]. Non-union may be difficult to see on the CT, but shows focal uptake. There may be new levels of degenerative changes, facet disease, or areas of vertebral instability on the 18F sodium fluoride PET-CT that is causing new back pain. Evaluating adjacent vertebral levels from the surgical implant are important. There may be areas of micro-instability which could be a precursor to subsequent discogenic disease. Often invasive procedures such as facet blocks or screw removal may be necessary to confirm spinal pathology after PET bone imaging.
Normal physiological uptake of 18F can be seen in every viable tissue. Increased uptake of 18F can be seen in benign and malignant tumors, infections, healing sites and other inflammatory processes. 18F radiotracer localization depends on regional blood flow and well as new bone formation [10,12]. The radiologist or clinician interpreting the 18F sodium fluoride bone should look for focal areas of increased uptake that is greater than the similar background activity. For example, uptake at the insertion site of a vertebral screw should be compared to its contralateral side, as well as, other insertion sites at different vertebral levels. 18F uptake and CT findings should also be correlated with timing and type of surgical history, physical examination, localization of pain, and other relevant clinical data. In addition, any other imaging modality should be directly visualized and not to be reliance on the official report. Recent surgeries can have intense uptake in the first few weeks in the postoperative period. Care must be taken in interpreting high osteoblastic activity from bone healing from high osteoblastic activity due to bone pathology. In some cases, a follow up PET-CT may be required if the interpretation is not clear. When viewing images, beware that at times tiny focal areas may be more prominent for no clear reason and should be treated as imaging noise rather than true pathologies especially when the site is distant from the region of true pathology. In addition, as patients adjust their posture and gait in relation to back pain, large areas of the spine may have generalized uptake due to muscle use inequality as well as inflammation. Because of the difficulty of reliable quantitative indices for 18F sodium, we do not rely on standardized uptake value or other method for quantification of bone uptake.
Younger patients that have not undergone surgery should have relative homogenous distribution of the radiotracer throughout the vertebral structures. To look for homogeneity within the osseous structures, the best images to view is the rotating maximum intensity projection. In the older patients, marked age related degenerative changes will develop with various amount of increased radiotracer activity that may not be clinically significant to the underlying problem. These areas of increased uptake can be confused with pathological areas of spinal distress and pain. All areas of the spine undergo degenerative changes. These areas of non-significant uptake include disc degenerative processes, facet joint arthritis and osteophytes formation. Other normal aging process may include mild degrees of disc herniation, degenerative spondylolisthesis, spondylolysis and stenosis [19,20]. Severe osteoporosis can cause quite heterogenous distribution of the radiotracer that can often be confusing with myeloinfiltrative processes as well as metastases. All benign pathologies found on a technetium labeled bone scan can also be encountered with PET bone scan [21-23].
Following spinal surgery, areas of implants and repair have increased radiotracer activity that can last up to a year and sometimes longer. Vertebral bodies that have undergone kyphoplasty have prominent radiotracer uptake lasting longer than a year [24]. Areas of screw insertion or other type of insertion into the bone start with intense uptake during the first few weeks and gradually change to a small mild area surrounding the screws or other metal [25-27].
When evaluating patients with prior surgical spinal fusion, the radiologist must confirm intact surgical fusion on the CT scan without abnormal focal FDG uptake in comparison to other similar sites [28]. All sites of the surgical implant must be analyzed with emphasis on the sites of recurring pain. The PET-CT scan must look for abnormal uptake that may be caused by hardware impingement or other complications of surgical fusion [22,29]. Non-union may be difficult to see on the CT, but shows focal uptake. There may be new levels of degenerative changes, facet disease, or areas of vertebral instability on the 18F sodium fluoride PET-CT that is causing new back pain. Evaluating adjacent vertebral levels from the surgical implant are important. There may be areas of micro-instability which could be a precursor to subsequent discogenic disease. Often invasive procedures such as facet blocks or screw removal may be necessary to confirm spinal pathology after PET bone imaging.
CASE EXAMPLES
In the examples below, we highlight 4 cases where 18F sodium fluoride PET-CT altered the diagnosis, management and treatment of patients with spinal pathology.
Case 1: This 59 year old male patient presented with persistent right lower extremity radiculopathy and progressive back pain with a history of spinal fusion at L4-L5 and L5-S1 levels. Initial X-rays at the time of presentation demonstrated solid fusion at L4-L5 with lucency along the superior aspect of the bone graft at L5-S1 level that was questionable for pseudarthrosis. F18 sodium fluoride PET-CT scan was then performed demonstrating solid fusion at L4-L5 and L5-S1 without increased activity. Interestingly, there was intense increased uptake within the right S1 pedicle screw head region with underlying bone sclerosis (Figure 1). Based on these findings it was elected to perform a right S1 screw removal. At 1 year follow-up the patient demonstrated a 40% global improvement of his back and leg pain.
Case 1: This 59 year old male patient presented with persistent right lower extremity radiculopathy and progressive back pain with a history of spinal fusion at L4-L5 and L5-S1 levels. Initial X-rays at the time of presentation demonstrated solid fusion at L4-L5 with lucency along the superior aspect of the bone graft at L5-S1 level that was questionable for pseudarthrosis. F18 sodium fluoride PET-CT scan was then performed demonstrating solid fusion at L4-L5 and L5-S1 without increased activity. Interestingly, there was intense increased uptake within the right S1 pedicle screw head region with underlying bone sclerosis (Figure 1). Based on these findings it was elected to perform a right S1 screw removal. At 1 year follow-up the patient demonstrated a 40% global improvement of his back and leg pain.
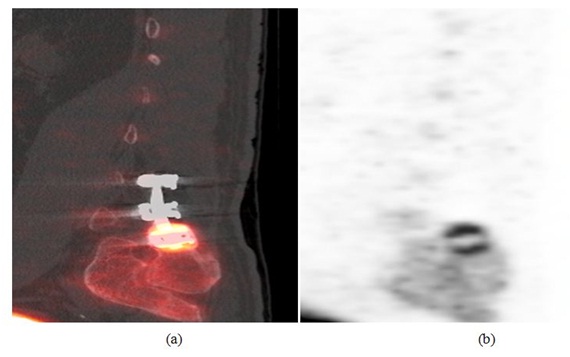
Figure 1: (a) Sagittal PET image (left) and (b) sagittal PET-CT fusion image (right) show intense increased uptake within the right S1 pedicle screw head region, adjacent to the right iliac bone related to a focal stress reaction.
Case 2: A 58 year old female who had previously undergone a L3, L4 and L5 transforaminal lumbar interbody fusions presented with persistent left lower extremity radiculopathy and new onset right lower extremity radiculopathy. The initial presumptive differential diagnosis included adjacent segment disease and progression of the spinal stenosis. A CT scan was subsequently performed that showed intact fusion at the L3-L4 and L4-L5 levels. Since the patient’s progressive pain was concerning, an 18F sodium fluoride PET-CT scan was performed. The PET scan demonstrated abnormal increased uptake at the bilateral L2-L3 facet joints, a spinal level adjacent to the surgically treated level confirming adjacent segment disease (Figure 2). Intra-operatively adjacent segment disease was confirmed as well as pseudarthrosis of the L3-L4 and L4-L5 levels with gross motion. A complete modified revision of the surgical fusion at L3-L4 and L4-L5 was performed. The patient’s overall pain relief at 1-year follow-up was 70% improved compared to prior to surgery.
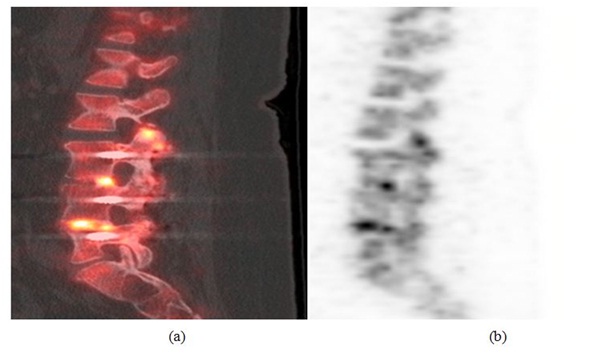
Figure 2: (a) Sagittal PET image (left) and (b) sagittal PET/CT fusion image (right) showing abnormal increased activity at L2-L3 facet joint compatible with adjacent segment disease. There is also increased activin in the disc space of L3-L4 and L4-L5 which is too intense for postoperative changes.
Case 3: A 60 year old female was seen with a history of multiple lumbar fusions including an initially failed anterior fusion using Ray cages. Following persistent back pain, a previously seen orthopedic surgeon performed a posterior fusion, hardware removal and laminectomy of the level above the fusion without pain relief. Based on this complex medical history, a PET/CT bone scan was immediately ordered which confirmed a solid fusion of the spine prosthesis without increased uptake. Unexpectedly, there was moderate increased uptake along the left sacroiliac joint (Figure 3). The patient subsequently underwent a left sacroiliac joint block which completely resolved her symptoms. Following this, the patient received a sacroiliac fusion with near complete resolution of symptoms at 1 year.
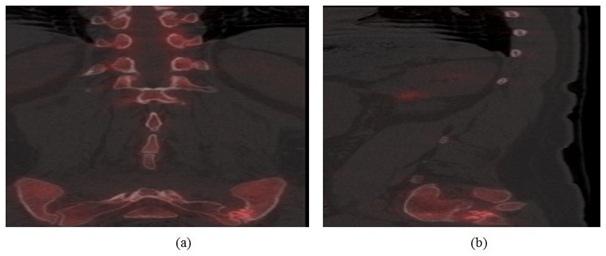
Figure 3: (a) sagittal PET/CT fusion image (left) and (b) Coronal PET/CT fusion image (right) demonstrating increased radiotracer uptake along the left sacroiliac joint.
Case 4: A 61 year old female who previously had undergone spinal fusion at L5-S1 presented with progressively worsening back pain over the course of several years. The presumptive diagnosis was adjacent segment disease. A sodium fluoride PET- CT scan was performed demonstrating bilateral facet degenerative changes and vacuuming at the L4-L5 level with increased radiotracer uptake (Figure 4). A facet block was implemented at L4-L5 level with complete resolution of the patient’s pain thus confirming adjacent segment disease.
Case 4: A 61 year old female who previously had undergone spinal fusion at L5-S1 presented with progressively worsening back pain over the course of several years. The presumptive diagnosis was adjacent segment disease. A sodium fluoride PET- CT scan was performed demonstrating bilateral facet degenerative changes and vacuuming at the L4-L5 level with increased radiotracer uptake (Figure 4). A facet block was implemented at L4-L5 level with complete resolution of the patient’s pain thus confirming adjacent segment disease.
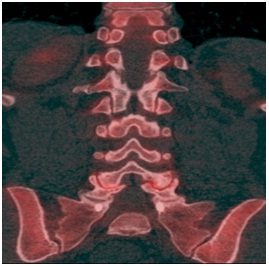
Figure 4: Coronal PET/CT fusion image demonstrating bilateral facet degenerative changes and vacuuming at the L4-L5 level with increased radiotracer activity.
CONCLUSION
The challenging diagnostic workup of post-surgical patients with recurrent back pain currently involves the use of x-rays, CT, bone scan and MRI, as well as various diagnostic block and invasive procedures. Our experience with 18F sodium fluoride PET-CT shows us that with the combination of anatomic findings with metabolic data, additional useful information can be aid in identifying spinal pathology and pain generators in the postoperative spine.
18F sodium fluoride PET-CT imaging is an evolving modality in spine surgery. Education and communication with patients, family members, physicians and technologists is vital. It is important for techniques to be improved, image acquisition times to be decreased, and patient comfort to be increased while performing these studies. This article intends to aid the radiologist and technologists in understanding what is involved in performing 18F sodium fluoride PET-CT studies of the spine. By using this article, we hope this technology can be beneficial in the evaluation of the spinal pathology especially the postoperative spine.
18F sodium fluoride PET-CT imaging is an evolving modality in spine surgery. Education and communication with patients, family members, physicians and technologists is vital. It is important for techniques to be improved, image acquisition times to be decreased, and patient comfort to be increased while performing these studies. This article intends to aid the radiologist and technologists in understanding what is involved in performing 18F sodium fluoride PET-CT studies of the spine. By using this article, we hope this technology can be beneficial in the evaluation of the spinal pathology especially the postoperative spine.
CONFLICT OF INTEREST, ETHICAL TREATMENT OF HUMAN SUBJECTS AND RESEARCH INVOLVING ANIMALS
This research did not receive any outside funding. The authors David Pouldar, Robert Matthews, Vijendra Rao, Guita Rahbar, Eric Chen and Harshwardhan Jain declare that they have no conflicts of interest. The authors disclose that there are no relationships or interests that may directly or potentially influence or impart bias on the work.
REFERENCES
- Last AR, Hulbert K (2009) Chronic Low Back Pain: Evaluation and Management. Am Fam Physician 79: 1067-1074.
- Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, et al. (2007) Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 147: 478-491.
- International Society for the Advancement of Spine Surgery (ISASS) (2011) Policy Statement on Lumbar Spinal Fusion Surgery, International Society for the Advancement of Spine Surgery. Aurora, IL, USA.
- Young PM, Berquist TH, Bancroft LW, Peterson JJ (2007) Complications of spinal instrumentation. Radiographics 27: 775-789.
- Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, et al. (2006) The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 47: 287-297.
- Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, et al. (2008) Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med 49: 68-78.
- Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, et al. (2010) FDG PET and PET/CT: EANM Procedure Guidelines for Tumour PET Imaging: Version 1.0. Eur J Nucl Med Mol Imaging 37: 181-200.
- Kawaguchi M, Tateishi U, Shizukuishi K, Suzuki A, Inoue T (2010) 18F-fluoride uptake in bone metastasis: morphologic and metabolic analysis on integrated PET/CT. Ann Nucl Med 24: 241-247.
- Fischer DR, Maquieira GJ, Espinosa N, Zanetti M, Hesselmann R, et al. (2010) Therapeutic impact of [(18)F]fluoride positron-emission tomography/computed tomography on patients with unclear foot pain. Skeletal Radiol 39: 987-997.
- Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, et al. (2010) SNM Practice Guideline for Sodium 18F-fluoride PET/CT Bone Scans 1.0*. J Nucl Med 51: 1813-1820.
- Temmerman OP, , Raijmakers PG, Heyligers IC, Comans EF, Lubberink M, et al. (2008) Bone metabolism after total hip revision surgery with impacted grafting: evaluation using H2 15O and [18F]fluoride PET; a pilot study. Mol Imaging Biol 10: 288-293.
- Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, et al. (2006) Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med 47: 885-895.
- Beyer T, Antoch G, Müller S, Egelhof T, Freudenberg LS, et al. (2004) Acquisition protocol considerations for combined PET/CT imaging. J Nucl Med 45: 25-35.
- Tins B (2010) Technical aspects of CT imaging of the spine. Insights Imaging 1: 349-359.
- Townsend DW, Beyer T, Blodgett TM (2003) PET/CT scanners: a hardware approach to image fusion. Semin Nucl Med 33: 193-204.
- Quon A, Dodd R, Iagaru A, de Abreu MR, Hennemann S, et al. (2012) Initial investigation of 18F-NaF PET/CT for identification of vertebral sites amenable to surgical revision after spinal fusion surgery. Eur J Nucl Med Mol Imaging 39: 1737-1744.
- Andersson GB (1998) What are the age-related changes in the spine? Baillieres Clin Rheumatol 12: 161-173.
- Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, et al. (2000) A combined PET/CT scanner for clinical oncology. J Nucl Med 41: 1369-1379.
- von Schulthess GK, Steinert HC, Hany TF (2006) Integrated PET/CT: current applications and future directions. Radiology 238: 405-422.
- Rosenbaum SJ, Lind T, Antoch G, Bockisch A (2006) False-positive FDG PET uptake--the role of PET/CT. Eur Radiol 16: 1054-1065.
- Alamo L, Funke M, Grabbe E, Herold T, Kopka L, et al. (2002) Imagine skeletal anatomy of injured cervical spine specimens: comparison of single-slice vs multi-slice helical CT. European Radiology 12: 2107-2111.
- Douglas-Akinwande AC, Buckwalter KA, Rydberg J, Rankin JL, Choplin RH (2006) Multichannel CT: evaluating the spine in postoperative patients with orthopedic hardware. Radiographics 26: 97-110.
- Rydberg J, Liang Y, Teague SD (2004) Fundamentals of multichannel CT. Semin Musculoskelet Radiol 8: 137-146.
- White LM, Buckwalter KA (2002) Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol 6: 5-17.
- Brenner W, Bohuslavizki KH, Eary JF (2003) PET imaging of osteosarcoma. J Nucl Med 44: 930-942.
- Even-Sapir E, Metser U, Flusser G, Zuriel L, Kollender Y, et al. (2004) Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med 45: 272-278.
- Lim R, Fahey FH, Drubach LA, Connolly LP, Treves ST (2007) Early experience with fluorine-18 sodium fluoride bone PET in young patients with back pain. J Pediatr Orthop 27: 277-282.
- Czernin J, Schelbert H (2004) PET/CT Imaging: Facts, Opinions, Hopes, and Questions. J Nucl Med 45: 1-3.
- Hamblen SM, Lowe VJ (2003) Clinical 18F-FDG oncology patient preparation techniques. J Nucl Med Technol 31: 3-7.
Citation: Pouldar D, Matthews R, Rahbar G, Chen E, Jain H, et al. (2016) Pearls and Pitfalls of 18F Sodium Fluoride PET-CT Bone Imaging for Evaluation of Back Pain and Spine Pathology. J Nucl Med Radiol Radiat Ther 1: 005.
Copyright: © 2016 David Pouldar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
