
Laryngeal Actinomycosis: A Case Report and Systematic Review of 32 Cases in the Literature
*Corresponding Author(s):
Benjamin GoogeDepartment Of Otolaryngology, University Of Mississippi Medical Center, 2500 N State St, Jackson, MS 39216, United States
Tel:+1 6623165224,
Email:bgooge@umc.edu
Abstract
Objective
To report a case of vocal cord actinomycosis and provide a systematic review of the literature to provide a reference for its diagnosis and management.
Review methods
Relevant cases from a PubMed search were reviewed for age/gender, risk factors, clinical manifestations, and treatment.
Results
Thirty-two cases of laryngeal actinomycosis have been reported in the literature. Most (80%) cases presented in patient with known risk factors. The majority presented with dysphonia (61.5%). Thirteen (58.3%) involved the true vocal cords. Penicillin based therapy was treatment of choice.
Conclusion
A structured assessment revealed 32 cases of laryngeal actinomycosis in the literature. Actinomycosis should be considered on the differential, even in healthy individuals with symptoms of laryngeal impairment.
Keywords
INTRODUCTION
CASE REPORT
On exam the patient expressed mild dysphonia. No adenopathy, thyroid enlargement, or lesions on the inside of the mouth were noted. Laryngoscopy revealed a sessile whitish plaque-like lesion on the superior aspect of the middle-third right true vocal cord with asymmetric mobility but complete glottis closure (Figure 1). Additional nasopharyngeal and physical exam findings were otherwise unremarkable. The patient was taken to operating room for biopsy of lesion suspicious for vocal cord leukoplakia.
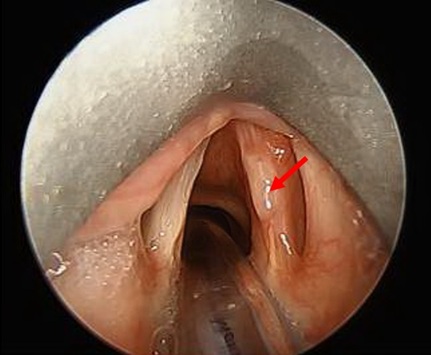
Figure 1: Nodule in middle third of right true vocal cord in our patient in clinic later diagnosed with laryngeal actinomycosis (arrow).
A 0.5 × 0.2 × 0.1 cm lesion was excised from the superior surface of the right true vocal cord. The patient was placed on voice rest two weeks following the procedure. Histopathology revealed a benign specimen consisting largely of a mass of fibrinopurulent exudate with granulation tissue with Actinomyces present. All findings were benign with no evidence of neoplasia or virocytes (Figure 2). The patient was started on amoxicillin 500 mg orally three times per day for one month with scheduled follow-up to monitor continued resolution of actinomycosis. The patient was seen in clinic at one month and two months following the procedure and achieved full resolution of her dysphonia. She denied any additional problems or complaints following the operation.
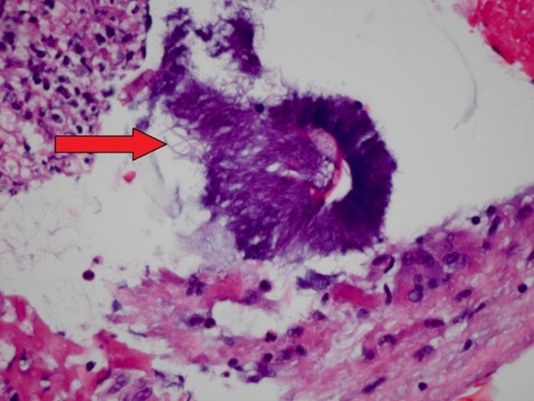
Figure 2: Histopathological image taken from our patient’s vocal cord biopsy. The fibrinopurulent exudate in the vocal cords is centered somewhat on an aggregate of filamentous Actinomyces (arrow).
SYSTEMATIC REVIEW
Methods
RESULTS
| Variable | n | % |
| Age (n=30) | ||
| Average age | 53.7 | |
| Oldest | 77 | |
| Youngest | 14 | |
| Sex (n=30) | ||
| Males | 26 | 86.70% |
| Females | 4 | 13.30% |
| Risk factors (n=30) | ||
| Radiotherapy | 8 | 26.70% |
| None | 6 | 20.00% |
| Immunocompromised | 6 | 20.00% |
| Chronic steroid use | 2 | 6.70% |
| Chemotherapy | 2 | 6.70% |
| Leukemia | 1 | 3.30% |
| HIV | 1 | 3.30% |
| Oropharyngeal trauma | 5 | 16.70% |
| Airway dilation | 2 | 6.70% |
| Tooth extraction | 1 | 3.30% |
| Prolonged intubation | 1 | 3.30% |
| Recent laryngeal surgery | 1 | 3.30% |
| Concurrent laryngeal carcinoma | 3 | 10.00% |
| Concurrent secondary actinomycosis infection | 2 | 6.70% |
| Diabetes | 2 | 6.70% |
| Environmental exposure | 1 | 3.30% |
| Site of involvement (n=27) | ||
| Cricoid region | 3 | 11.10% |
| Anterior commissure | 4 | 16.70% |
| Aryepiglottal fold | 4 | 16.70% |
| Epiglottis | 3 | 12.50% |
| Posterior commissure | 1 | 4.20% |
| Pyriform sinus | 3 | 12.50% |
| Subglottic region | 1 | 4.20% |
| Thyroid cartilage | 1 | 4.20% |
| Vestibular fold | 5 | 20.80% |
| Vocal cord | 14 | 58.30% |
| Clinical presentation (n=29) | ||
| Dyspnea | 5 | 17.20% |
| Cough | 3 | 11.50% |
| Dysphagia | 9 | 34.60% |
| Dysphonia | 16 | 61.50% |
| Edema of ear | 1 | 3.80% |
| Fever | 1 | 3.80% |
| Odynophagia | 3 | 11.50% |
| Pharyngitis | 5 | 19.20% |
| Stridor | 3 | 11.50% |
| Weight loss | 5 | 19.20% |
| Author | Age | Gender | Risk Factors | Site of Involvement | Clinical Presentation | Treatment |
| Lensing F, et al. [8] | 24 | M | Oropharyngeal trauma: airway dilation | Cricoid cartilage and adjacent fat pad | Acute dyspnea and laryngeal pain 2 days after recent airway dilation | IV penicillin 6 weeks |
| Patel S, et al. [9] | 74 | M | Immunocompromised (neutropenia secondary to chemotherapy) | Vocal cord and Vestibular fold | Odynophagia and dysphagia | Ciprofloxacin and amoxicillin/clavulanic acid for 1° month |
| Sari M, et al. [10] | 21 | M | None | Vocal cord | Dysphonia for 6 months | Amoxicillin-clavulanate 625 mg TID for 8 weeks |
| Shaheen SO, et al. [11] | 45 | M | Hx of recent tooth extraction | Cricoid area and vocal cord | Recurrent pharyngitis | IV penicillin 1 mega unit 6 hours for 23 days, then PO 2 mega units daily for 10 days |
| Yoshihama K, et al. [12] | 49 | M | None | Vocal cord | Dysphonia 2 years | Amoxicillin-clavulanate 625 mg orally three times a day for 8 weeks |
| Syed MA, et al. [13] | 74 | M | Post radiotherapy for laryngeal carcinoma | Cricoid region | Dysphagia | Amoxicillin 500 mg three times daily |
| Sims HS, et al. [14] | 47 | M | Immunocompromised (post-transplant recipient, chronic steroid use for SLE) | Vocal cord | Dysphonia and dysphagia | IV penicillin for 2 wks. then PO penicillin for 3 months |
| Artesi L, et al. [15] | 75 | M | None (smoker) | Epiglottis and aryepiglottal fold | Dysphagia progressing to slight dyspnea over 2 months | IV penicillin for 15 days then PO clindamycin 600 mg TID for 4 months |
| Khademi B, et al. [16] | 14 | M | Hx of recent tooth extraction | Vocal cord | Dysphonia worsening over 2 months | IV penicillin for 2 wks. then PO penicillin for 3 months |
| Batur Çali? A, et al. [17] | 66 | M | Concurrent cancer diagnosis (smoker) | Vocal cord and Vestibular fold | Dysphonia 4 months and dyspnea for 2 months | Surgical excision of lesion (for cancer) |
| Batur Çali? A, et al. [17] | 45 | M | Concurrent cancer diagnosis (smoker) | Vestibular fold and Vocal cord | Dysphonia, dyspnea, dysphagia for 1 month | Surgical excision of lesion (for cancer) |
| Ferry T, et al. [18] | 67 | M | Hx of laryngeal carcinoma, chemotherapy and Radiotherapy (also smoker and previous MI) | Vocal cord and Vestibular fold | Dysphonia 2 yrs | Amoxicillin 6g/day for 4 months |
| Meidani M, et al. [19] | 77 | M | Prolonged intubation (from heart surgery) and Pulmonary actinomycosis | Fever, Cough, Weight loss | Penicillin | |
| Menezes MC, et al. [20] | 77 | M | None (smoker) | Aryepiglottic fold | Dysphonia and pain worsening over 2 months | Antibiotics for 6 months |
| Moreno PJM, et al. [21] | 52 | F | Diabetes | Posterior commissure and Vocal cord | Cough 2 months | Cefuroxime-axetil 250 mg twice a day for 3 weeks |
| Wierzbicka M, et al. [22] | 20 | M | None | Epiglottis | Dysphagia and weight loss over several months | IV penicillin and clindamycin |
| Silvestri SB, et al. [23] | 69 | M | Concurrent cancer diagnosis (smoker) | Thyroid cartilage | Dysphonia, persistent cough, weight loss over 8 months | IV ampicillin 500 mg every 6 hours for 12 weeks, then PO for 6 to 12 months |
| Schumann R, et al. [24] | 56 | M | Environmental exposure | Aryepiglottic fold and pyriform sinus | Mild dysphagia and increased edema of ear | Surgical resection of abscess then amoxicillin and clavulanic acid for 3 weeks |
| Yasuda M [25] | 53 | M | Immunocompromised (adult T cell leukemia) | Anterior commissure | Dysphonia | Penicillin 20 million IU daily for 35 days |
| Fernandez SH [26] | 30 | F | Concurrent pulmonary actinomycosis infection | Vocal cord | Dysphonia 1 month | IV penicillin 6 wks. |
| Abed T, et al. [27] | 35 | F | Immunocompromised (chronic steroid use for SLE) | Anterior commissure | Dysphonia | Oral penicillin |
| Tsuji DH, et al. [28] | 68 | M | Post radiotherapy for laryngeal carcinoma | Vocal cord | Dysphonia, odynophagia, and pharyngitis for 3 weeks | Penicillin 10 million IU daily for 40 days |
| García Lozano MC, et al. [29] | 53 | M | None (smoker) | Vocal cord | Dysphonia | Penicillin 600,000 units orally q6 hours for 3 weeks |
| Brandenburg JH, et al. [32] | 67 | M | Post radiotherapy for laryngeal carcinoma | Subglottic | Dyspnea, stridor, dysphonia, weight loss over 7 months | Penicillin |
| Hughes RA Jr, et al. [33] | 66 | M | Diabetes | Pyriform sinus, aryepiglottic fold, hypopharyngeal wall | Pharyngitis and dysphagia over 5 days and weight loss, stridor | Cephalexin 4 months |
Ages of the patient at the time of diagnosis ranged from 14 to 77 with a mean of 53.7 years. The sex of the patients was predominantly male (88.5%). Six (20%) of the patients had no predisposing risk factors reported. Immunocompromised status was present in six (20%) of the cases, most commonly as result of radiotherapy for previous laryngeal carcinoma. Other cases included chronic steroid use, chemotherapy, T-cell leukemia, and Human Immunodeficiency Virus (HIV). Five (16.7%) of the patents had a known history oropharyngeal trauma, two as a result of recent tooth extraction, one due to airway dilation, one due to recent surgery of the larynx and one due to prolonged intubation. Two (6.7%) patients had a secondary actinomycosis infection, both of the more common pulmonary subtype. Three (10%) of the cases were diagnosed in patients in addition to an initial diagnosis of laryngeal carcinoma. Two patients had a diagnosis of type II diabetes mellitus within the past three years. Finally, there was unusual case of suspected environmental exposure in which a patient inadvertently inhaled part of an ear of corn that formed a laryngeal abscess that was later found to be host to an actinomycosis infection.
Specific sub-site of laryngeal involvement was reported in 27 cases. Laryngeal actinomycosis was most commonly reported on or around the substructures of the glottis, the most common being the vocal folds (58.3%). Other common sites included the vestibular folds (20.8%), cricoid (11.1%), anterior/posterior commissures (20.8%), aryepiglottal fold (16.7%), epiglottis (12.5%), and pyriform sinus (12.5%). One case each of invasion into the thyroid cartilage and subglottic region was reported.
Laryngeal actinomycosis presents in a non-specific manner similar to other mass lesions in the larynx or oropharynx. After reviewing the cases, the most common clinical presentation was dysphonia (61.5%), reported in 16 of 29 cases. Nine (34.6%) presented with dysphagia and five (17.2%) with dyspnea. Other presentations included cough, odynophagia, pharyngitis, weight loss, stridor and fever.
Treatment involved excisional biopsy followed by penicillin based therapy in almost all cases. Therapeutic dose, timeframe, and method of administration varied from case to case. Earlier cases usually involved short term IV antibiotics followed by long-term oral administration for prophylaxis against secondary involvement. No cases reported seeding of disease to new site when the primary lesion was restricted only to the larynx. Cases with involvement limited to the larynx were treated with a shorter term dose of oral only antibiotics: 500-625 mg oral penicillin based therapy three times a day for up to 8 weeks following excisional biopsy [10,12,13,21].
DISCUSSION
Although our patient was female, a male predominance of laryngeal actinomycosis exists in the literature (86.7%) [8-30,32-36]. This is not different that the prevalence of pulmonary actinomycosis. A 94 patient, 10-year retrospective study on pulmonary actinomycosis identified a 70.2% male majority [37]. Similarly, the mean age was 52.1 years, just under the 53.7 years in our laryngeal actinomycosis review [37]. The exact causation of these factors has not been definitively established. Due to the retrospective nature of the study, all predisposing factors cannot be known, but aspiration of oral substances has been suggested as previous studies have demonstrated a higher incidence of pulmonary actinomycosis in alcoholics [37].
Known risk factors including immunodeficiency, oropharyngeal trauma, and diabetes, accounted for most patients in our study. Around one fourth of the patients did not have a known risk factor.
Clinical manifestations of the disease are variable, though analogous to other lesions in or around the glottis. Lesions can present both symptomatically and physically as laryngeal tumors. Considering the age of the population and risk factors, the exclusion of laryngeal carcinoma cannot be over emphasized. Patients should be followed in clinic to insure a carcinoma is not present in addition to the actinomycosis. Ongoing symptomology following adequate antibiotic therapy for actinomycosis should warrant prompt swift laryngoscopic evaluation and biopsy for suspected laryngeal carcinoma as 10% of patients with laryngeal actinomycosis have been found to have a current malignancy in our review of the literature [17,23].
Treatment of laryngeal actinomycosis very much depends on extent of involvement. Our case and analysis of similar cases shows that primary infection of the larynx restricted only to the glottis or its substructures, has been successfully treated and suppressed with excision of the lesion and eight weeks of oral penicillin based therapy [10,12,13,21]. More involved lesions, such as those with concurrent pulmonary involvement require more invasive therapy, including a course of intravenous antibiotic therapy.
CONCLUSION
ACKNOWLEDGMENT
REFERENCES
- Smego RA Jr, Foglia G (1998) Actinomycosis. Clin Infect Dis 26: 1255-1261.
- Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, et al. (2014) Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 7: 183-197.
- Sharkawy A, Chow A (2014) Cervicofacial actinomycosis. UpToDate.
- Belmont MJ, Behar PM, Wax MK (1999) Atypical presentations of actinomycosis. Head Neck 21: 264-268.
- Zajc I, Orihovac Z, Bagatin M (1999) Temporal actinomycosis: report of a case. J Oral Maxillofac Surg 57: 1370-1372.
- Feder HM Jr (1990) Actinomycosis manifesting as an acute painless lump of the jaw. Pediatrics 85: 858-864.
- Schaal KP, Beaman BL (1984) Clinical significance of actinomycetes. In: Goodfellow M, Mordarski M, Williams ST (eds.). The Biology of the Actinomycetes, Academic Press, New York, USA. Pg: 391.
- Lensing F, Abele T, Wiggins R 3rd, Quigley E (2014) Laryngeal actinomycosis. Proc (Bayl Univ Med Cent) 27: 35-36.
- Patel S, Jaworek AJ, Patel V, Duckworth LV, Sawhney R, et al. (2014) Laryngeal actinomycosis in an immunocompromised patient. J Voice 28: 838-840.
- Sari M, Yazici M, Ba?lam T, Inanli S, Eren F (2007) Actinomycosis of the larynx. Acta Otolaryngol 127: 550-552.
- Shaheen SO, Ellis FG (1983) Actinomycosis of the larynx. J R Soc Med 76: 226-228.
- Yoshihama K, Kato Y, Baba Y (2013) Vocal cord actinomycosis mimicking a laryngeal tumor. Case Rep Otolaryngol 2013: 361986.
- Syed MA, Ayshford CA, Uppal HS, Cullen RJ (2001) Actinomycosis of the post-cricoid space: an unusual cause of dysphagia. J Laryngol Otol 115: 428-429.
- Sims HS, Heywood BB (2007) Post-transplant actinomycosis of the posterior glottis involving both vocal processes. Otolaryngol Head Neck Surg 137: 967-968.
- Artesi L, Gorini E, Lecce S, Mullace M, Sbrocca M, et al. (2006) Laryngeal actinomycosis. Otolaryngol Head Neck Surg 135: 161-162.
- Khademi B, Dastgheib-Hosseini S, Ashraf M (2011) Vocal Cord Actinomycosis: A Case Report. Iranian Journal of Otorhinolaryngology 23: 49-52.
- Batur Cali? A, Ozbal AE, Ba?ak T, Turgut S (2006) Laryngeal actinomycosis accompanying laryngeal carcinoma: Report of two cases. Eur Arch Otorhinolaryngol 263: 783-785.
- Ferry T, Buiret G, Pignat JC, Chidiac C (2012) Laryngeal actinomycosis mimicking relapse of laryngeal carcinoma in a 67-year-old man. BMJ Case Rep 2012.
- Meidani M, Berjis N, Mokhtari M, Ahmadi N, Rikhtegar MH, et al. (2011) A Rare Case Of Laryngeal and Pulmonary Actinomycosis Co-Infection. Journal of Isfahan Medical School 29: 1-6.
- Menezes MC, Tornin ODS, Botelho RA, De Brito Jr JP, Ortellado DK, et al. (2006) Actinomycosis of the larynx: A case report. Radiol Bras 39: 309-311.
- Melgarejo Moreno PJ, Hellín Meseguer D, Gil Vélez M, Ruiz Macia JA (1997) [Primary laryngeal actinomycosis]. Acta Otorrinolaringol Esp 48: 237-238.
- Wierzbicka M, Bartochowska A, Nowak K, Szyfter W (2013) [Laryngeal actinomycosis - a case report and the review of the literature]. Otolaryngol Pol 67: 308-311.
- Silvestri SB, Carrau RL, Peel R, Hunt JL (2006) Spindle cell carcinoma of the larynx with Actinomyces chondritis of the larynx and trachea. Otolaryngol Head Neck Surg 134: 345-347.
- Schumann R, Lorenz KJ, Tisch M, Maier H (2010) [Laryngeal and pharyngeal actinomycosis]. HNO 58: 867-871.
- Yasuda M (2000) Actinomycosis accompanied with adult T cell leukemia. Otolaryngology - Head and Neck Surgery (Tokyo) 72: 105-108.
- Fernandez SH (1999) Actinomycosis of the vocal cord: a case report. Malays J Pathol 21: 111-115.
- Abed T, Ahmed J, O’Shea N, Payne S, Watters GW (2013) Primary laryngeal actinomycosis in an immunosuppressed woman: a case report. Ear Nose Throat J 92: 301-303.
- Tsuji DH, Fukuda H, Kawasaki Y, Kawaida M, Ohira T (1991) Actinomycosis of the larynx. Auris Nasus Larynx 18: 79-85.
- García Lozano MC, Pérez Sánchez C, Ayala Martínez L (2004) [Laryngeal actinomycosis]. An Otorrinolaringol Ibero Am 31: 237-244.
- Nelson EG, Tybor AG (1992) Actinomycosis of the larynx. Ear Nose Throat J 71: 356-358.
- Tami TA, Ferlito A, Lee KC, Rinaldo A, Singh B (1999) Clinicopathological Consultation Laryngeal pathology in the acquired immunodeficiency syndrome: Diagnostic and therapeutic dilemmas. Ann Otol Rhinol Laryngol 108: 214-220.
- Brandenburg JH, Finch WW, Kirkham WR (1978) Actinomycosis of the larynx and pharynx. Otolaryngology 86: ORL-739-742.
- Hughes RA Jr, Paonessa DF, Conway WF Jr (1984) Actinomycosis of the larynx. Ann Otol Rhinol Laryngol 93: 520-524.
- Langnickel R (1972) [Primary actinomycosis of the larynx]. Z Laryngol Rhinol Otol 51: 147-152.
- Koegel L Jr, Tucker HM (1983) Postoperative actinomycotic infection of the larynx. Otolaryngol Head Neck Surg 91: 213-216.
- Pavlov P, Tenchov G (1956) [Diagnosis and therapy of pharyngolaryngeal actinomycosis]. Khirurgiia (Sofiia) 9: 454-456.
- Kim SR, Jung LY, Oh IJ, Kim YC, Shin KC, et al. (2013) Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infect Dis 13: 216.
Citation: Benjamin G, David K, Kim G, John S (2016) Laryngeal Actinomycosis: A Case Report and Systematic Review of 32 Cases in the Literature. J Otolaryng Head Neck Surg 2: 006.
Copyright: © 2016 Benjamin Googe, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

